Abstract
Insects, unlike vertebrates, use heteromeric complexes of odorant receptors and co-receptors for olfactory signal transduction. However, the secondary messengers involved in this process are largely unknown. Here, we use the olfactory signal transduction of the aggregation pheromone 4-vinylanisole (4VA) as a model to address this question. When locusts detect 4VA, the pheromone is transported by OBP10 and OBP13 to the OR35–Orco receptor complex, thereby activating downstream pathways in the antenna. A pivotal downstream molecule, the lipid-binding protein Clvs2, facilitates phosphatidylinositol 4,5-bisphosphate transportation across the cytolemma, providing more substrates for inositol trisphosphate (IP3) production. PLCe1, a biosynthetic enzyme, boosts IP3 levels in the antennal lobe of the brain. IP3 is responsible for converting chemical signals from the antenna into neural signals, confirming IP3 as a secondary messenger in olfaction perception instead of GPCR in locusts. These findings elucidate the universal function of IP3 in olfactory signal perception, shedding light on the key nodes of insect olfactory signal transduction.
Unraveling how locusts sense and respond to pheromones without G protein–coupled receptors.
INTRODUCTION
Olfaction is critical for the survival and reproduction of animals, enabling the sensitive detection of pheromone and environmental signals. In vertebrates, odor perception is controlled by odorant receptors (ORs), a class of heterotrimeric guanine nucleotide–binding protein (G protein)–coupled receptors (GPCRs) (1, 2). When an odor ligand binds to a receptor, activated adenylyl cyclase converts adenosine 5′-triphosphate to cyclic adenosine monophosphate (cAMP), which acts as a second messenger to open cyclic nucleotide–gated ion channels. The activation of the receptor can initiate a G protein–coupled phospholipase C (PLC) signaling cascade that results in Ca2+ entry and further signal cascade (3).
Unlike vertebrates, insects use heteromeric complexes composed of odor-specific OrX proteins and highly conserved odorant co-receptor proteins (Orco) for olfactory perception (4–7). Although insect OR heteromers function as a ligand-gated ion channel eliciting Ca2+ influx (8, 9), cAMP has been reported to be involved in the perception via ORs (10). Besides the direct modulation of the sensitivity and desensitization of ORs through phosphorylation events, cAMP can initiate intracellular signaling cascades that influence the temporal dynamics of olfactory sensory neuron responses to odor stimuli (11, 12). In human embryonic kidney (HEK) 293 cells, fly Or22a and Or83b complexes conduct ionic currents in response to cAMP (9). Moreover, the expression of Or83b and Or43a in HEK293 cells results in increased cAMP levels upon ligand exposure, which elevates cell firing rates, indicating a cAMP-dependent excitatory signaling pathway in insect olfactory receptor neurons (13). Previous studies implied the involvement of possible secondary messengers in the signaling cascade downstream of insect ORs. However, what molecules serve as secondary messengers in insects to act in the olfactory perception is largely unknown.
Locust aggregations often result in severe plagues that cause huge economic losses. 4-Vinylanisole (4VA) has been identified as an aggregation pheromone in the migratory locusts (14). 4VA perception via its specific OR35 can accelerate behavioral aggregation and synchronous sexual maturation of female adults (15, 16). The specific knockout of Or35 by CRISPR-Cas9 causes the locusts to lose their ability to perceive 4VA and to aggregate (14). Moreover, the knockout of Orco results in olfactory deficiency (17) and mating location (18). Therefore, 4VA perception in locusts provides an ideal case study to investigate the mechanism of the olfactory signal transduction in insects. Moreover, the signaling cascade downstream of the OR-Orco complexes is not yet explored in locusts.
In this study, we investigated the molecular dynamics both upstream and downstream of the specific receptor complex OR35 and Orco in locusts. We elucidated the signal transduction pathway of 4VA-OR35-Orco from the antenna to the antennal lobe, an olfactory center in the brain, by combining transcriptome screening, quantitative polymerase chain reaction (PCR) validation, protein structure modeling, molecular docking simulations, RNA interference (RNAi) experiments, electrophysiological recordings, pharmacological interventions, and behavioral assays. Ultimately, a comprehensive multigene olfactory signaling pathway was identified. Inositol trisphosphate (IP3) was confirmed as a crucial secondary messenger in the perception of 4VA and other pheromones. These findings provide valuable insights into the role of second messengers in the transduction of olfactory signals in insects.
RESULTS
OBPs and Clvs2 respond to 4VA in locust antennae
Olfactory signal transduction is bifurcated into two main steps: peripheral processing and central nervous system integration. We first investigated 4VA perception within the peripheral nervous system, specifically in the antenna. Transcriptomic analysis has been widely used to identify the genes involved in olfactory downstream signaling pathways in various insect species (19, 20). To explore the key genes associated with 4VA detection, we performed RNA sequencing on antennae from fifth-instar locusts following 4VA exposure (Fig. 1A). A total of 307 differentially expressed genes (DEGs; P value < 0.01) was identified, of which 145 genes exhibited increased expression following 4VA exposure (Fig. 1B). InterPro (IPR) enrichment analysis of DEGs (adjusted P < 0.01, Fisher’s exact test) revealed the notable enrichment of genes in pathways associated with binding proteins, enzymes, and other biological processes. We found 14 key genes related to binding proteins, encompassing those encoding the insect odorant-binding protein, CRAL-TRIO domains, and PBP/GOBP domains (Fig. 1C and table S1). Among these genes, LOCMI16966 (OBP10), LOCMI16977 (OBP13), and LOCMI04198 (Clvs2) exhibited significant up-regulation upon exposure to 4VA fumigation compared to the mineral oil control group by quantitative PCR (qPCR) assays (two-tailed unpaired t test, t = 6.089, P = 0.0003 for OBP13; t = 3.925, P = 0.0024 for OBP10; t = 3.365, P = 0.0051 for Clvs2) (Fig. 1D and fig. S1). To determine the relationship between these genes and behavior, we performed a dual-choice behavioral assay following the RNAi knockdown of OBP10, OBP13, and Clvs2 (two-tailed unpaired t test, t = 7.015, P < 0.001 for dsOBP10; t = 6.683, P < 0.001 for dsOBP13; t = 6.419, P < 0.001 for dsClvs2) (fig. S2A). The knockdown of these genes resulted in a loss of attractive behavior in locusts toward 4VA [Mann-Whitney U (MWU) test, U = 191, P = 0.469 for dsOBP10; U = 166, P = 0.075 for dsOBP13; U = 1000, P = 0.267 for dsClvs2], while the dsGFP [double-stranded RNA (dsRNA) complementary to gfp mRNA]–injected group showed significant preference for 4VA (MWU test, U = 86, P = 0.001 for OBP10; U = 169.5, P < 0.001 for OBP13; U = 596, P = 0.002 for Clvs2) (Fig. 1, E to G, and fig. S2B). Thus, the two OBP and Clvs2 genes are involved in 4VA detection in locusts.
Fig. 1. OBPs and Clvs2 participate in the perception for 4VA in locust antennae.
(A) Schematic of antennal transcriptome sampling and sequencing. (B) Volcano plot of DEGs after 4VA exposure versus oil control in the antennae. Red points denote up-regulated genes, and green points indicate down-regulated genes (P value < 0.01, log2 fold change (log2FC) ≥ 1.0, n = 3). (C) IPR enrichment analysis of up-regulated DEGs. Only IPR terms with P < 0.05 are shown. (D) qPCR verification of DEGs in the IPR enrichment pathway (n = 8). (E) Behavioral responses of gregarious locusts injected with dsGFP and dsOBP10 to 4VA (n = 28). (F) Behavioral responses of gregarious locusts injected with dsGFP and dsOBP13 to 4VA (n = 26). (G) Behavioral responses of gregarious locusts to 4VA after RNAi of Clvs2 (n = 26). (H and I) Predicted structure models of OBP10 and OBP13 complexed with 4VA; α helices are shown in blue (H) and red (I) and loops in gray. BFE, binding free energy. (J and K) Competitive fluorescence binding curve of OBPs with 4VA, with 1-NPN as the fluorescent probe. The dissociation constant of OBP10 was 1.89 μM (J), and that of OBP13 was 3.46 μM (K) (n = 3). (L) Antenna response to 4VA at different concentrations after RNAi of OBP10 and OBP13. (M) Representative spike traces and responses of basiconic sensilla with OBP10 RNAi knockdown to 4VA. (N) Representative spike traces and the response intensity of basiconic sensilla in response to 4VA after injection of dsOBP13. Gene expression differences were analyzed by two-tailed unpaired t test (D), behavioral assays by MWU test [(E) to (G)], and electrophysiological recordings by two-tailed unpaired t test [(L) to (N)]. Data are presented as the means ± SEM. NS, not significant; **P < 0.01; ***P < 0.001.
OBP10 and OBP13 are responsible for transporting 4VA
Olfactory binding proteins (OBPs) play roles in recognizing, binding, and transporting odors to ORs. We predicted the protein structures of OBP10 and OBP13 and analyzed their interactions with 4VA. Both OBP10 and OBP13 contain the PBP-GOBP structural domain typical of insect OBPs, characterized by six conserved cysteine residues (C1 to C6), a three-amino-acid interval between C2 and C3, and an eight-amino-acid interval between C5 and C6 (fig. S3, A and B). The three-dimensional structures of OBP10 and OBP13 were modeled using AlphaFold 2.0, and AutoDock Vina was used to dock 4VA into binding pockets predicted by DeepSite. The predicted structures indicated that OBP10 and OBP13 can bind to 4VA. The predicted binding free energies were −4.8 kcal/mol for OBP13 and −5.6 kcal/mol for OBP10. In general, the more negative the binding free energy, the higher the binding affinity (21, 22), indicating that both OBP10 and OBP13 exhibit strong binding affinity (Fig. 1, H and I). Subsequently, the computational predictions were validated through in vitro binding assays. In vitro, competitive fluorescence binding assays were conducted to evaluate the binding of the fluorescent probe N-phenyl-1-naphthylamine (1-NPN) to OBP10 and OBP13. Both OBPs exhibited significant enhancements in fluorescence intensity upon binding to 1-NPN, with the dissociation constants of the OBPs/1-NPN complex (K1-NPN) of 3.565 μM for OBP10 and 13.75 μM for OBP13 (fig. S4). The competitive fluorescence binding assays confirmed the binding capabilities of OBP10 and OBP13 to 4VA, with OBP10 showing higher binding activity [ligand’s binding affinity (Ki) = 1.89 μM] (Fig. 1J) compared to OBP13 (Ki = 3.46 μM) (Fig. 1K). This matches computational predictions and validates the docking models. So, OBP10 and OBP13 have relatively high binding affinities to 4VA.
