Abstract
Purpose:
The purpose of this study was to identify the axillary lymph nodes on pre-treatment diagnostic computed tomography (CT) of the chest to determine their position relative to the anatomic axillary borders as defined by the Radiation Therapy Oncology Group (RTOG) breast cancer atlas for radiation therapy planning.
Methods and Materials:
Pretreatment diagnostic CT chest scans available for 30 breast cancer patients with clinically involved lymph nodes were fused with simulation CT. Contouring of axillary levels I, II, and III according to the RTOG guidelines was performed. Measurements were made from the area of distal tumor to the anatomic borders in 6 dimensions for each level.
Results:
Of the 30 patients, 100%, 93%, and 37% had clinical involvement of levels I, II, and III, respectively. The mean number of lymph nodes dissected was 13.6. The mean size of the largest lymph node was 2.4 cm. Extracapsular extension was seen in 23% of patients. In 97% of patients, an aspect of the involved lymph node lay outside of the anatomic border of a level. In 80% and 83% of patients, tumor extension was seen outside the cranial (1.78 ± 1.0 cm; range, 0.28–3.58 cm) and anterior (1.27 ± 0.92 cm; range, 0.24–3.58 cm) borders of level I, respectively. In 80% of patients, tumor extension was seen outside the caudal border of level II (1.36 ± 1.0 cm, range, 0.27–3.86 cm), and 0% to 33% of patients had tumor extension outside the remaining borders of all levels.
Conclusions:
To cover 95% of lymph nodes at the cranial and anterior borders of level I, an additional clinical target volume margin of 3.78 cm and 3.11 cm, respectively, is necessary. The RTOG guidelines may be insufficient for coverage of axillary disease in patients with clinical nodal involvement who are undergoing neoadjuvant chemotherapy, incomplete axillary dissection, or treatment with intensity modulated radiation therapy. In patients with pretreatment diagnostic CT chest scans, fusion with simulation CT should be considered for tumor delineation.
Summary
This study measured the distance of lymph nodes on diagnostic CT at presentation fused with CT simulation relative to the axillary borders defined by the RTOG breast cancer atlas for radiation therapy planning. Tumor extension was noted outside level I cranial and anterior borders, and level II caudal border, requiring additional CTV margin for coverage. This may be relevant for patients with clinical axillary nodal involvement undergoing neoadjuvant chemotherapy, incomplete axillary dissection, or treatment with intensity modulated radiation therapy.
Introduction
Twenty-five percent to 30% of breast cancer patients present with locally advanced breast cancer (LABC) and many additional patients present with lymph node involvement (1). Regional nodal radiation therapy (RT) is often a component of adjuvant RT in patients with pathologic lymph node involvement, with adjuvant RT shown to improve local control (LC) and breast cancer–specific mortality when compared with patients who did receive adjuvant RT (2, 3). In addition, studies have shown a disease-free survival (DFS) benefit specifically attributable to adjuvant regional RT (4, 5). Adjuvant regional RT may also be considered for patients who present with clinical lymph node involvement undergoing neoadjuvant chemotherapy (NAC) with incomplete response (6, 7), or it may be used in place of axillary lymph node dissection (ALND). With increasing use of neoadjuvant chemotherapy for patients to allow for breast conservation and improve cosmetic outcome (8), and increasing reliance on sentinel lymph node dissection (SLND) in place of ALND when nodal RT is a component of therapy (9–11), accurate delineation of the axilla is critical for delivering conformal regional RT to prevent recurrences (12).
The Radiation Therapy Oncology Group (RTOG) published a breast cancer atlas for RT planning with consensus definitions for clinical tumor volumes (CTV) including axillary levels I, II, and III based on anatomic boundaries for the cranial, caudal, anterior, posterior, lateral, and medial dimensions for each axillary level. However, the consensus guidelines may be inadequate in the context of NAC, where the extent of bulky clinical disease involvement may be uncertain, or in the case of residual disease such as with incomplete ALND, or with the use of intensity modulated radiation therapy (IMRT), where precise target definition in required (13, 14).
In the present study, we sought to identify the location of the pretreatment gross tumor volume (GTV) on pretreatment diagnostic CT and to determine the distance relative to the anatomic borders of the axilla. As a primary aim, we planned to use these measurements to determine the optimal margin around the anatomic borders of levels I, II, and III to cover 95% of lymph nodes. As a secondary aim, we assessed dose–volume histogram (DVH) coverage of the pretreatment GTV at axillary levels I, II, and III to determine whether the pre-treatment GTV received an adequate dose.
Methods and Materials
This study was approved by our institutional review board and identified 30 patients with clinical axillary nodal involvement with CT chest scans before receiving NAC treated between August 2004 and March 2015. Patients were simulated for RT in the supine position using a Vac-Lok and wingboard to support arms above the head while 3-mm slice CT simulation data were captured.
Diagnostic CT chest scans were fused with simulation CT for each patient using the Pinnacle Treatment Planning System version 7.4–9.0 (Philips, Andover, MA). Image fusion between the CT chest scans and CT simulation was performed using adjacent ribs as target structures. The pretreatment GTV was contoured based on the fused diagnostic CT findings and was verified by a single attending radiologist specializing in breast and CT body imaging. Axillary levels I, II, and III were contoured according to the RTOG breast cancer atlas (Table 1). Measurements were made from the area of distal tumor to the anatomic borders in the cranial, caudal, anterior, posterior, medial, and lateral dimensions for each level. The data were analyzed separately for measurements of tumor that extended outside the borders or remained within the borders for each level. The DVHs were generated to determine coverage of axillary levels I, II, and III pretreatment GTVs. The DVH information was available for 24 patients. Only 1 patient was treated with IMRT. In addition, patient characteristics, tumor characteristics, and treatment parameters including age, clinical/pathologic stage, type of surgery, number of lymph nodes dissected, size of largest lymph node and axillary lymph nodes involved per pretreatment CT chest scan, presence of ECE, receptor status, and systemic therapy were also recorded.
