Abstract
Synthesis of the new bulky phosphine ligand di(1‑adamantyl)-2-trifluoromethyphenylphosphine is reported, which coordinates with the trifluoromethyl group projected toward the metal center and exhibits a %V bur of 47.3%. Well-defined gold(I) derivatives demonstrate higher catalytic activity for the cycloisomerization of 4-fluoro-N-(prop-2-yn-1-yl)benzamide than di(1‑adamantyl)-2-biphenylphosphine (AdJohnPhos) and di(1‑adamantyl)phenylphosphine analogues.
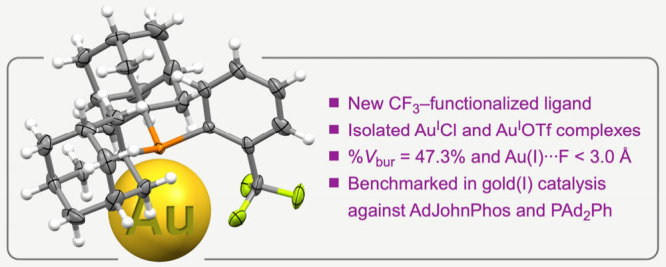
Homogeneous gold catalysis is a powerful synthetic tool, which enables the efficient preparation of a wide variety of organic molecules and natural products. The electrophilic activation of alkynes by monoligated gold(I) cations, in particular, is a widely exploited strategy for the construction of carbon–carbon and carbon–heteroatom bonds. Tuning the reactivity and stability of these catalytically active low coordinate gold(I) fragments is key to effective catalysis and is typically realized by selection of a bulky phosphine or N-heterocyclic carbene ancillary ligand, in combination with a weakly coordinating anion and solvent. , In this context, gold(I) complexes of biaryl-substituted, Buchwald-type, phosphine ligands are often the precatalysts of choice with the sterically imposing profile of these ligands conferred by projection of the ortho-aryl group into the coordination sphere of the metal (Figure A). , As part of our work exploring the coordination chemistry of ortho-trifluoromethylphenyl substituted phosphine ligands, we speculated that this bulky, electron-deficient P-substituent could be beneficial in electrophilic gold(I) catalysis. We herein present our preliminary findings evaluating this hypothesis using conformationally rigid phosphine ligand L1 (Figure B), in which the weakly interacting trifluoromethyl appendage is directed at the metal binding site.
1.
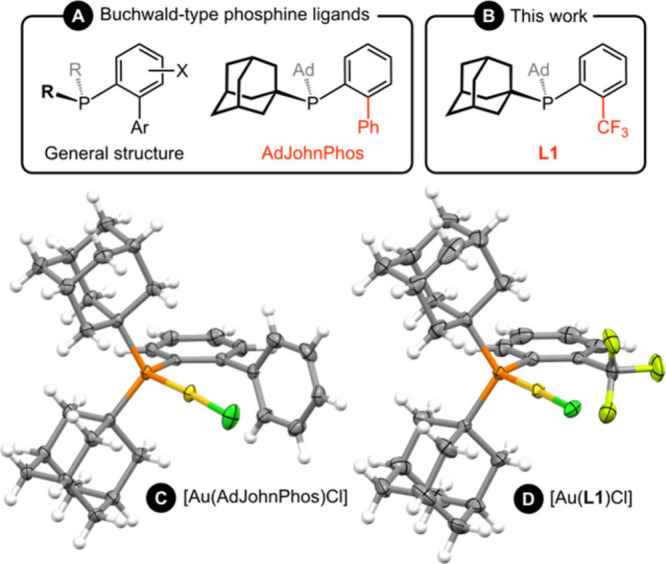
Buchwald-type phosphine ligands (A), the ortho-trifluoromethylphenyl substituted phosphine ligand L1 (B), and solid-state structures of gold(I) chloride complexes of AdJohnPhos (C, CCDC 1550231) and L1 (D) with thermal ellipsoids at 50%.