Electrophysiological assays following RNAi knockdown further determined the influence of the two OBPs on 4VA perception. The whole antenna of locusts injected with dsOBP10 or dsOBP13 exhibited significantly reduced responses to 4VA compared to control groups injected with dsGFP (OBP10: two-tailed unpaired t test, t = 1.055, P = 0.295 for 0.05 ng/μl; t = 1.528, P = 0.1323 for 0.5 ng/μl; t = 2.603, P = 0.0115 for 5 ng/μl; t = 2.156, P = 0.0349 for 50 ng/μl; t = 2.718, P = 0.0085 for 500 ng/μl; and t = 3.869, P < 0.001 for 5000 ng/μl; OBP13: two-tailed unpaired t test, t = 3.188, P = 0.0026 for 0.05 ng/μl; t = 4.253, P < 0.0013 for 0.5 ng/μl; t = 5.075, P < 0.001 for 5 ng/μl; t = 6.365, P < 0.001 for 50 ng/μl; t = 7.044, P < 0.001 for 500 ng/μl; and t = 5.253, P < 0.001 for 5000 ng/μl) (Fig. 1L). In addition, the electrophysiological responses of basiconic sensilla, where OR35 is located, were tested after injection of dsOBPs (Fig. 1, M and N). The locusts injected with dsOBP10 and dsOBP13 displayed significantly reduced responses from the concentration of 5 ng/μl compared to the dsGFP groups (two-tailed unpaired t test: t = 0.2665, P = 0.7920 for 0.05 ng/μl; t = 0.0656, P = 0.9482 for 0.5 ng/μl; t = 3.702, P = 0.0011 for 5 ng/μl; t = 2.231, P = 0.0353 for 50 ng/μl; t = 2.543, P = 0.0176 for 500 ng/μl; and t = 4.412, P < 0.001 for 5000 ng/μl for OBP10; t = 1.835, P = 0.0755 for 0.05 ng/μl; t = 1.855, P = 0.0728 for 0.5 ng/μl; t = 1.855, P = 0.0228 for 5 ng/μl; t = 3.473, P = 0.0016 for 50 ng/μl; t = 5.232, P < 0.001 for 500 ng/μl; and t = 7.012, P < 0.001 for 5000 ng/μl for OBP13) (Fig. 1, M and N). Therefore, OBP10 and OBP13 are binding proteins responsible for 4VA signal transduction in the peripheral nervous system of locusts.
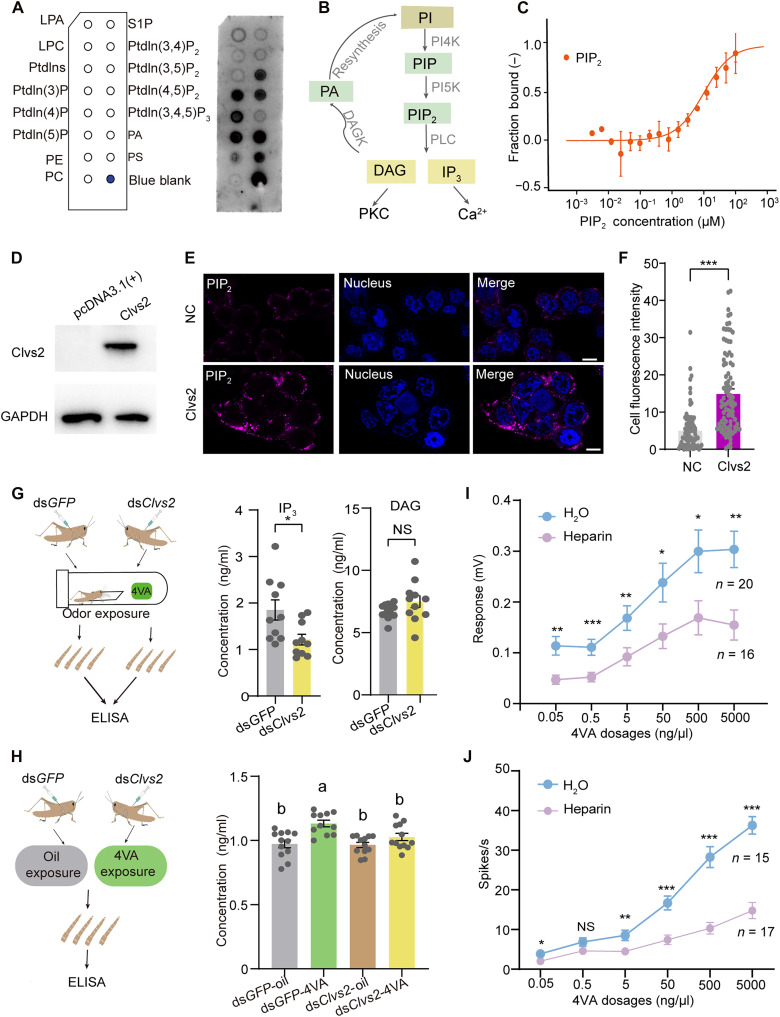
Clvs2 facilitates 4VA perception through enhancing IP3 production
Clavesin 2 (Clvs2) is another gene for significant up-regulation in response to 4VA exposure mentioned above. To investigate the function of Clvs2 in relation to 4VA, we conducted domain analysis and protein structure prediction. Clvs2 contains a prominent SEC14 structural domain (fig. S5A). The secondary structures of Clvs2 adopt the characteristic folds of the SEC14 domain, known as the Sec14-fold, with a core consisting of five parallel β strands arranged in a β sheet, sandwiched by α helices on both sides (Fig. 2A). Following the purification of Clvs2 recombinant protein via eukaryotic expression (fig. S5B), we quantified the binding affinity between Clvs2 and 4VA using a microscale thermophoresis (MST) assay. The binding curve indicated that the Clvs2 protein does not directly bind to 4VA (Fig. 2B). Furthermore, Clvs2 expression did not respond to 4VA when Or35 expression significantly decreased after RNAi knockdown, suggesting that OR35 is necessary for the activation of Clvs2 (Fig. 2C). Immunohistochemistry showed that Clvs2 and OR35 colocated within the shafts of basiconic sensilla, where the outer dendrites of the olfactory receptor neurons are located (Fig. 2D). The colocalization of OR35 and Clvs2 was confirmed when we expressed OR35 and Clvs2 simultaneously in HEK293T cells (Fig. 2E). Membrane labeling with DiI (1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate) further revealed the membrane localization of Clvs2 (Fig. 2, E and F). To investigate the function of Clvs2 in neural signal transmission, we conducted electrophysiological assays after RNAi knockdown. The antenna responses in locusts injected with dsClvs2 exhibited a significant decrease from a concentration of 0.05 ng/μl compared to the control group injected with dsGFP (two-tailed unpaired t test, t = 2.769, P = 0.0084 for 0.05 ng/μl; t = 2.631, P = 0.0119 for 0.5 ng/μl; t = 2.624, P = 0.0121 for 5 ng/μl; t = 2.388, P = 0.0216 for 50 ng/μl; t = 2.035, P = 0.0483 for 500 ng/μl; and t = 2.549, P = 0.015 for 5000 ng/μl) (Fig. 2G). In addition, we observed markedly reduced responses of basiconic sensilla to 4VA from the concentration of 0.05 ng/μl relative to the dsGFP-injected group (two-tailed unpaired t test, t = 2.362, P = 0.0279 for 0.05 ng/μl; t = 2.506, P = 0.0425 for 0.5 ng/μl; t = 2.408, P = 0.0253 for 5 ng/μl; t = 2.334, P = 0.0296 for 50 ng/μl; t = 2.226, P = 0.0371 for 500 ng/μl; and t = 4.167, P < 0.001 for 5000 ng/μl) (Fig. 2, H and I). Thus, the function of Clvs2 is closely related to 4VA sensing, although it cannot directly bind to 4VA.
Fig. 2. Clvs2 participates in 4VA signal transduction downstream of OR35.
(A) Structure model of Clvs2 protein. α helices are shown in orange, loops in gray, and β barrel in light blue. (B) Binding affinity of Clvs2 protein to 4VA was determined using the MST analysis (n = 3). The solid curve was fit to the standard KD-fit function. (C) Clvs2 expression following 4VA exposure and Or35 RNAi (n = 7). “+” and “−” symbols below each bar indicate the presence and absence of the corresponding treatment, respectively: dsRNA injection (dsGFP or dsOr35) and odorant exposure (oil or 4VA). (D) OR35 (magenta) and Clvs2 (green) colocate under basiconic sensilla in antennae of gregarious locusts. NC, negative control; BF, bright field of image. (E) Immunofluorescence labeling of recombinant fusion OR35-mOrange and Clvs2-eGFP in HEK293T cells. Cells transfected with the empty pcDNA3.1(+) plasmid were used as negative controls. Scale bars, 10 μm. (F) Immunofluorescence localization of recombinant fusion Clvs2-eGFP in HEK293T cells. Cytolemma were counterstained with DiI, and nuclei were counterstained with Hoechst 33342. Scale bars, 10 μm. (G) Electroantennography (EAG) responses of antennae to 4VA in gregarious locusts with Clvs2 RNAi knockdown. (H) Representative spike traces of basiconic sensilla to 4VA after RNAi of Clvs2. (I) Responses of basiconic sensilla to 4VA at different concentrations in gregarious locusts with Clvs2 RNAi knockdown. Gene expressions were analyzed by the one-way analysis of variance (ANOVA). The comparisons of electrophysiological recordings were analyzed by the two-tailed unpaired t test [(G) and (I)]. Data in [(G) and (I)] are presented as the means ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001.