Table 1.
RTOG regional nodal contours: anatomic boundaries
| Axilla level | Cranial | Caudal | Anterior | Posterior | Lateral | Medial |
|---|---|---|---|---|---|---|
|
| ||||||
| I | Axillary vessels cross lateral edge of pectoralis minor muscle m. | Pectoralis major muscle inserts into ribs | Plane defined by anterior surface of pectoralis major m. and latissimus dorsi m. | Anterior surface of subscapularis m. | Medial border of latissimus dorsi m. | Lateral border of pectoralis minor m. |
| II | Axillary vessels cross medial edge of pectoralis minor m. | Axillary vessels cross lateral edge of pectoralis minor m. | Anterior surface pectoralis minor m. | Ribs and intercostal muscles | Lateral border of pectoralis minor m. | Medial border of pectoralis minor m. |
| III | Pectoralis minor m. insert on coracoid process | Axillary vessels cross medial edge of pectoralis minor m. | Posterior surface pectoralis major m. | Ribs and intercostal muscles | Medial border of pectoralis minor m. | Thoracic inlet |
Abbreviation: RTOG = Radiation Therapy Oncology Group.
Results
The patient and tumor characteristics are shown in Table 2. The average age was 51.9 years (range, 26–73 years). All patients had clinical lymph node involvement classified according to the following clinical stages as given by the American Joint Committee on Cancer 7th edition staging: IIB (n=7), IIIA (n=8), IIIB (n=8), and IIIC (n=4). Three patients (10%) presented with M1 disease and were treated to curative doses for the purpose of LC. Eight patients (27%), including those with M1 disease, had inflammatory breast cancer (IBC). There were 11 (37%) left-sided and 19 (63%) right-sided breast cancers. All patients underwent NAC, most commonly with doxorubicin/cyclophosphamide/paclitaxel in 18 patients (60%). Twenty-eight patients (93%) received a taxol-based chemotherapy as a component of systemic therapy. Eleven tumors (37%) were HER2/neu positive, and 10 of these patients (90%) received trastuzumab as a component of systemic therapy. Fourteen tumors (47%) were estrogen receptor–positive, progesterone receptor–positive, or both. Of patients undergoing surgery, 15 (56%) underwent a modified radical mastectomy and 12 (44%) underwent breast conservation surgery (BCS). Three patients (10%) with M1 disease underwent NAC followed by RT alone.
Table 2.
Patient characteristics
| Patient | Age | Stage | Surgery | No. of lymph nodes dissected | Size of largest lymph node (cm) | Involved lymph node levels | ECE+ | Receptor status | Neoadjuvant chemotherapy |
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| 1 | 35 | cT2N1/ypT0N0 | BCS | 5 | 1.4 | I, II | N | ER−/PR−/H2N− | ACT |
| 2 | 51 | cT3N1/ypT2N1c | MRM | 4 | 2.3 | I, II | N | ER+/PR+/H2N− | ACT |
| 3 | 47 | cT3N1/ypTisN0 | MRM | 16 | 1.2 | I | N | ER+/PR+/H2N+ | TH, abraxane, FECH |
| 4 | 73 | cT3N1/ypT1cN2a | MRM | 14 | 2.2 | I, II | Y | ER+/PR+/H2N− | ACT |
| 5 | 57 | cT3N1/ypT0N1a | MRM | 8 | 6.3 | I, II, III | Y | ER−/PR−/H2N− | ACT |
| 6 | 49 | cT2N1/ypT1cN2a | BCS | 15 | 1 | I, II | N | ER+/PR−/H2N− | ACT |
| 7 | 47 | cT2N1/ypT3N1a | MRM | NS | 6.2 | I, II, III | Y | ER+/PR+/H2N− | ACT |
| 8 | 52 | cT3N3c*/ypT1N1a | MRM | 11 | 1.8 | I, II, SCV | N | ER−/PR−/H2N+ | ACTH |
| 9 | 48 | cT3N1/ypT3N0 | MRM | 9 | 4.4 | I, II, III | N | ER−/PR−/H2N− | ACT |
| 10 | 52 | cT4aN1/ypT3N0 | BCS | 5 | 1 | I, II, III | N | ER−/PR−/H2N− | ACT |
| 11 | 38 | cT4aN1/ypT2N0 | MRM | 4 | 1.9 | I, II, III | N | ER−/PR+/H2N− | ACT |
| 12 | 53 | cT3N1/ypT1aN0 | MRM | 20 | 1.5 | I, II | N | ER+/PR−/H2N+ | ACTH |
| 13 | 62 | cT1cN2a/ypT0N0 | BCS | 15 | 1.7 | I, II, III | N | ER+/PR−/H2N− | ACT |
| 14 | 54 | cT2N1/ypT1cN3a | BCS | 19 | 2.3 | I, II | Y | ER−/PR−/H2N− | ACT |
| 15 | 56 | cT3N1/ypT0N0 | BCS | 11 | 2.6 | I, II, III | N | ER−/PR−/H2N+ | ACTH |
| 16 | 50 | cT4dN1/ypT2N3a | MRM | 19 | 2.2 | I, II | Y | ER+/PR+/H2N− | ACT |
| 17 | 47 | cT4dN1/ypTisN0 | MRM | 13 | 2.2 | I, II | N | ER−/PR−/H2N+ | CTH |
| 18 | 52 | cT4dN1/ypT1bN1mic | MRM | 18 | 1.8 | I, II, III | N | ER−/PR−/H2N− | TC |
| 19 | 37 | cT4dN1/ypT0N1 | MRM | 24 | 2.3 | I, II, III | N | ER−/PR−/H2N+ | ACTH |
| 20 | 67 | cT2N3c*/ypT2N2a | MRM | 9 | 1.2 | I, II, SCV, IM | N | ER−/PR−/H2N− | ACT |
| 21 | 70 | cT2N3c*/ypT1aN2 | BCS | 36 | 5 | I, II, SCV | N | ER−/PR−/H2N− | Carboplatin/eribulin |