The new phosphine ligand L1 was prepared following an adapted literature procedure, involving palladium-catalyzed coupling of PAd2H and 2-bromobenzotrifluoride, and isolated in 53% yield. The gold(I) chloride complex was thereafter obtained by reaction with [Au(SMe2)Cl], isolated in 78% yield, and fully characterized. Coordination of the phosphine is marked by a large downfield shift of the quartet 31P resonance δ 24.9 → 96.4 and reduction in the TS J PF coupling constant (57 → 14 Hz) but only a minor perturbation to the corresponding 19F resonance δ –54.3 → –57.0. The solid-state structure demonstrates a close approach of the CF3 appendage to the gold(I) center, which is straddled by two C–F bonds with Au···F contacts of 2.986(6) and 2.988(7) Å (Figure D). Crystallographically characterized instances of noble metal M···F3C bonding are scarce and, using Plenio’s distance threshold of 3.0 Å for a significant M···F interaction, limited to a handful of silver-cation-based systems, epitomized by Ag[Al(OC(CF3)3)4] and group 9 complexes from our group (M···F = 2.36–2.54 Å; CSD v5.46). Further analysis using the SambVca2 program enabled the steric profile of L1 to be quantified, with the resulting %V bur value of 47.3% only slightly smaller than that measured for AdJohnPhos (51.4%) from the known gold(I) chloride derivative (Figure C).
The cycloisomerization of N-propargyl benzamides is a useful model reaction for assessing the relative performance of homogeneous gold(I) catalysts. The transformation of 4-fluoro-N-(prop-2-yn-1-yl)benzamide can be conveniently followed by 19F NMR spectroscopy and was selected to benchmark the utility of L1 in catalysis relative to AdJohnPhos (Scheme ). Under the mild conditions used, [Au(L1)Cl] proved to be an active precatalyst, in combination with the halide abstracting agent Na[BArF 4] (ArF = 3,5-(CF3)2C6H3), delivering the 5-exo-cyclization product with an initial TOF of ∼ 40 h–1: over three times greater than the Buchwald system. Complete consumption of the substrate was observed within 6 h (1 mol % catalyst loading), and comparable catalytic activity was observed when the isolated “preactivated” triflate derivative [Au(L1)(OTf)] was used instead. Emphasizing the decisive role of ligand sterics in this reaction, low catalytic activity was observed when using PAd2Ph (%V bur = 37.6%) as the ancillary ligand.
1. Gold(I) Catalyzed Cycloisomerization of 4-Fluoro-N-(prop-2-yn-1-yl)benzamide .
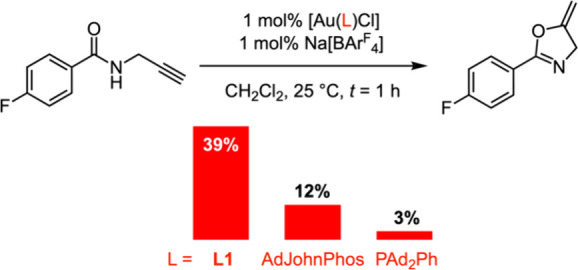
a Conditions: 1.5 mM of precatalyst, 1.5 mM of Na[BArF 4], and 0.15 M substrate in CH2Cl2 stirred under argon. Conversion determined by analysis of aliquots diluted with CH3CN using 19F NMR spectroscopy.
We hope these findings stimulate further investigation into the late transition metal coordination chemistry of L1 and its applications in homogeneous catalysis.
Supplementary Material
Acknowledgments
We thank the Spanish Ministry of Universities and the European Union (Margarita Salas grant funded by the European Union-NextGenerationEU, I.B.) and EUTOPIA alliance (N.R.) for financial support. Crystallographic data were collected using an instrument that received funding from the ERC under the European Union’s Horizon 2020 research and innovation programme (grant agreement No. 637313).
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.organomet.5c00240.
Full experimental details and characterization data for new complexes and selected reactions (PDF)
Deposition Numbers 2467804–2467807 contain the supplementary crystallographic data for this paper. These data can be obtained free of charge via the joint Cambridge Crystallographic Data Centre (CCDC) and Fachinformationszentrum Karlsruhe Access Structures service.
The authors declare no competing financial interest.