Given the presence of a critical SEC14 domain in Clvs2 (fig. S5A), we suspect that Clvs2 functions as a lipid transport protein (23–25). To test this hypothesis, we used recombinant affinity-purified Clvs2 proteins (fig. S5B) and performed protein-lipid blot assays (Fig. 3A). The results demonstrated that Clvs2 exhibited high specificity in binding to phosphatidic acid (PA), phosphatidylserine (PS), phosphatidylinositol 3,5-bisphosphate [PI(3,5)P2], phosphatidylinositol 4,5-bisphosphate [PI(4,5)P2 (PIP2)], and phosphatidylinositol phosphates (PIPs) (Fig. 3A). According to the Kyoto Encyclopedia of Genes and Genomes database, PA, PIP, and PI(4,5)P2 are involved in inositol phosphate metabolism. In the canonical phosphatidylinositol (PtdIns) pathway, PI(4,5)P2 can be enzymatically converted to generate IP3 and diacylglycerol (DAG), serving as key messengers in cellular signaling (Fig. 3B) (26, 27). To confirm the direct binding between PI(4,5)P2 and Clvs2, we performed an MST assay using fluorescently labeled Clvs2 protein and varying concentrations of PI(4,5)P2. The concentration-dependent effects of PI(4,5)P2 on the fluorescently labeled Clvs2 were observed with a dissociation constant (KD) of 5.86 ± 0.59 μM (Fig. 3C). Subsequently, we performed fluorescence quantification of PI(4,5)P2 on the membranes of HEK293T cells transfected with Clvs2. The PI(4,5)P2 content on the cell membrane increased with Clvs2 expression, suggesting that Clvs2 facilitates the translocation of PI(4,5)P2 to the membrane (Fig. 3, D to F). We then conducted an enzyme-linked immunosorbent assay (ELISA) to quantify the concentration of IP3 and DAG in locust antennae after Clvs2 RNAi knockdown. A significant decrease in IP3 levels was observed in the dsClvs2 group compared to the control group (two-tailed unpaired t test, t = 2.600, P = 0.018). However, DAG concentrations remained unchanged after dsClvs2 injection (two-tailed unpaired t test, t = 1.592, P = 0.126) (Fig. 3G). When dsGFP-injected locusts were exposed to 4VA, a significant elevation in IP3 levels was observed compared to the control treatment. Compared to the control, IP3 levels in the antennae of locusts with Clvs2 knockdown did not change after 4VA exposure (Fig. 3H). These results suggest that IP3 is responsible for the signal transduction within locust antennae. To further validate this, we recorded the antenna and single sensillum responses of locusts after injecting an IP3 receptor inhibitor, heparin (28). From the concentration of 0.05 to 5000 ng/μl, heparin elicited a significant decrease in the responses of locust antennae to 4VA (two-tailed unpaired t test, t = 3.380, P = 0.002 for 0.05 ng/μl; t = 3.944, P < 0.0015 for 0.5 ng/μl; t = 2.417, P = 0.0214 for 5 ng/μl; t = 2.282, P = 0.0291 for 50 ng/μl; t = 2.301, P = 0.0278 for 500 ng/μl; and t = 3.039, P < 0.001 for 5000 ng/μl) (Fig. 3I). Similarly, heparin injection significantly attenuated the responses of basiconic sensilla to 4VA compared to the control group (two-tailed unpaired t test, t = 2.683, P = 0.0118 for 0.05 ng/μl; t = 1.517, P = 0.140 for 0.5 ng/μl; t = 2.809, P = 0.0087 for 5 ng/μl; t = 4.409, P < 0.001 for 50 ng/μl; t = 5.636, P < 0.0001 for 500 ng/μl; and t = 7.352, P < 0.001 for 5000 ng/μl) (Fig. 3J). Moreover, to further clarify the role of OR35 in IP3 signaling, we measured IP3 levels after Or35 knockdown. 4VA treatment failed to increase IP3 levels in dsOr35-injected locusts (fig. S6). Therefore, Clvs2-mediated IP3 signaling emerges as a crucial component downstream of the OR35-Orco complex of 4VA perception.
Fig. 3. Clvs2-mediated IP3 signaling regulates 4VA perception downstream of OR35.
(A) Lipid blot overly assay of Clvs2 (right) and schematic diagram of biomembrane lipids on strips (left), including lysophosphatidic acid (LPA), lysophosphocholine (LPC), PtdIns, phosphatidylinositol (3)-phosphate [PI(3)P, PtdIn(3)P], phosphatidylinositol (4)-phosphate [PI(4)P, PtdIn(4)P], phosphatidylinositol (5)-phosphate [PI(5)P, PtdIn(5)P], phosphatidylethanolamine (PE), phosphatidylcholine (PC), phosphatidylinositol (3,4)-bisphosphate [PI(3,4)P2, PtdIn(3,4)P2], phosphatidylinositol (3,5)-bisphosphate [PI(3,5)P2, PtdIn(3,5)P2], PI(4,5)P2 [PtdIn(4,5)P2], phosphatidylinositol (3,4,5)-trisphosphate [PI(3,4,5)P3, PtdIn(3,4,5)P3], PA, and phosphatidylserine (PS). (B) Schematic of the PtdIns metabolic pathway. The green frame highlights Clvs2-binding lipids. (C) The MST assay reveals specific binding between recombinant protein Clvs2 and PI(4,5)P2 (PIP2). The PI(4,5)P2 (ligand) was gradient diluted. The solid curve represents the standard KD fit (n = 3). (D) Western blot validation of Clvs2 expression in HEK293T cells. GAPDH, glyceraldehyde-3-phosphate dehydrogenase. (E and F) Clvs2 expression enhances membrane-associated PI(4,5)P2 in HEK293T cells compared with negative control [empty pcDNA3.1(+)] (G) Quantification of second messenger contents (IP3 and DAG) in locust antennae following Clvs2 RNAi (n = 10). (H) Measurement of antenna IP3 levels after 4VA exposure (n = 11). (I and J) Dose-response curves of antennal and basiconic sensilla responses to 4VA after heparin (IP3 receptor inhibitor) treatment. The comparisons of the ELISA of IP3 and DAG were analyzed by the two-tailed unpaired t test (G). The comparisons of electrophysiological recordings were analyzed by the two-tailed unpaired t test [(I) and (J)]. Different letters indicate multiple comparisons of significant differences based on the one-way ANOVA (P < 0.05). Data in [(F) to (J)] are presented as the means ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001.
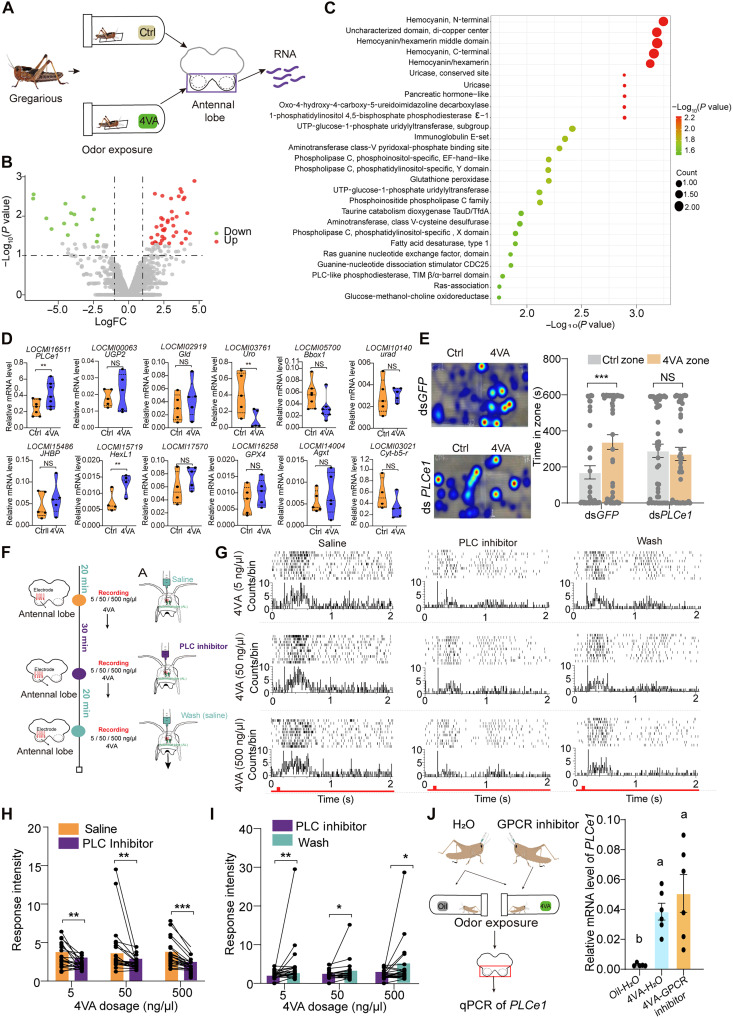
PLCe1 as a synthetic enzyme of IP3 participates in the olfactory processing of 4VA in the antennal lobe
To investigate the key genes involved in regulating the perception of 4VA in the central nervous system, we conducted transcriptomic analyses on the antennal lobes of fifth-instar locusts after 4VA fumigation (Fig. 4A). A total of 109 DEGs was identified with a P value of < 0.01 (Fig. 4B). Enrichment analysis of these DEGs using IPR analysis (adjusted P < 0.05, Fisher’s exact test) identified 25 pathways associated with the PLC domain and enzymes (Fig. 4C), involving 12 genes (table S2). qPCR verification indicated that the expression level of LOCMI16511 (PLCe1) and LOCMI15719 (HexL1) exhibited significant up-regulation following 4VA exposure compared to the control group (two-tailed unpaired t test, t = 2.392, P = 0.034 for PLCe1; t = 2.537, P = 0.0443 for HexL1) (Fig. 4D). PLCe1 contains a pleckstrin homology domain, a sequence region between two PLCs (X and Y), a C2 domain, and two Ras-binding domains (fig. S6). Phylogenetic analysis indicates that PLC epsilon 1 (PLCe1) belongs to the PLCε subtype of PLCs, which are biosynthetic enzymes of IP3 and DAG (figs. S7 and S8). To verify the function of PLCe1 in 4VA perception, we injected the dsRNA of PLCe1 into the brains of locusts. The knockdown of PLCe1 (Student’s t test, t = 2.727, P = 0.0213) (fig. S9A) significantly impeded the attraction response of locusts to 4VA compared to the dsGFP-injected group (MWU test, U = 604, P = 0.002 for dsGFP time; U = 1044, P = 0.915 for dsPLCe1 time; U = 623, P = 0.015 for dsGFP distance; U = 860.5, P = 0.381 for dsPLCe1 distance) (Fig. 4E and fig. S9B). Therefore, PLCe1 participates in the olfactory processing of 4VA in the antennal lobe of locusts.