| 22 | 73 | cT4dN1M1 | n/a | n/a | 2 | I, II | n/a | ER+/PR−/H2N+ | CMFH, gemcitabine |
| 23 | 58 | cT4dN1M1 | n/a | n/a | 1.5 | I, II, III | n/a | ER+/PR−/H2N+ | ACT |
| 24 | 62 | cT4dN1M1 | n/a | n/a | 1 | I, II | n/a | ER−/PR−/H2N+ | TH |
| 25 | 26 | cT4dN1/ypT0N0 | MRM | 15 | 2.8 | I, II | N | ER−/PR−/H2N+ | CTH |
| 26 | 62 | cT2N1/ypT0N0 | BCS | 3 | 1.1 | I, II, III | N | ER−/PR−/H2N− | ACT |
| 27 | 49 | cT4aN1/ypT1bN2a | BCS | 12 | 5.3 | I, II | Y | ER+/PR−/H2N− | ACT |
| 28 | 60 | cT2N1/ypTisN2a | BCS | 20 | 2.8 | I | Y | ER+/PR+/H2N− | ACT |
| 29 | 34 | cT2N3b/ypT0N0 | BCS | 18 | 1.7 | I, II | N | ER−/PR+/H2N+ | TCH |
| 30 | 37 | cT2N1/ypT0N1mic | BCS | 11 | 1.2 | I, II | N | ER+/PR−/H2N− | ACT |
Abbreviations: ACT = doxorubicin/cyclophosphamide/paclitaxel; BCS = breast conservation surgery; CMF = cyclophosphamide/methotrexate/ fluorouracil; ECE = extracapsular extension; ER = estrogen receptor; FEC = fluorouracil/epirubicin/cyclophosphamide; H = Herceptin; H2N = HER2/neu; IM = internal mammary; LRR = local-regional recurrence; MRM = modified radical mastectomy; n/a = not applicable; PR = progesterone receptor; SCV = supraclavicular.
All patients received targeted nodal RT; 16 patients (53%) were treated with an anterior-posterior or anterior oblique field and 13 patients (43%) with opposed oblique or posterior axillary boost fields. The RT to the chest wall or intact breast included tangents in 22 patients (73%), a photon-electron match in 6 patients (20%), and multifield conformal 3-dimensional conformal RT in 1 patient (3%). One patient (3%) was treated with IMRT to the chest wall and nodal region. The internal mammary nodal (IM) chain was targeted in 11 patients (37%).
The mean number of lymph nodes dissected was 13.6 (range, 3–36). The mean size of the largest lymph node was 2.4 cm. Extracapsular extension was found in 23% of patients, and 100%, 93%, and 37% of patients had clinical involvement of levels I, II, and III, respectively, as determined by pretreatment CT chest scans.
A total of 309 measurements were made: 140 measurements for level I, 114 measurements for level II, and 55 measurements for level III. In 97% of patients, aspect of the presumed involved lymph node lay outside of the anatomic borders of a level. A summary of statistics of tumor extension at all levels is shown in Table 3. The data are reported in centimeters as mean ± standard deviation and minimum-maximum. Eighty-three percent and 80% of patients showed tumor extension outside the cranial (1.78 ± 1.0, 0.28–3.58) (Fig. 1A) and anterior (1.27 ± 0.92, 0.24–3.58) (Figs. 1B and 2) borders, respectively of level I. Ninety-five percent of lymph nodes lay within 3.78 cm of the cranial border and 3.11 cm of the anterior border, respectively, of level I. Eighty percent of patients showed tumor extension outside the caudal border of level II (1.36 ± 1.0, 0.27–3.86). Ninety-five percent of lymph nodes lay within 3.36 cm of the caudal border of level II. Tumor extension was seen to a lesser extent at level I with 33%, 30%m and 30% outside the caudal (0.87 ± 0.39, 0.32–1.18), posterior (0.47 ± 0.34, 0.04–1.12) and lateral (0.56 ± 0.38, 0.29–1.50) borders, respectively. Ninety-five percent of lymph nodes lay within 1.65 cm, 1.15 cm, and 1.32 cm of the caudal, posterior, and lateral borders, respectively, of level I. Within level II, there was tumor extension in 33% and 30% of patients outside the cranial (1.13 ± 0.56, 0.26–2.10) (Fig. 3) and posterior (0.42 ± 0.19, 0.25–0.70) borders, respectively. Ninety-five percent of lymph nodes lay within 2.25 cm and 0.8cm of the cranial and posterior borders of level II, respectively. Within level III, there was tumor extension in 30% of patients outside the caudal (1.25 ± 0.48, 0.60–2.07) border, and 95% of the lymph nodes lay within 2.21 cm of the caudal border of level III. Tumor extension outside the remaining borders of all levels occurred in 0% to 13% of patients. There were no statistically significant differences in terms of presence or degree of tumor extension at a border of axillary levels I, II, or III between patients with left-sided or right-sided breast cancers or between patients with IBC compared with patients with non-IBC (P>.05).
Table 3.