References
- a Hashmi A. S. K.. Introduction: Gold Chemistry. Chem. Rev. 2021;121:8309–8310. doi: 10.1021/acs.chemrev.1c00393. [DOI] [PubMed] [Google Scholar]; b Zi W., Toste F. D.. Recent Advances in Enantioselective Gold Catalysis. Chem. Soc. Rev. 2016;45:4567–4589. doi: 10.1039/C5CS00929D. [DOI] [PubMed] [Google Scholar]; c Pflästerer D., Hashmi A. S. K.. Gold Catalysis in Total Synthesis – Recent Achievements. Chem. Soc. Rev. 2016;45:1331–1367. doi: 10.1039/C5CS00721F. [DOI] [PubMed] [Google Scholar]; d Rudolph M., Hashmi A. S. K.. Gold Catalysis in Total Synthesis – an Update. Chem. Soc. Rev. 2012;41:2448–2462. doi: 10.1039/C1CS15279C. [DOI] [PubMed] [Google Scholar]; e Hashmi A. S. K.. Gold-Catalyzed Organic Reactions. Chem. Rev. 2007;107:3180–3211. doi: 10.1021/cr000436x. [DOI] [PubMed] [Google Scholar]; f Gorin D. J., Toste F. D.. Relativistic Effects in Homogeneous Gold Catalysis. Nature. 2007;446:395–403. doi: 10.1038/nature05592. [DOI] [PubMed] [Google Scholar]
- a Dorel R., Echavarren A. M.. Gold(I)-Catalyzed Activation of Alkynes for the Construction of Molecular Complexity. Chem. Rev. 2015;115:9028–9072. doi: 10.1021/cr500691k. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Fürstner A.. From Understanding to Prediction: Gold- and Platinum-Based π-Acid Catalysis for Target Oriented Synthesis. Acc. Chem. Res. 2014;47:925–938. doi: 10.1021/ar4001789. [DOI] [PubMed] [Google Scholar]
- a Collado A., Nelson D. J., Nolan S. P.. Optimizing Catalyst and Reaction Conditions in Gold(I) Catalysis – Ligand Development. Chem. Rev. 2021;121:8559–8612. doi: 10.1021/acs.chemrev.0c01320. [DOI] [PubMed] [Google Scholar]; b Gorin D. J., Sherry B. D., Toste F. D.. Ligand Effects in Homogeneous Au Catalysis. Chem. Rev. 2008;108:3351–3378. doi: 10.1021/cr068430g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Z., Li T., Mudshinge S. R., Xu B., Hammond G. B.. Optimization of Catalysts and Conditions in Gold(I) Catalysis – Counterion and Additive Effects. Chem. Rev. 2021;121:8452–8477. doi: 10.1021/acs.chemrev.0c00713. [DOI] [PubMed] [Google Scholar]
- a Zuccarello G., Zanini M., Echavarren A. M.. Buchwald-Type Ligands on Gold(I) Catalysis. Isr. J. Chem. 2020;60:360–372. doi: 10.1002/ijch.201900179. [DOI] [Google Scholar]; b Nieto-Oberhuber C., López S., Echavarren A. M.. Intramolecular [4 + 2] Cycloadditions of 1,3-Enynes or Arylalkynes with Alkenes with Highly Reactive Cationic Phosphine Au(I) Complexes. J. Am. Chem. Soc. 2005;127:6178–6179. doi: 10.1021/ja042257t. [DOI] [PubMed] [Google Scholar]
- a Poole E. W., Bustos I., Hood T. M., Smart J. E., Chaplin A. B.. Iridium Complexes of an ortho-Trifluoromethylphenyl Substituted PONOP Pincer Ligand. Dalton Trans. 2023;52:1096–1104. doi: 10.1039/D2DT03608H. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Emerson-King J., Prokes I., Chaplin A. B.. Rhodium(III) Complexes Featuring Coordinated CF3 Appendages. Chem.Eur. J. 2019;25:6317–6319. doi: 10.1002/chem.201901184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Hu H., Wang Y., Qian D., Zhang Z.-M., Liu L., Zhang J.. Enantioselective Gold-Catalyzed Intermolecular [2+2]-Cycloadditions of 3-Styrylindoles with N-Allenyl Oxazolidinone. Org. Chem. Front. 2016;3:759–763. doi: 10.1039/C6QO00087H. [DOI] [Google Scholar]; b Lundgren R. J., Peters B. D., Alsabeh P. G., Stradiotto M. A P.. N-Ligand for Palladium-Catalyzed Ammonia Arylation: Coupling of Deactivated Aryl Chlorides, Chemoselective Arylations, and Room Temperature Reactions. Angew. Chem., Int. Ed. 2010;49:4071–4074. doi: 10.1002/anie.201000526. [DOI] [PubMed] [Google Scholar]