Fig. 4. PLCe1 participates in the locust response to 4VA in the antennal lobe.
(A) Schematic diagram of antennal lobe transcriptome sequencing and sampling. (B) Volcano plot of transcriptome sequencing in the antennal lobe. Red points denote up-regulated genes in the antennae lobe exposed to 4VA compared to those exposed to oil, while green points indicate down-regulated genes (P value < 0.01, log2FC ≥ 1.0, n = 3). (C) IPR enrichment analysis of up-regulated DEGs. Only IPR terms with P < 0.05 are shown. (D) qPCR verification of DEGs in the IPR enrichment pathway (n = 6). (E) Behavioral responses of gregarious locusts to 4VA after PLCe1 knockdown in the dual-choice behavior assay (n = 26). The pseudocolored areas represent the regions where insects spent more time or were more active. (F) Diagram of multichannel electrophysiological recording with pharmacological treatments. (G) Representative raster plots of PNs before and after PLC inhibitor (U71322) treatment. The red bar indicates the period of odor presentation. (H and I) Intensity of PN response to 4VA at different concentrations after PLC inhibitor (U171322) (H) treatment and washing rescue (I) (n = 22 neurons). (J) Relative mRNA levels of PLCe1 after injection of GPCR inhibitors [pertussis toxin (PTX) and YM25980] and odor exposure (n = 7). Data of dual-choice behavior experiment were analyzed by the MWU test (E). Comparisons of pharmacological treatments were analyzed by the Wilcoxon matched-pair signed-rank test [(H) and (I)]. Comparisons of gene expression difference were analyzed by the two-tailed unpaired t test [(D) and (J)]. Data are presented as the means ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001.
PLCe1-induced IP3 regulates the responses of projection neurons
To further investigate the role of PLCe1 in the responses of locust antennal lobe to 4VA, we conducted multichannel electrophysiological recordings to investigate the PLCe1 responses to 4VA in the antennal lobe (Fig. 4F). The representative in vivo electrophysiological responses of projection neurons (PNs) before and after the application of the PLC inhibitor U71322 are shown in Fig. 4G. The initial baseline recording of untreated locust antennal lobe established a stable neural response to 4VA, serving as a reference point for subsequent observations. At 30 min after the application of U71322, a noticeable decrease in neural activity to 4VA was observed from the concentrations of 5 to 500 ng/μl (Wilcoxon matched-pair signed-rank test, P = 0.001 for 5 ng/μl, P = 0.026 for 50 ng/μl, and P = 0.011 for 500 ng/μl) (Fig. 4H). Over the 20-min washout period, the neural activity significantly recovered (Wilcoxon matched-pair signed-rank test, P = 0.007 for 5 ng/μl, P < 0.001 for 50 ng/μl, and P = 0.006 for 500 ng/μl) (Fig. 4I). Meanwhile, we assessed whether the up-regulation of PLCe1 is regulated by GPCRs using broad-spectrum inhibitors. The results showed that inhibiting GPCRs does not affect the up-regulation of PLCe1 genes (Fig. 4J), suggesting that the increase in PLC transcription is regulated by factors that bypass GPCR signaling. Thus, PLCe1 affects the electrophysiological responses of PNs in the locust antennal lobe to 4VA. Furthermore, we examined the changes in the levels of IP3 and DAG after the RNAi knockdown of PLCe1 (Fig. 5A). The concentration of IP3 markedly decreased in the brains of dsPLCe1-injected locusts (two-tailed unpaired t test, t = 3.292, P = 0.0032) (Fig. 5B); however, the concentration of DAG was not affected (two-tailed unpaired t test, t = 1.73, P = 0.098) (Fig. 5C). In addition, IP3 levels significantly increased in the brains of locusts following 4VA exposure (two-tailed unpaired t test, t = 1.73, P = 0.098), indicating that IP3 is also responsible for signal transduction in the antennal lobe (Fig. 5, D and E). We further validated the involvement of IP3 in the antennal lobe response through multichannel electrophysiological recording. The representative electrophysiological responses of PNs before and after the application of the IP3 inhibitor heparin are shown in Fig. 5F. After 30 min of heparin treatment at a concentration of 5 ng/μl, a significant reduction in neural activity was observed (Wilcoxon matched-pair signed-rank test, P = 0.002 for 5 ng/μl, P < 0.011 for 50 ng/μl, and P < 0.002 for 500 ng/μl) (Fig. 5G). During the subsequent 20-min washout period, neural activity significantly recovered (Wilcoxon matched-pair signed-rank test, P = 0.005 for 5 ng/μl, P = 0.024 for 50 ng/μl, and P = 0.012 for 500 ng/μl) (Fig. 5H). Notably, heparin treatment also reduced baseline firing between 1 and 2 s, indicating a role of IP3 signaling in maintaining spontaneous neuronal activity. To eliminate this effect, 4VA-evoked responses were normalized to the respective background baseline, confirming that IP3 blockade specifically impaired odor coding. The blocking of IP3 signaling by injecting an IP3 receptor inhibitor led to the loss of 4VA attractiveness in locusts (MWU test, U = 152.5, P = 0.007 for H2O; U = 218, P = 0.955 for heparin) (Fig. 5I). Therefore, PLCe1-induced IP3 affects the responses of PNs and facilitates the processing of 4VA in the locust antennal lobe.
Fig. 5. IP3 signaling is responsible for 4VA processing in the locust antennal lobe.
(A) Diagram for the measurement of IP3 and DAG content after RNAi of PLCe1. (B and C) Changes of IP3 and DAG contents in the locust brain after PLCe1 knockdown (n = 12). (D) Diagram for the measurement of IP3 content after 4VA exposure in the brain. (E) Changes of IP3 concentration in the brains of 4VA-exposured locusts (n = 12). (F) Raster plot examples of PNs before and after IP3 inhibitor (heparin) treatment. (G and H) Response intensity of PNs to 4VA at various concentrations, following heparin application (G) and after washout recovery (H) (n = 27 neurons). (I) Behavioral responses of gregarious locusts to 4VA after injection of the IP3 receptor inhibitor (heparin) in the dual-choice behavior assay (n = 24). Comparisons of ELISA were analyzed by the two-tailed unpaired t test [(B) to (E)]. Comparisons of pharmacological treatments were analyzed by the Wilcoxon matched-pair signed-rank test [(G) and (H)]. Data of dual-choice behavior experiment were analyzed by the MWU comparison test (I). Data are presented as the means ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001.
IP3 is responsible for the perception of 4VA and other odors
To determine whether the IP3 signaling pathway is a common mechanism for the transduction of various odorant signals, we assessed the function of IP3 in nymphs perceiving plant volatile (Z)-3-hexenyl acetate [(Z)-3-HA] and phenylacetonitrile (PAN; aposematic pheromone of locusts) (29), as well as in adults perceiving dibutyl phthalate (DBP; locust sex pheromone) (18). We measured the IP3 concentration in the antennae and brains of locusts exposed to these odors. The results showed that IP3 levels in these tissues significantly increased following exposure to (Z)-3-HA, PAN, and DBP (two-tailed unpaired t test, t = 2.661, P = 0.014 for PAN in the antenna; t = 2.669, P = 0.015 for PAN in the antennal lobe; t = 2.317; P = 0.030 for (Z)-3-HA in the antenna; t = 2.108; P = 0.046 for (Z)-3-HA in the antennal lobe; t = 6.863; P < 0.011 for DBP in the adult antenna; t = 2.682; P = 0.014 for DBP in the antennal lobe) (Fig. 6, A to D). We then recorded the responses of locust antennae after injecting with heparin. The responses to (Z)-3-HA, PAN, and DBP significantly reduced in the heparin-injected group compared to the control group at different concentrations (two-tailed unpaired t test, t = 3.248, P = 0.0024 for 0.5 ng/μl; t = 2.948, P = 0.0053 for 5 ng/μl; t = 2.335, P = 0.0247 for 50 ng/μl; t = 3.373, P = 0.0017 for 500 ng/μl; and t = 3.159, P = 0.0030 for 5000 ng/μl for (Z)-3-HA; t = 3.052, P = 0.0041 for 0.5 ng/μl; t = 2.200, P = 0.034 for 5 ng/μl; t = 2.614, P = 0.0128 for 50 ng/μl; t = 3.457, P = 0.0013 for 500 ng/μl; and t = 3.751, P < 0.0001 for 5000 ng/μl for PAN; t = 2.732, P = 0.011 for 0.5 ng/μl; t = 2.648, P = 0.013 for 5 ng/μl; t = 2.771, P = 0.010 for 50 ng/μl; t = 2.635, P = 0.0138 for 500 ng/μl; and t = 3.774, P = 0.0008 for 5000 ng/μl for DBP) (Fig. 6, E to G). Simultaneously, nymphs injected with heparin lost their attractiveness for (Z)-3-HA and no longer exhibited repellent behavior to PAN. In addition, adult locusts injected with heparin showed no preference to DBP (MWU test, (Z)-3-HA: U = 392, P = 0.005 for H2O; U = 224.5, P = 0.689 for heparin; PAN: U = 129.5, P = 0.019 for H2O; U = 235, P = 0.875 for heparin; DBP: U = 679.5, P = 0.049 for H2O; U = 258, P = 0.546 for heparin) (Fig. 6, H to J, and fig. S10). Consequently, IP3 exerts a pivotal role in the olfactory processing of locusts, functioning downstream of the OR-Orco complex.