Summary statistics of tumor extension at axillary levels I, II, III
| Axillary level I |
Axillary level II |
Axillary level III |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cranial | Caudal | Anterior | Posterior | Lateral | Medial | Cranial | Caudal | Anterior | Posterior | Lateral | Medial | Cranial | Caudal | Anterior | Posterior | Lateral | Medial | |
|
| ||||||||||||||||||
| Patients with extension at border (%) | 83 | 33 | 80 | 30 | 30 | 0 | 33 | 80 | 13 | 30 | 0 | 0 | 3 | 30 | 0 | 10 | 0 | 0 |
| Mean (cm) | 1.78 | 0.87 | 1.27 | 0.47 | 0.56 | 0 | 1.13 | 1.36 | 1.16 | 0.42 | 0 | 0 | 1.03 | 1.26 | 0 | 0.46 | 0 | 0 |
| Standard deviation | 1 | 1.43 | 0.92 | 0.58 | 0.95 | n/a | 0.74 | 0.99 | 0.55 | 0.23 | n/a | n/a | 0.5 | 0.62 | n/a | 0.35 | n/a | n/a |
| Range (minimum-maximum,) | 0.28–3.58 | 0.32–1.49 | 0.24–3.58 | 0.04–1.12 | 0.25–1.50 | n/a | 0.26–2.10 | 0.27–3.86 | 0.72–2.01 | 0.25–0.70 | n/a | n/a | 0.88–1.86 | 0.63–2.07 | n/a | 0.25–0.69 | n/a | n/a |
Abbreviation: n/a = not applicable.
Fig. 1.
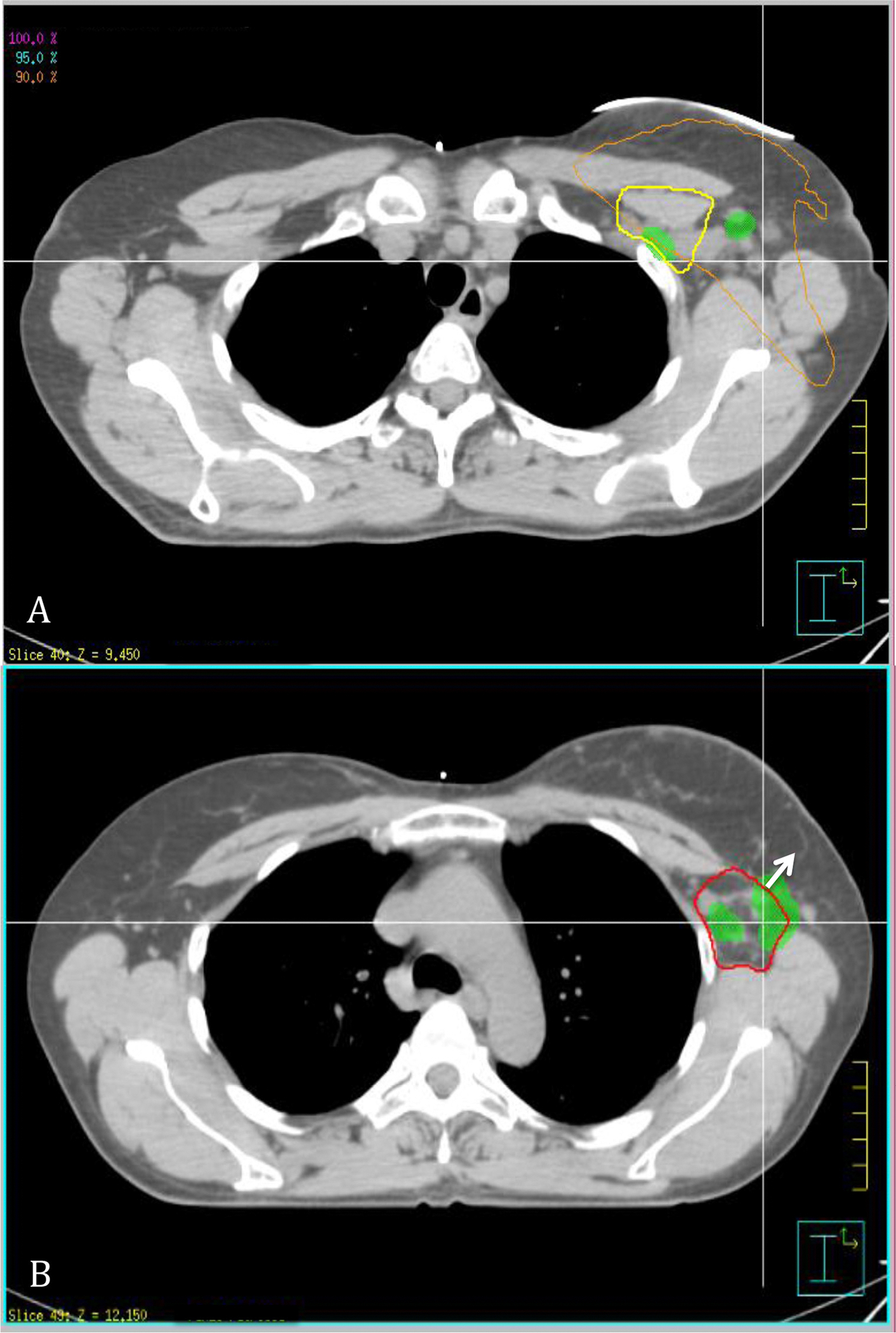
Axial view of pretreatment computed tomographic (CT) chest scan that has been fused with CT simulation. Abbreviations: Green = pretreatment gross tumor volume (GTV); red = level I axilla; yellow = level II axilla. (A) Before treatment, areas of GTV extension outside cranial border of level I axilla with isodose line in orange showing 90% coverage of prescribed dose in this region. (B) Arrow represents area of pretreatment GTV extension outside anterior border of level I axilla. A color version of this figure is available at www.redjournal.org.
Fig. 2.