- Plenio H.. The Coordination Chemistry of the CF Unit in Fluorocarbons. Chem. Rev. 1997;97:3363–3384. doi: 10.1021/cr970465g. [DOI] [PubMed] [Google Scholar]
- Krossing I.. The Facile Preparation of Weakly Coordinating Anions: Structure and Characterisation of Silverpolyfluoroalkoxyaluminates AgAl(ORF)4, Calculation of the Alkoxide Ion Affinity. Chem.Eur. J. 2001;7:490–502. doi: 10.1002/1521-3765(20010119)7:2<490::AID-CHEM490>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Falivene L., Credendino R., Poater A., Petta A., Serra L., Oliva R., Scarano V., Cavallo L.. SambVca 2. A Web Tool for Analyzing Catalytic Pockets with Topographic Steric Maps. Organometallics. 2016;35:2286–2293. doi: 10.1021/acs.organomet.6b00371. [DOI] [Google Scholar]
- Rotta-Loria N. L., Chisholm A. J., MacQueen P. M., McDonald R., Ferguson M. J., Stradiotto M.. Exploring the Influence of Phosphine Ligation on the Gold-Catalyzed Hydrohydrazination of Terminal Alkynes at Room Temperature. Organometallics. 2017;36:2470–2475. doi: 10.1021/acs.organomet.7b00373. [DOI] [Google Scholar]
- For representative examples see:; a Yu C., Hsiao Y., Löffler J., Kaiser N., Huang B., Lee C., Hung C., Shen J., Yap G. P. A., Gessner V. H., Ong T.. Increasing the Donor Strength of Alkenylphosphines by Twisting the CC Double Bond. Angew. Chem., Int. Ed. 2025;64:e202416764. doi: 10.1002/anie.202416764. [DOI] [PubMed] [Google Scholar]; b Bárta O., Císařová I., Schulz J., Štěpnička P.. Assessing the Influence of Phosphine Substituents on the Catalytic Properties of Self-Stabilised Digold(I) Complexes with Supporting Ferrocene Phosphinonitrile Ligands. N. J. Chem. 2019;43:11258–11262. doi: 10.1039/C9NJ02555C. [DOI] [Google Scholar]; c Rigo M., Hettmanczyk L., Heutz F. J. L., Hohloch S., Lutz M., Sarkar B., Müller C.. Phosphinines versus Mesoionic Carbenes: A Comparison of Structurally Related Ligands in Au(I)-Catalysis. Dalton Trans. 2017;46:86–95. doi: 10.1039/C6DT03766F. [DOI] [PubMed] [Google Scholar]; d Biasiolo L., Del Zotto A., Zuccaccia D.. Toward Optimizing the Performance of Homogeneous L-Au-X Catalysts through Appropriate Matching of the Ligand (L) and Counterion (X–) Organometallics. 2015;34:1759–1765. doi: 10.1021/acs.organomet.5b00308. [DOI] [Google Scholar]; e Kumar M., Jasinski J., Hammond G. B., Xu B.. Alkyne/Alkene/Allene-Induced Disproportionation of Cationic Gold(I) Catalyst. Chem.Eur. J. 2014;20:3113–3119. doi: 10.1002/chem.201304271. [DOI] [PubMed] [Google Scholar]
- Wilkins L. C., Kim Y., Litle E. D., Gabbaï F. P.. Stabilized Carbenium Ions as Latent, Z-type Ligands. Angew. Chem., Int. Ed. 2019;58:18266–18270. doi: 10.1002/anie.201911662. [DOI] [PubMed] [Google Scholar]
- No turnover was observed in the absence of Na[BArF 4].
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