Fig. 6. The IP3 signaling pathway is involved in the perception of multiple odor signals.
(A) Diagram for the measurement of IP3 content after odor exposure in locust antennae. (B) IP3 concentration in locust antennae after plant volatile (Z)-3-HA, aposematic pheromone PAN, and locust sex pheromone DBP exposure (n = 12). (C) Diagram for the measurement of IP3 content after odor exposure in the locust brain. (D) IP3 concentration in the locust brains of (Z)-3-HA–, PAN-, and DBP-exposed locusts (n = 12). (E to G) Dose-response curves of antennae to (Z)-3-HA (E), PAN (F), and DBP (G) in gregarious locusts after being injected with heparin. (H to J) Behavioral responses of gregarious locusts to (Z)-3-HA (H), PAN (I), and DBP (J) after being injected with heparin (n = 23). Comparisons of IP3 content were analyzed by the two-tailed unpaired t test (B and D). P values in electrophysiological recordings were determined by the two-tailed unpaired t test (D to F). Data of dual-choice behavior experiments were analyzed by the MWU test (H to J). Data are presented as the means ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001.
DISCUSSION
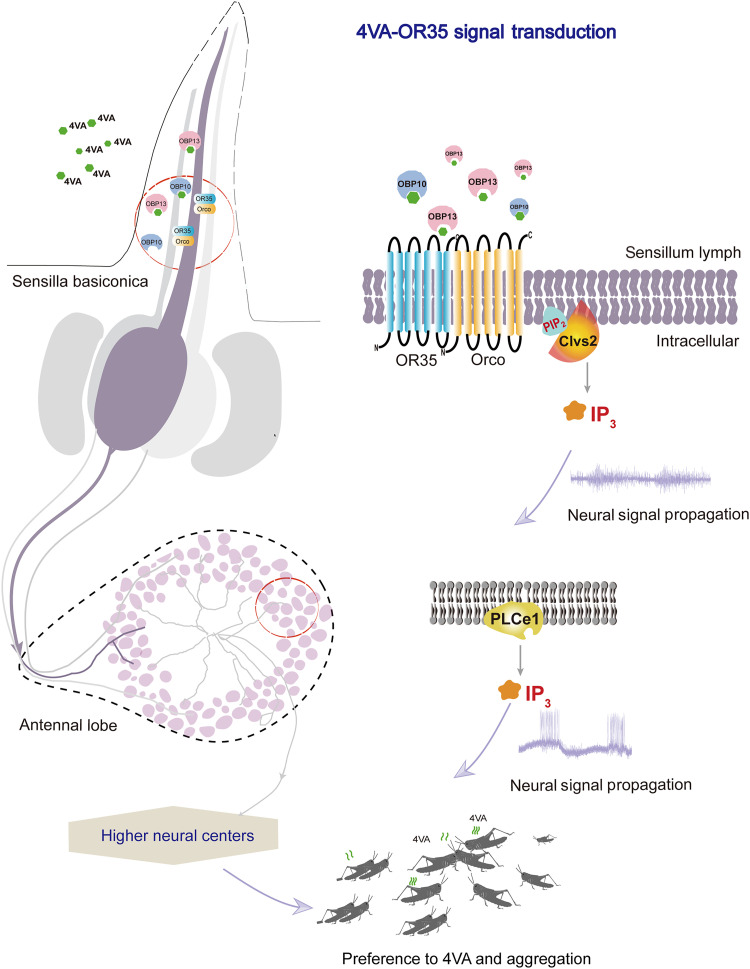
The present study reveals the molecular signaling circuit of 4VA from the antenna to the antennal lobe in locusts. In particular, olfactory signal processing in locusts involves sequential activation of OBPs and ORs, ultimately activating Clvs2 and generating IP3 as the second messenger. This is different from olfactory perceptions in vertebrates. OBP10 and OBP13 convey 4VA and activate OR35 in basiconic sensilla of locust antennae. Subsequently, activating Clvs2 provides more substrates for IP3 production. IP3 acts as the second messenger and triggers the conversion of odorant information from chemical to electrical signals without the involvement of GPCRs. The transmitting electrical signals to the antennal lobe elevate PLCe1 expression to increase IP3 levels, which elicits the responses of PNs, and lastly facilitates the olfactory and behavioral decision of locusts (Fig. 7).
Fig. 7. Molecular circuit of 4VA signal transduction in the locust olfactory system.
Within the antenna, OBP10 and OBP13, situated upstream of OR35, undertake the responsibility of conveying 4VA within basiconic sensilla and instigating the activation of OR35. Simultaneously, downstream of OR35, Clvs2 binds to PIPs, resulting in the modulation of PLC activity, which catalyzes the conversion of PI(4,5)P2 into IP3. Via the IP3 signaling cascade, signal transduction at the antennal lobe triggers the up-regulation of PLCe1 expression. The up-regulated IP3 pathway therefore facilitates the transduction of signals to a higher center and decision-making for aggregation.
Two OBPs act in concert within 4VA signal transduction
Both OBP10 and OBP13 directly bind to 4VA and play key roles in 4VA signal transduction. OBP10 and OBP13 were categorized within the same clade on the evolutionary tree, representing antenna-rich expression OBPs (30). Our study further revealed significant structural similarities and strong binding capabilities of the two OBPs to 4VA, suggesting their equally important roles in 4VA delivery. In several critical biological processes, such as the essential signaling transduction of important pheromone and pivotal synthetic metabolic pathways, it is common to observe the participation of the two key regulators. These two crucial factors frequently operate synergistically, acting as a backup to each other in the regulation of these processes. In Agrotis ipsilon, both AipsGOBP1 and AipsGOBP2 were proved to actively bind to the same sex pheromones and bioactive plant volatiles, including Z9-14:Ald and Z11-14:Ald (31, 32). As well as in Bombyx mori, both GOBP1 and GOBP2 respond to the sex pheromones (33, 34), suggesting their double insurance function in recognizing the sex pheromones. Two OBPs in 4VA transport guarantee the successful signal transduction of 4VA in locust aggregation.
A novel lipid-binding protein, Clvs2, plays crucial roles in olfactory signal transduction in insects
In this study, we demonstrate that Clvs2, which processes the SEC14 domain, is involved in the 4VA signal transduction in the antennae through regulating the production of IP3. The SEC14 family includes proteins known to bind lipids, vitamins, and retinoids (24, 35). The yeast protein Sec14p was the first discovery showcasing lipid transport functionality, extracting PtdIns and phosphatidylcholine from membranes in vitro, and plays a crucial regulatory role in cellular lipid metabolism (36, 37). The PtdIns transfer activities of lipid transfer proteins have been required for PI3K (phosphoinositide 3-kinase)– and PLC-mediated signaling. Db1 in mouse, Sec14l3/SEC14L2 in zebrafish and humans, and Patellin1 in Arabidopsis thalianas, belonging to the SEC14 family, are reported as lipid transfer proteins to transport PtdIns, serving at the interface between lipid metabolism, cellular signaling and vesicular trafficking (38, 39). Notably, our study identified Clvs2 as a previously unidentified lipid-binding protein with a SEC14 domain that specifically participates in the insect OR signaling pathway. This highlights the distinctive contributions of lipid-binding proteins in insect olfactory signal transduction and confirms the role of IP3 signaling cascade downstream of OR35-Orco complexes. These findings contribute to a more comprehensive understanding of cellular signaling of insect OR signal transduction and provide direct evidence for the canonical second messenger IP3 in non-GPCR signaling of insect olfactory signal transduction pathways. Clvs2 directly activates the IP3 pathway instead of GPCRs, revealing a distinct molecular mechanism for lipid-binding proteins in animals.
Building on the identification of Clvs2 as a key mediator of IP3 signaling in insect olfactory transduction, we also observed that OR35 activation leads to the up-regulation of Clvs2 mRNA in response to 4VA stimulation. The olfactory signaling pathway in sensory neurons is activated within seconds to minutes, while transcriptional changes in genes encoding OBPs and Clvs2 occur over much longer periods. Rapid transcriptional responses occurring within a short time frame may be explained by several mechanisms, including alternative splicing of preexisting transcripts, which enables swift posttranscriptional modifications (40, 41); liquid-liquid phase separation that locally enriches transcripts or RNA binding proteins to accelerate mRNA accumulation (42, 43); and the regulation by noncoding RNAs, such as circular RNAs and microRNAs (44, 45). In addition, epigenetic modifications and calcium signaling may also contribute to bridging these processes, ensuring that the rapid electrical signals in olfactory sensory neurons are effectively converted into sustained transcriptional outputs. In the context of olfaction, rapid electrophysiological responses are initiated within seconds by the activation of ORs, whereas the transcriptional changes, such as those observed for Clvs2, may be facilitated by one or more of these mechanisms acting in concert. However, a full resolution of this intriguing question will require further in-depth mechanistic studies.