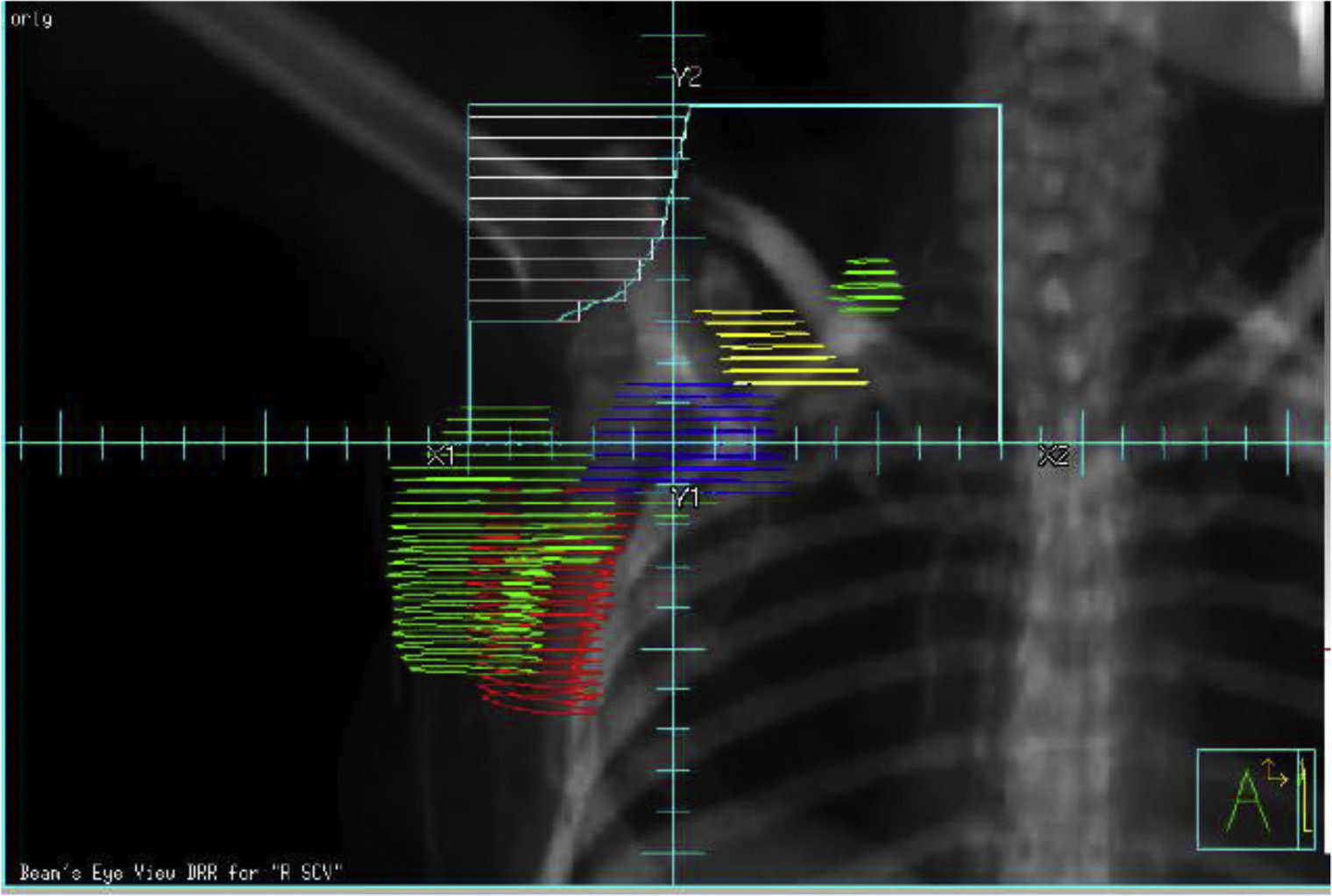
Digitally reconstructed radiograph (DRR) of a conventional low axillary/supraclavicular field showing that aspect of anterior border of axillary level I is out of field. Abbreviations: Green = GTV; red = level I axilla; yellow = level II axilla; blue = level III axilla. A color version of this figure is available at www.redjournal.org.
Fig. 3.
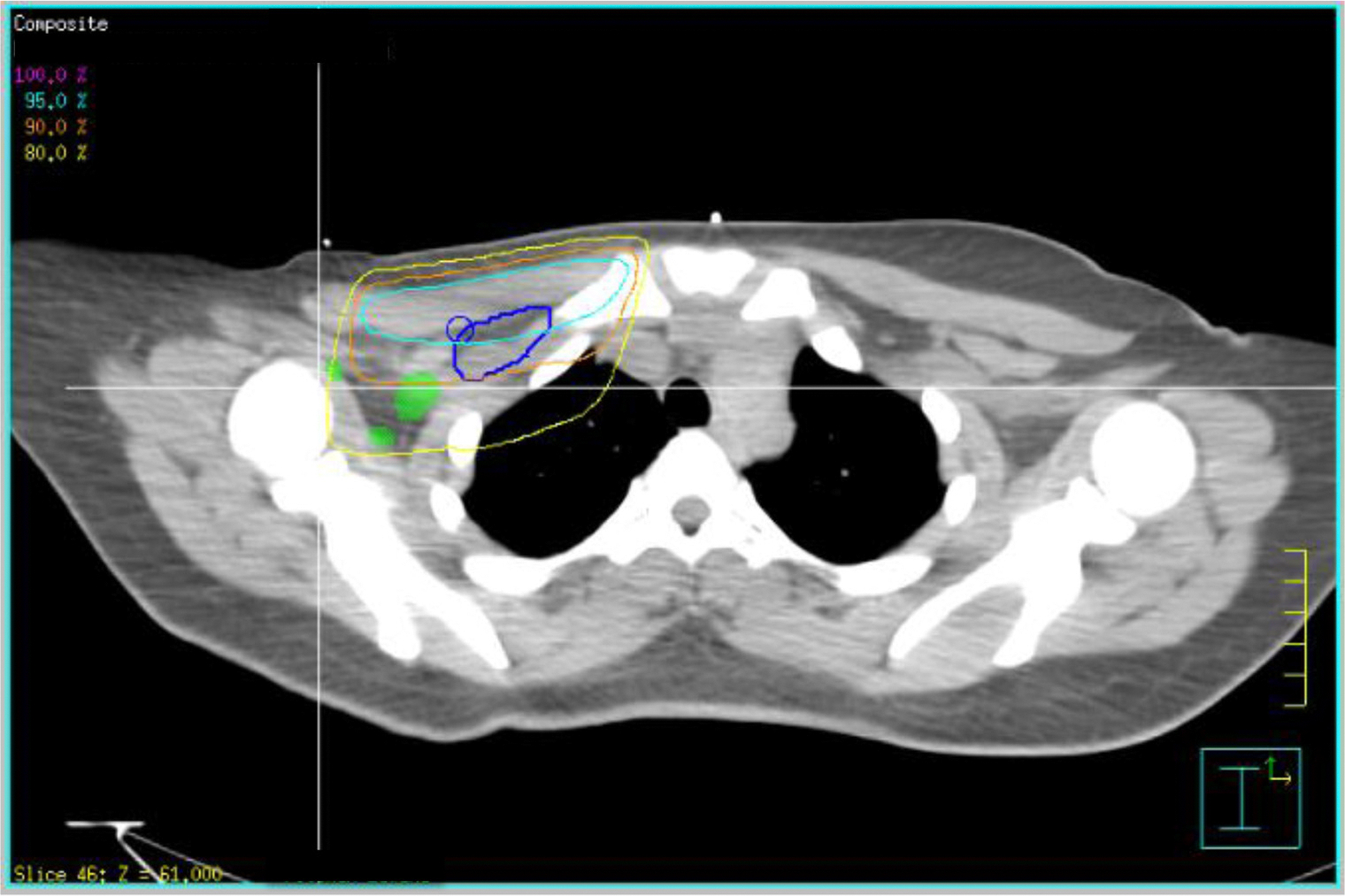
Axial view showing area of pretreatment gross tumor volume (GTV) extension outside cranial borders of level I and level II axillae with 90% isodose line in orange and 80% isodose line in yellow, showing undercoverage of prescribed dose in these regions. Abbreviations: Green = pretreatment GTV; blue = level III axilla. A color version of this figure is available at www.redjournal.org.
For all patients, the mean and median dose prescribed to the chest wall or intact breast, excluding a boost, was 50 Gy and 50.4 Gy, respectively (range, 45–59.4 Gy). The mean and median dose prescribed to the supraclavicular (SCV) fossa was 49 and 50.4 Gy, respectively (range, 45–50.4 Gy). According to each axillary level, the mean and median percentage of level I pretreatment GTV receiving the prescribed dose was 52.1% and 51.2%, respectively (range, 0% to 98.5%); the mean and median percentage of level I pretreatment GTV receiving 45 Gy (V45) was 85.8% and 97.2%, respectively (range, 0% to 100%). The mean and median percentage of level II pretreatment GTV receiving the prescribed dose was 23.3% and 1.0%, respectively (range, 0% to 88.6%); the mean and median percentage of level II V45 was 78.9% and 99.5%, respectively (range, 0% to 100%). The mean and median percentage of level III pretreatment GTV receiving the prescribed dose was 29.3% and 4.4%, respectively (range 0% to 80.4%); the mean and median percentage of level III V45 was 73.0% and 100%, respectively (range, 0% to 100%).
Discussion
In this study, we identified 30 breast cancer patients with clinically involved axillary lymph nodes with diagnostic CT chest scans before receiving NAC. By fusing this with CT simulation, we measured from distal tumor to the anatomic borders of levels I, II, and III according to the RTOG breast cancer atlas guidelines. In 97% of patients, an aspect of the presumed involved lymph node lay outside the anatomic borders of an axillary level. Whereas tumor extension was found in patients at most anatomic borders, it was most notable at the cranial and anterior borders of level I in 80% and 83% of patients, respectively, and the caudal border of level II in 80% of patients. In addition, the pretreatment GTV was often not adequately treated to doses considered acceptable for even microscopic disease through standard nodal fields; the mean percentage of level I, II, and II pre-treatment GTV receiving the full prescribed dose and 45 Gy was consistently below 90% and in many cases was far lower. To cover 95% of lymph nodes, we recommend that an additional CTV margin of 3.78 cm and 3.11 cm, respectively, may be needed in the cranial and anterior borders of level I. Not all areas of tumor extension are at risk for recurrence, including the caudal borders of axillary II, which is within the tangent fields targeting the intact breast or chest wall. Modification of the CTV may be particularly appropriate for patients with clinical nodal involvement undergoing NAC, incomplete ALND, and use of IMRT.
Regional nodal RT has become increasingly important for several reasons. There is evidence that RT of the axillary lymph nodes may be as effective as ALND. The NSABP B-04 randomized breast cancer patients with clinically negative lymph nodes to radical mastectomy, simple mastectomy alone, or simple mastectomy followed by nodal RT and at 25-year follow-up showed no statistically significant differences in local-regional recurrence (LRR), DFS, or overall survival (OS) (11). Given the potential morbidity of ALND, more recently the ACSOG Z0011 randomized trial was conducted to determine the OS of patients with early-stage lymph node–positive breast cancer treated with BCS and SLND alone versus SLND followed by complete ALND. All patients underwent adjuvant whole breast RT using tangential fields. There were no statistically significant differences in 5-year OS or DFS. Furthermore, there was no statistically significant difference in the 5-year LRR with a 0.9% axillary nodal recurrence rate in the SLND-alone group, despite an estimated 27% of patients harboring additional metastases in the undissected axilla (9). Although systemic therapy may play a role in treatment of undissected axillary disease, RT delivered using high-tangent fields targeting levels I and II may have contributed to improved regional control in the SLND-alone group (15, 16). Although the ACSOG Z0011 findings apply to patients with early-stage breast cancer and clinically negative axillae, the increased use of SLND alone will require improved delineation of nodal regions for RT planning. In addition, the AMAROS trial randomized patients with SLND to additional full ALND or axillary RT (10). Regional nodal RT targeted axillary levels I and II, and the inclusion of level III was optional. At a median follow-up time of 6.1 years, the axillary recurrence rates were extremely low in both groups, with no statistically significant differences in DFS or OS. There was a statistically significant increase in lymphedema in patients receiving ALND and a nonsignificant trend toward shoulder movement impairment.