IP3 signaling in insect olfactory signal transduction
Our study provides crucial evidence illustrating the molecular cascades downstream of insect OR-Orco and the explicit linkage between an ion channel and a second messenger. The involvement of the IP3 signaling pathway through GPCRs in vertebrate olfactory signal transduction is well supported by ample evidence (46). However, the role of IP3 in the olfactory processing of insects remains insufficiently investigated, although intracellular IP3 levels can rapidly and temporarily increase in response to olfactory stimuli in locust antenna tissue, antennal neuron cells, and the antennae of Periplaneta americana (47). IP3 plays a critical role in rapidly mobilizing intracellular Ca2+ stores, which are essential for various cellular processes. In the context of olfactory signaling, the transient increase in intracellular Ca2+ levels mediated by IP3 enhances the sensitivity and specificity of the olfactory response. Thus, the precise and rapid signal transduction mediated by IP3 is particularly relevant for locusts in making behavioral decisions. The IP3 signaling pathway regulates olfactory signal transduction in locusts for many odors. Our findings demonstrate that the downstream olfactory signal transduction pathways of locust OR-Orco use a conserved mechanism of IP3 signaling. In locusts, this IP3-mediated metabolic olfactory pathway facilitates both signal amplification and sustained transmission, which may be essential for regulating behavioral transitions and phase change. Notably, during the transition from solitary to gregarious phases, continuous integration of environmental stimuli is critical, and the cascade amplification effect of this pathway efficiently coordinates the process.
Our findings indicate that the downstream transduction pathway of the locust OR-Orco complex is predominantly mediated by Clvs2-dependent IP3 signaling, which contrasts with canonical models described in other insect systems. In moths such as Manduca sexta, pioneering work by Stengl and colleagues (48, 49) demonstrated that pheromone detection is primarily mediated by a GPCR-dependent cAMP cascade, which regulates cyclic nucleotide–gated channels and intracellular Ca2+ oscillations. In parallel, studies using diverse techniques in Drosophila and various moth species have supported the involvement of a G protein–coupled, PLCβ-dependent signaling cascade. PLCβ activity generates IP3, which subsequently regulates store-operated Ca2+ entry and fine-tunes neuronal excitability (50, 51). These stand in sharp contrast to our findings in locusts, where IP3 signaling appears to be independent of conventional GPCR activation and is instead mediated through the Clvs2 protein. This suggests that locusts may use IP3-mediated calcium mobilization to support rapid behavioral responses associated with phase change. These comparative insights highlight the evolutionary plasticity of insect olfactory systems. Although our understanding of IP3 involvement in olfactory signaling transduction remains limited in other insects, IP3 potentially acts as a second messenger and is a conserved intrinsic factor in the transduction of olfactory signals across insect species. Conserved molecular elements such as OR-Orco and IP3 have been repurposed into distinct signaling architectures adapted to species-specific ecological demands.
GPCRs and olfactory signaling in locusts
We show how Clvs2 directly activates the IP3 pathway in the antenna, functioning independently of GPCRs. These findings unveil a previously unreported mode of action within the second messenger signaling cascade that occurs downstream of the non–GPCR-structured insect OR complex. In locust olfactory transduction, IP3 generation may bypass GPCRs entirely. We observed that Clvs2-mediated PI(4,5)P2 transport facilitates PLC-dependent IP3 synthesis without the involvement of GPCRs. For instance, in Drosophila, Ca2+ influx has been shown to regulate intracellular cAMP levels and, in turn, modulate OR activation through a reverse signaling mechanism (9). Similarly, in locusts, the OR35-Orco complex itself could directly activate PLC via ion flux (e.g., Ca2+) or Clvs2. Moreover, in the locust antennal lobe, up-regulation of PLCe1 was observed in the absence of GPCR involvement, further implying that alternative, non-GPCR pathways may contribute to 4VA perception. Although the inhibitor cocktails were selected to target more GPCR subtypes, the extent to which these cocktails inhibit the full spectrum of GPCRs remains unverifiable. Thus, there is still a possibility that some subtypes contribute to the IP3 production. Future studies using genetic knockouts of specific GPCRs or downstream effectors (e.g., Gα subunits) will be crucial to verify these pending questions. Although the potential role of GPCRs in olfactory signal transduction in insects has been proposed (52), the specific GPCRs that might regulate 4VA signal transduction and trigger corresponding behavioral responses, as well as the underlying molecular mechanisms, need to be further explored.
MATERIALS AND METHODS
Insects
The gregarious locusts used in this experiment were sourced from colonies maintained at the Institute of Zoology, Chinese Academy of Sciences, China. They were reared under a 14:10 light/dark cycle at 30 ± 2°C and fed fresh wheat seedlings grown in a greenhouse. Rearing took place in cages measuring 30 by 30 by 30 cm, each containing ~1000 insects. All experiments were conducted under the license of the Animal Experimental Committee of the Institute of Zoology, Chinese Academy of Science (IOZ20170071).
Odor exposure treatment and sample collection
Fifth-instar nymphs were used for the odor exposure treatment. A single nymph was placed in a 50-ml centrifuge tube with a filter paper sheet, and 10 μl of mineral oil was added to the filter paper as a control group. In comparison, the treatment group was treated with an equal amount of 4VA at a concentration of 50 ng/μl for 5 min for odor fumigation. After the odor exposure treatments, the antennae and antennal lobes were collected for transcriptome analysis.
RNA sequencing and data analysis
Total RNA was extracted from the collected antennae (three samples with six antennae in each sample) and the antennal lobes (three samples with 30 antennal lobes in each sample) using TRIzol reagent (Invitrogen, Carlsbad, US). Transcriptome sequencing libraries were generated using the TIANSeq Fast RNA Library Kit (Illumina) following the manufacturer’s instructions. The clustering of the index-coded samples used the TruSeq PE Cluster Kit v3-cBot-HS (Illumina) on a cBot Cluster Generation System. After cluster generation, the library preparations were sequenced on an Illumina sequencing platform. The raw data were filtered to obtain clean data, which were then mapped to the locust genome using HISAT2 software. Gene expression levels were indicated using transcripts per kilobase million. Subsequently, EdgeR software was used for differential gene expression analysis, and the IPR enrichment analysis of DEGs was carried out.
Quantitative PCR
RNA extraction methods are described above. RNA reverse transcription reactions were conducted by using the FastKing RT Kit (with gDNase) (Tiangen, China) according to the manufacturer’s instructions. The relative mRNA expression level was quantified by using the FastFire qPCR PreMix Kit (SYBR Green) (Tiangen, China) and LightCycler 480 (Roche, Mannheim, Germany). Rp49 served as an endogenous control for mRNAs. The 2−ΔCt method was used to calculate the gene expression data. All qPCR analyses were performed in six to eight biological replicates. All qPCR primers used in this assay are listed in table S3.
RNAi assay
The T7 RiboMAX Express RNAi system (Promega, WI, P1700) was used to synthesize dsRNA molecules targeting the green fluorescent protein (GFP), Clvs2, OBP13, OBP10, and PLCe1 following the manufacturer’s instructions. To ensure the specificity of RNAi knockdown and identify homologous target genes, a comparison was made between all experimentally used fragments and the genome database. The primers for dsRNA synthesis can be found in table S4. Each fifth-instar gregarious locust received an injection of 10 μg of dsRNA directly into the thoracic hemocoels (with a minimum of 30 individuals per treatment). Subsequently, the behavioral assay or electrophysiological experiments were conducted 72 hours postinjection. Following the assay, the decrease in relative expression of the target genes was determined using qPCR.
Behavioral assay
We used a dual-choice arena system, as detailed in previous studies (14, 16), to perform the behavioral assay. This system provided clean oil vertical airflow as the control zone and adjacent 4VA vertical steam as the treatment zone. Fifth-instar gregarious locusts injected with dsRNA or an inhibitor were subjected to the assay. Each locust was exposed to 10 μl of diluted 4VA (50 ng/μl) on one side of the funnel, with an equal volume of mineral oil on the other side as the control treatment. After a 10-min exposure period for each of the 15 tested individuals, the positions of the treatments were reversed to mitigate potential position bias. At least 30 locusts were tested for each candidate gene or inhibitor. Locust behaviors were recorded using a Panasonic video camera and VCR software (version 2, Noldus Information Technology) at 25 frames per second for 10 min. Subsequently, EthoVision XT software (version 11.5, Noldus Information Technology) was used to objectively measure the total distance of movement (unit: cm) and the total time spent on each side (unit: s).
Electroantennograms
The root of the antenna of a healthy locust was cut off with a razor blade, and then the top of the antenna was trimmed about 2 mm; the trimmed antenna was attached to both ends of the electrode using conductive adhesive, and then the circuit was connected for recording. The airflow pump was started, and the recording baseline was left to stabilize before starting the recording. A blank value was recorded by stimulating the antenna with a Pasteur tube containing mineral oil, an odor solvent. Then, the antenna was stimulated with odor from low to high concentrations using a Pasteur tube. After all stimulations, the sample antenna was replaced for recording. Before each recording, 4VA was diluted to 1/10 (v/v) with mineral oil for electrophysiological stimulation, with mineral oil as the negative control. Syntech EAG software version 2.6c was used for signal recording and analysis. For statistical analysis, the voltage value of odor stimulation was subtracted from that of blank mineral oil stimulation.
Single-sensillum recordings
We conducted single-sensillum recordings on fifth-instar gregarious nymphs. To immobilize the locust, we placed it inside a narrow plastic tube with a diameter of 1 cm and secured its antennae with dental wax. We inserted a sharpened tungsten wire electrode into the bottom of the sensillum using a Narishige micromanipulator to record the sensillum responses, while the reference electrode was inserted into the eye. We amplified the signals using IDAC4 amplifiers from Syntech and recorded and analyzed the frequency variation using Autospike32 (version 3.9, Syntech). The stimuli used in single-sensillum recording were the same as in electroantennography (EAG) experiments.
Protein structure prediction and molecular docking
The protein structures of OBP10, OBP13, and Clvs2 were predicted using AlphaFold 2.0 (53, 54). AutoDock Tools (55) was used to prepare the protein structures for docking by adding hydrogen atoms and Gasteiger charges. The three-dimensional atomic structure of the ligand 4VA was retrieved from the PubChem database (http://pubchem.ncbi.nlm.nih.gov/compound/12507) and saved in SDF format, subsequently converted to PDB format using Open Babel (56). DeepSite software was then used to analyze the target protein structures and predict potential binding sites for the ligand. Subsequently, AutoDock Vina 1.2.0 (57) was used to perform molecular docking between the target proteins and the ligand 4VA, aiming to predict the binding mode and affinity between the protein and the ligand. In molecular docking studies using AutoDock Vina, the binding free energy is a critical metric for evaluating the binding capacity between molecules. A more negative binding free energy value suggests greater stability of the molecular complex and a stronger binding affinity (21). Last, PyMOL 2.0 software (Schrödinger Inc., New York, NY) was used for the visualization of the binding mode and binding sites between the protein and the ligand.
Expression and purification of recombinant proteins
The full-length open reading frame sequence of OBP13 and OBP10 was obtained by PCR amplification, and the resulting PCR products were ligated into the pET28a vector between the BamH1 and Xho1 restriction sites to generate the recombinant plasmids encoding His-tagged recombinant proteins. The recombinant plasmids were introduced into Escherichia coli BL21 (DE3.0 pLysS; Rosetta) for expression. Protein expression was induced using 0.5 mM isopropyl-1-thio-β-d-thiogalactopyranoside (Thermo Fisher Scientific) when the optical density at 600 nm reached 0.5 to 0.8. The cultures were incubated at 20°C for 15 hours to facilitate protein synthesis. Bacterial cells were harvested by centrifugation (3000g, 4°C, 30 min), and the resulting pellets were lysed by ultrasonic disruption at 4°C. The supernatant was collected for subsequent protein purification. OBP-His from locusts was purified using Ni Sepharose (GE Healthcare, Buckinghamshire, UK) with 100 mM imidazole according to the manufacturer’s instructions. The purified protein was then concentrated using a 10-kDa-cutoff Amicon Ultra Centrifugal Filter (Burlington, MA) and dissolved in 10 mM tris-HCl (pH 8.0). Purified proteins were confirmed using SDS–polyacrylamide gel electrophoresis, and their concentrations were measured using the bovine serum albumin method before being used in competitive fluorescence binding assays.
For the expression of Clvs2 protein, a eukaryotic expression system was used. The full length of the Clvs2 open reading frame was subcloned into the pFastbac1 vector (Invitrogen) with a His tag. The Bac-to-Bac expression system was used to construct the recombinant Bacmid vector using the pFastbac1 plasmid, followed by PCR validation. Positive clones containing the recombinant Bacmid were selected, and the Bacmid was extracted on a large scale for transfection. Subsequently, the diluted transfection reagent and recombinant plasmid were mixed and incubated at room temperature for 5 min before being added dropwise to Sf9 cells. The cells were incubated for 4 days at 28°C, after which the culture was centrifuged at 1500 rpm for 5 min to collect the P0 generation virus particles. These were stored at 4°C in the dark or aliquoted for long-term storage at −80°C. Afterward, large-scale protein expression and extraction were performed. The P0 generation of virus particles was added dropwise to Sf9 cells and incubated for 3 days at 28°C. The cells were harvested by centrifugation at 1500 rpm for 5 min, lysed with lysis buffer at 4°C for 30 min, and centrifuged at 11,000 rpm for 20 min to separate the supernatant and pellet. Protein verification and purification were carried out using methods similar to those described above.
Competitive fluorescence binding assays
The fluorescent probe 1-NPN (Sigma-Aldrich; purity ≥95%) and the ligand 4VA were dissolved in high-performance liquid chromatography–grade methanol at a final concentration of 1 mM. First, the affinity of OBPs to 1-NPN was first determined. A final concentration of 2 μM protein was added to 1 ml of 50 mM tris-HCl buffer (pH 7.4) in a 1-cm light path quartz cuvette, and the excitation wavelength was set to 337 nm. Emission spectra were recorded from 390 to 500 nm as the 1-NPN probe was added sequentially to final concentrations of 1, 2, 4, 6, 10, 12, 14, 16, and 20 μM. Each concentration was measured three times to obtain the maximum fluorescence intensity. A fluorescence spectrophotometer F-4600 (Tianjin, China) was used in this experiment. The Scatchard method was applied to calculate the binding constant between OBPs and 1-NPN. The binding ability of 4VA and 1-NPN was then assessed by adding 4VA at concentrations ranging from 0.5 to 20 μM to the 1-NPN/OBP solution, with both the probe and protein present at 2 μM. The Ki value was calculated to represent the binding ability of OBPs and 4VA. A Ki value of less than 10 μM indicates strong binding between the protein and 4VA, a Ki value between 10 and 20 μM indicates weak binding, and a Ki value greater than 30 μM suggests no binding interaction (58).
MST assay
The MST assay was used to measure the binding affinity of Clvs2 protein and 4VA/PI(4,5)P2 in vitro, following previously described methods (59). The Clvs2 protein was fluorescently labeled using the Monolith Protein Labeling Kit (RED-NHS 2nd Generation MO-L011, Nano Temper), following the manufacturer’s recommended procedures. The concentration of Clvs2 protein was maintained at 125 nM, while the concentrations of PI(4,5)P2 and 4VA were systematically diluted across a gradient from 50 μM to 0.0417 nM. All samples were diluted with MST buffer, consisting of phosphate-buffered saline (PBS) with Tween 20 (PBST) buffer and 0.05% n-dodecyl-β-d-maltoside. After 30-min incubation at room temperature, the samples were loaded and measured using a Monolith NT.115 device (Nano Temper Technologies GmbH, Munich, Germany) at 24°C, with 40% excitation power and medium light-emitting diode power. Three independent measurements were performed as three biological replicates. The acquired data were analyzed using Monolith Affinity Analysis version 2.2.4 software to determine the KD.
Lipid blot assay
Lipid-blotted membranes were purchased from Echelon prepared by spot fixing equal amounts of lipids onto nylon membranes. To block the membranes, we incubated them in TBS buffer [50 mM tris-HCl, pH 7.5, 150 mM NaCl, and 0.1% (v/v) Tween 20] containing 3% skimmed milk powder at room temperature for 1 hour. Purified Clvs2 protein was diluted in the blocking solution to a concentration of 1 μg/ml. The membrane was incubated with protein for 1 hour at room temperature on a shaking platform. Then, the membrane was washed three times with PBST buffer for 5 to 10 min each. The membrane was then incubated with a 1:2000 dilution of Clvs2 mouse monoclonal antibody (ABclonal) in the blocking solution for 1 hour at room temperature. After three washes with PBST buffer for 5 to 10 min each, the membrane was incubated with a secondary mouse antibody (Kangwei Century) conjugated with horseradish peroxidase at a dilution of 1:5000 in the blocking solution at room temperature with shaking for 1 hour. Following two additional washes with PBST buffer for 5 to 10 min each, the membrane was visualized using an eECL Western Blot Kit (Kangwei Century) and exposed using an Image Station System (Carestream).
Quantitative measurement of lipids (ELISA)
The concentrations of IP3 and DAG were measured using the IP3 Assay Kit (CUSABIO, CSB-E12636h) and DAG/DG ELISA Kit (MM-92525701), respectively. The assay shown in fig. S6A was performed with the Cloud-Clone Corp kit (CEC037Ge), while subsequent experiments (e.g., Figs. 3H and 5, B to H) used the CUSABIO kit (CSB-E12636h) because of supply limitations. All comparisons were performed within the same batch and kit to ensure consistency in relative quantification. The experiments were carried out according to the manufacturer’s instructions. First, six antennae were cut into small pieces and ground on ice with precooled tissue lysis buffer (Cloud-Clone Corp, IS007). After thorough grinding, the mixture was kept on ice and lysed for 10 min before being sonicated and centrifuged at 4°C for 10 min. The supernatant was then transferred to a new tube for further use. For each assay, 50 μl of the prepared tissue homogenate was added to a well of a 96-well ELISA plate and gently mixed with 50 μl of detection solution A. The plate was covered and incubated at 37°C for 1 hour, allowing competition between the specific antibody and the biotinylated antigen in the tissue homogenate. After incubation, the liquid was discarded, and the plate was washed three times with wash buffer for 1 to 2 min per wash. After removing the residual wash buffer, 100 μl of detection solution B was added to each well. The plate was then covered and incubated at 37°C for 30 min, followed by five washes. Subsequently, 90 μl of substrate solution was added to each well and incubated at 37°C for 10 to 20 min. After that, 50 μl of stop solution was added to terminate the reaction. Last, the optical density value of each well was immediately measured at a 450-nm wavelength using a microplate reader (SpectraMax Paradigm, US), and the lipid concentrations in the samples were calculated.