There has also been greater reliance on NAC for breast cancer patients in recent years. It has been estimated that in 2011, 20.5% of patients with invasive breast cancer were treated with NAC compared with 13.9% in 2006 (8). Neoadjuvant therapies allow for a greater degree of breast conservation and improved cosmetic outcomes, and the degree of response to neoadjuvant therapies may lend valuable prognostic information. Decisions for when to use RT and treatment design in the NAC setting, however, have become more complex (7). Of patients with clinical lymph node–positive disease, 20% to 40% undergo conversion to pathologic lymph node–negative disease after receiving neoadjuvant therapies, and the numbers may be greater in patients with HER2/neu-overepressing tumors who receive directed therapies (17, 18). Given that the pathologic extent of disease is often modified with NAC, a precise knowledge of the clinical extent of disease may be unknown without pretreatment imaging, and standard nodal RT fields may not be appropriate in this patient population.
In the past, support for regional nodal RT in patients undergoing BCS with 1 to 3 positive lymph nodes had been extrapolated from the postmastectomy setting (3). Recently, the results of the MA20 and EORTC 22922 trials in abstract format have lent greater support to the benefit of regional RT (4, 5). The MA20 trial compared outcomes in breast cancer patients with a moderate to high risk of regional recurrence undergoing BCS with ALND and systemic therapy randomized to adjuvant breast RT alone versus adjuvant breast and nodal RT. Regional nodal RT targeted the SCV, infraclavicular, and ipsilateral IM lymph nodes in the first to third interspaces and the high axillary lymph nodes. With a median follow-up time of 5.1 years, LRRs were similar between the 2 groups, but there was a marked reduction in regional recurrence, with 21 versus 4 favoring the adjuvant breast and regional nodal RT arm. Of these isolated regional recurrences, 67% occurred in the axilla. This improvement in regional recurrence translated to statistically significant improvements in DFS and distant DFS, and a trend toward OS. Patients who received regional nodal RT had a statistically significant increase in ≥grade 2 pneumonitis and lymphedema and worse cosmetic outcomes (4). Similarly, the EORTC 22922 trial evaluated outcomes in a similar patient population randomized to adjuvant breast or chest wall RT alone versus adjuvant breast or chest wall and nodal RT. Regional nodal RT targeted the medial SCV and internal mammary lymph nodes. With a median follow-up time of 10.9 years, there were statistically significant improvements in DFS, metastasis-free survival, and OS. Moreover, the OS benefit was independent of the number of lymph nodes involved, including lymph node–negative patients (5).
Furthermore, the feasibility of IMRT has been studied in breast cancer (13, 14), and it may be used when other RT techniques prove inadequate, necessitating precise nodal and other target delineation.
Previous studies have evaluated the applicability of the RTOG contouring guidelines for gynecologic malignancies. Consensus guidelines for delineation of the CTV in the postoperative treatment of endometrial and cervical cancer using IMRT have been published (19). To evaluate the anatomic distribution of lymph node metastases, involved pelvic and para-aortic nodes were contoured for 50 cervical cancer patients with pretreatment positron emission tomography (PET)/CT imaging and mapped to the CT simulation imaging used for their postoperative IMRT planning. The authors found that 12 of the 122 nodes were not fully covered by the CTV as defined by the RTOG guidelines (20). Recommendations for increased accuracy of para-aortic and inguinal nodal CTV contouring for conformal RT planning in patients with gynecologic malignancies have been proposed by multiple studies (21–23).
The limitations of the study include that only 1 investigator was involved in contouring, which may have increased accuracy but did not assess reproducibility. Surgical clips demarcating the surgical cavity were not specifically encompassed in the axillary contours, as might be in a clinical setting. Furthermore, the accuracy of fusion may have been limited by a difference in arm position between the CT chest scan and CT simulation, although care was taken to fuse each axillary level independently if this was found to be the case. In this study, 67% of patients had LABC, with a relatively large tumor burden. Thus, results may be limited to this patient population.
Accurate delineation of axillary nodal CTVs is imperative, given its benefit to improve DFS and its increasing use in many clinical contexts. Prevention of regional recurrence is also important, given the limited salvage options in the setting of prior RT (24). Modification of the axillary nodal CTV, especially at the cranial and anterior borders of axillary level I, may be necessary in the setting of clinical nodal involvement followed by NAC, with insufficient or no ALND, and with use of IMRT. This data suggests that in patients with a pretreatment diagnostic CT chest scan available, fusion with simulation CT should be considered.
In conclusion, this study showed that pretreatment diagnostic CT chest imaging fused with CT simulation imaging identified the location of axillary lymph nodes and revealed that most patients had an aspect of the presumed involved lymph node lying outside the anatomic borders of an axillary level. Tumor extension was most notable outside the cranial and anterior borders of level I and was not adequately covered by standard RT nodal fields. To cover 95% of lymph nodes, an additional CTV margin of 3.78 cm and 3.11 cm, respectively would have been needed. This may be particularly relevant in patients with clinical nodal involvement undergoing NAC, incomplete ALND, and with the use of IMRT. In patients with a diagnostic CT chest scan available at presentation, fusion with simulation CT should be considered.
Footnotes
Conflict of interest: none.
Presented in abstract form at the 55th Annual Meeting of the American Society for Radiation Oncology, Atlanta, GA, Sept 22–25, 2013.
References
- 1.Howlader N, Noone AM, Krapcho M, et al. SEER cancer statistics review, 1975–2010. National Cancer Institute. Bethesda, MD. http://seer.cancer.gov/csr/1975_2010/, based on November 2012 SEER data submission, posted to the SEER web site, 2013. [Google Scholar]
- 2.Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: An overview of the randomized trials. Lancet 2005;366:2087–2106. [DOI] [PubMed] [Google Scholar]
- 3.Whelan TJ, Julian J, Wright J, et al. Does locoregional radiation therapy improve survival in breast cancer? A meta-analysis. J Clin Oncol 2000;18:1220–1229. [DOI] [PubMed] [Google Scholar]
- 4.Struikmans H, Collette S, Van den Bogaert W, et al. The benefit of regional irradiation in stage I to III breast cancer: 10 years results of the EORTC Radiation Oncology and Breast Cancer Groups phase III trial 22922/10925 [abstract]. Eur J Cancer 2013;50:S3. [Google Scholar]
- 5.Whelan TJ, Olivotto I, Ackerman J, et al. NCIC CTG MA.20 An intergroup trial of regional nodal irradiation in early breast cancer [abstract]. J Clin Oncol 2011;29:LBA1003. [Google Scholar]
- 6.Mamounas EP, Anderson SJ, Dignam JJ, et al. Predictors of locoregional recurrence after neoadjuvant chemotherapy: Results from combined analysis of National Surgical Adjuvant Breast and Bowel Project B-18 and B-27. J Clin Oncol 2012;30:3960–3966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fowble BL, Einck JP, Kim DN, et al. Role of postmastectomy radiation after neoadjuvant chemotherapy in stage II-III breast cancer. Int J Radiat Oncol Biol Phys 2012;83:494–503. [DOI] [PubMed] [Google Scholar]
- 8.Killelea BK, Yang VQ, Mougalian S, et al. Neoadjuvant chemotherapy for breast cancer increases the rate of breast conservation: Results from the National Cancer Database. J Am Coll Surg 2015;220:1063–1069. [DOI] [PubMed] [Google Scholar]
- 9.Giuliano AE, Hunt KK, Ballman KV, et al. Axillary dissection vs no axillary dissection in women with invasive breast cancer and sentinel lymph node metastasis: A randomized clinical trial. JAMA 2011;305:569–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Straver ME, Meijnen P, van Tienhoven G, et al. Role of axillary clearance after a tumor-positive sentinel node in the administration of adjuvant therapy in early breast cancer. J Clin Oncol 2010;28:731–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fisher B, Jeong JH, Anderson S, et al. Twenty-five year follow-up of a randomized trial comparing radical mastectomy, total mastectomy and total mastectomy followed by irradiation. N Engl J Med 2002;347:567–575. [DOI] [PubMed] [Google Scholar]
- 12.Chen SA, Schuster DM, Mister D, et al. Radiation field design and patterns of locoregional recurrence following definitive radiotherapy for breast cancer. Int J Radiat Oncol Biol Phys 2012;85:309–314. [DOI] [PubMed] [Google Scholar]
- 13.Mukesh MB, Barnett GC, Wilkinson JS, et al. Randomized controlled trial of intensity-modulated radiotherapy for early breast cancer: 5 year results confirm superior overall cosmesis. J Clin Oncol 2013;31:4488–4495. [DOI] [PubMed] [Google Scholar]
- 14.Keller LM, Sopka DM, Li T, et al. Five-year results of whole breast intensity modulated radiation therapy for the treatment of early state breast cancer: The Fox Chase Cancer Center experience. Int J Radiat Oncol Biol Phys 2012;84:881–887. [DOI] [PubMed] [Google Scholar]
- 15.Jagsi R, Chadha M, Moni J, et al. Radiation field design in the ACOSOG Z11 (Alliance) trial. J Clin Oncol 2014;32:3600–3606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hafty BG, Hunt KK, Harris JR, et al. Positive sentinel nodes without axillary dissection: Implications for the radiation oncologist. J Clin Oncol 2011;29:4479–4481. [DOI] [PubMed] [Google Scholar]
- 17.Mamounas T Sentinel node biopsy after neoadjuvant chemotherapy: The pros. Available at: http://ctep.cancer.gov/highlights/docs/mamounas.pdf. Accessed April 21, 2015. [Google Scholar]
- 18.Dominici LS, Negron Gonzalez VM, Buzdar AU, et al. Cytologically proven axillary lymph node metastases are eradicated in patients receiving preoperative chemotherapy with concurrent trastuzamab for HER2-positive breast cancer. Cancer 2010;116:2884–2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lim K, Small W Jr., Portelance L, et al. Consensus guidelines for delineation of clinical target volume for intensity-modulated pelvic radiotherapy for the definitive treatment of cervix cancer. Int J Radiat Oncol Biol Phys 2011;79:348–355. [DOI] [PubMed] [Google Scholar]
- 20.Fontanilla HP, Klopp AH, Lindberg ME, et al. Anatomic distribution of [(18)F] fluorodeoxyglucose-avid lymph nodes in patients with cervical cancer. Pract Radiat Oncol 2013;3:45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takier V, Fontanilla HP, Eifel PJ, et al. Anatomic distribution of fluorodeoxyglucose-avid para-aortic lymph nodes in patients with cervical cancer. Int J Radiat Oncol Biol Phys 2013;85:1045–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kabolizadeh P, Fulay S, Beriwal S. Are Radiation Therapy Oncology Group para-aortic contouring guidelines for pancreatic neoplasm applicable to other malignancies—assessment of nodal distribution in gynecological malignancies. Int J Radiat Oncol Biol Phys 2013;87:106–110. [DOI] [PubMed] [Google Scholar]
- 23.Kim CH, Olson AC, Kim H, et al. Contouring inguinal and femoral nodes; how much margin is needed around the vessels? Pract Radiat Oncol 2012;2:274–278. [DOI] [PubMed] [Google Scholar]
- 24.Lukens JN, Vapiwala N, Hwang WT, et al. Regional nodal recurrence after breast conservation with radiotherapy for women with early-stage breast carcinoma. Int J Radiat Oncol Biol Phys 2009;73:1475–1481. [DOI] [PubMed] [Google Scholar]