Immunofluorescence
Antennae of fifth-instar locust were collected, fixed in a cryoprotectant medium O.C.T. (optimal cutting temperature compound; Sakura Finetek), and rapidly frozen in liquid nitrogen. The fixed antenna tissue was cut into 10-μm-thick sections using a Leica CM1950 cryostat. The sections were then placed on SuperFrost Plus slides (Menzel-Gläser) and allowed to dry for 15 min before immunofluorescence staining. The immunofluorescence was performed according to the previously described protocol (14). The sections were first fixed in 4% paraformaldehyde for 30 min at room temperature and then washed twice for 5 min with PBS. After 1-hour incubation in 0.1 M PBS containing 5% normal goat serum (Boster), the sections were incubated with primary antibodies anti-OR35 and anti-Clvs2, which were diluted in 0.1 M PBS containing 2% normal goat serum at ratios of 1:500 and 1:2000, respectively, and incubated at 4°C for 48 hours. Then, they were washed three times with PBS for 5 min each. The sections were incubated overnight at 4°C with secondary antibodies goat anti-rabbit Alexa Fluor 488 (1:500, Life Technologies, A11034) and goat anti-mouse Alexa Fluor 566 (1:500, Life Technologies, A16086) together, which were used to label the primary antibodies. The cell nuclei of antennae were labeled with Hoechst 33342 (Life Technology), and the sections were washed three times. Last, we sealed the slides using a fluorescence quenching reagent. A Zeiss laser confocal scanning microscope (Oberkochen, Germany) captured all images.
293T cells were cultured on poly-l-lysine–coated coverslips in 24-well plates and seeded at a density of 8 × 105 cells per well. After 24-hour incubation at 37°C, transfection was performed when cell confluence reached ~80%. Plasmids encoding Or35-mCherry, Orco-BFP, and Clvs2-eGFP were mixed in equal proportions (800 ng total) and transfected into the cells using Lipofectamine 3000. Following a 36-hour incubation period, cells were fixed, permeabilized, and stained with Hoechst 33342 to label the nuclei. The coverslips were mounted onto glass slides using antifade mounting medium and sealed with nail polish. Images were captured using a Zeiss laser confocal scanning microscope (Oberkochen, Germany).
Multichannel recording
Multichannel electrophysiological recordings were conducted on fifth-instar migratory locust. To monitor the activity in the antennal lobe, locusts are immobilized on a surgical platform, ensuring the integrity of both antennae, followed by the removal of the head capsule and glandular tissues to expose the brain. Subsequently, the locusts are maintained at room temperature, with brain tissue moistened using a physiologically balanced locust saline solution. Multiunit tetrode recordings were conducted in the antennal lobe using commercial Neuronexus 16-channel silicon probes (4-by-4 configuration). The responses of PNs were recorded. Silver wire was used as a reference electrode and connected to the perfusion system. Three-way solenoid valves were used for precision odor stimulus delivery. During olfactory stimulation, the process begins with an initial exposure to mineral oil, followed by the sequential delivery of odors at increasing concentrations during exhalation. Each odor pulse is delivered at a frequency of 3.3 Hz, with each pulse lasting for 200 ms and repeated 10 times. After signal stabilization, the first round of odor stimulation was performed in ascending concentration order: mineral oil, 0.05 ng/μl, 0.5 ng/μl, 5 ng/μl, 50 ng/μl, 500 ng/μl, and 5000 ng/μl. Upon completion, the brain was perfused with 10 μM PLC or IP3 inhibitors for 30 min. A second round of odor stimulation was then conducted using the same concentration sequence. This was followed by a 20-min washout with saline to remove residual inhibitors, after which a third round of odor stimulation was carried out. Neuronal signals recorded throughout the experiment were exported and analyzed using Offline Sorter (Plexon, version 4.4.0) for spike sorting, and NeuroExplorer (version 5) for further data processing and statistical analysis.
Pharmacological experiments
Mid-fifth-instar gregarious locusts were subjected to microinjections into the brain. Each locust received an injection of 23 nl of either saline or GPCR inhibitors [pertussis toxin (PTX) and YM254980]. After 2 to 3 hours, we collected the antennal lobes for RNA extraction and qPCR analysis of PLCe1 gene expression. Before sampling, we treated 80 locusts in the saline group with mineral oil for 5 min, while the 80 locusts receiving the inhibitors or saline were subjected to 4VA exposure for 5 min. For each treatment, 12 antennal lobes were collected, with six replicates per condition.
Statistical analyses
The behavioral data of locusts were analyzed for statistical significance using the MWU test. Comparisons of gene expression difference and ELISA data were analyzed by the two-tailed unpaired t test. Electrophysiological data were analyzed using two-tailed unpaired t tests for independent comparisons between treatment and control groups. Comparisons of differences in nerve potential responses of locusts before and after pharmacological intervention in multichannel recordings were analyzed by the Wilcoxon matched-pair signed-rank test. SPSS 20.0 software (SPSS Inc.) was used for statistical analysis. Differences were considered statistically significant at P < 0.05.
Acknowledgments
We thank H. Liu for the assistance in transcriptomic analysis and protein structure prediction. We thank J. Wei and X. Chen for the assistance during the sampling process.
Funding: This research was supported by the National Natural Science Foundation of China (NSFC) (32088102 to L.K. and 32222072 to X.G.); the National Key R&D Program of China (2022YFD1400500 to X.G. and 2023YFA0916300 to X.G.); Initiative Scientific Research Program, Institute of Zoology, Chinese Academy of Sciences (2023IOZ0103 to X.G.); and the State Key Laboratory of Integrated Management of Pest Insects and Rodents (IPM2318 to X.G.).
Author contributions: Conceptualization: J.Y., X.G., and L.K. Methodology: J.Y., H.H., S.D., J.L., and Q.Y. Validation: J.Y., L.C., H.H., and J.L. Investigation: J.Y., H.H., and S.D. Resources: L.K. and S.D. Supervision: X.G. and L.K. Writing—original draft: J.Y. Writing—review and editing: J.Y., S.D., X.G., and L.K.
Competing interests: The authors declare that they have no competing interests.
Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. RNA sequencing data were deposited at the NCBI SRA database. All datasets under accession number PRJNA1144184 are available at the following link: www.ncbi.nlm.nih.gov/bioproject/PRJNA1144184/.
Supplementary Materials
This PDF file includes:
Figs. S1 to S10
Tables S1 to S4
REFERENCES AND NOTES
- 1.Zucchi R., Chiellini G., Scanlan T., Grandy D., Trace amine-associated receptors and their ligands. Br. J. Pharmacol. 149, 967–978 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Munger S. D., Leinders-Zufall T., Zufall F., Subsystem organization of the mammalian sense of smell. Annu. Rev. Physiol. 71, 115–140 (2009). [DOI] [PubMed] [Google Scholar]
- 3.Dhallan R. S., Yau K.-W., Schrader K. A., Reed R. R., Primary structure and functional expression of a cyclic nucleotide-activated channel from olfactory neurons. Nature 347, 184–187 (1990). [DOI] [PubMed] [Google Scholar]
- 4.Neuhaus E. M., Gisselmann G., Zhang W. Y., Dooley R., Stortkuhl K., Hatt H., Odorant receptor heterodimerization in the olfactory system of Drosophila melanogaster. Nat. Neurosci. 8, 15–17 (2005). [DOI] [PubMed] [Google Scholar]
- 5.Soffan A., Subandiyah S., Makino H., Watanabe T., Horiike T., Evolutionary analysis of the highly conserved insect odorant coreceptor (Orco) revealed a positive selection mode, implying functional flexibility. J. Insect Sci. 18, 18 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Larsson M. C., Domingos A. I., Jones W. D., Chiappe M. E., Amrein H., Vosshall L. B., Or83b encodes a broadly expressed odorant receptor essential for Drosophila olfaction. Neuron 43, 703–714 (2004). [DOI] [PubMed] [Google Scholar]
- 7.Benton R., Sachse S., Michnick S. W., Vosshall L. B., A typical membrane topology and heteromeric function of Drosophila odorant receptors in vivo. PLOS Biol. 4, e20 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sato K., Pellegrino M., Nakagawa T., Nakagawa T., Vosshall L. B., Touhara K., Insect olfactory receptors are heteromeric ligand-gated ion channels. Nature 452, 1002–1006 (2008). [DOI] [PubMed] [Google Scholar]
- 9.Wicher D., Schafer R., Bauernfeind R., Stensmyr M. C., Heller R., Heinemann S. H., Hansson B. S., Drosophila odorant receptors are both ligand-gated and cyclic-nucleotide-activated cation channels. Nature 452, 1007–1011 (2008). [DOI] [PubMed] [Google Scholar]
- 10.Wicher D., Miazzi F., Functional properties of insect olfactory receptors: Ionotropic receptors and odorant receptors. Cell Tissue Res. 383, 7–19 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wicher D., Olfactory signaling in insects. Prog. Mol. Biol. Transl. Sci. 130, 37–54 (2015). [DOI] [PubMed] [Google Scholar]
- 12.Prelic S., Getahun M. N., Kaltofen S., Hansson B. S., Wicher D., Modulation of the NO-cGMP pathway has no effect on olfactory responses in the Drosophila antenna. Front. Cell. Neurosci. 17, 1180798 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deng Y., Zhang W., Farhat K., Oberland S., Gisselmann G., Neuhaus E. M., The stimulatory Gαs protein is involved in olfactory signal transduction in Drosophila. PLOS ONE 6, e18605 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guo X., Yu Q., Chen D., Wei J., Yang P., Yu J., Wang X., Kang L., 4-Vinylanisole is an aggregation pheromone in locusts. Nature 584, 584–588 (2020). [DOI] [PubMed] [Google Scholar]
- 15.Chen D., Hou L., Wei J., Guo S., Cui W., Yang P., Kang L., Wang X., Aggregation pheromone 4-vinylanisole promotes the synchrony of sexual maturation in female locusts. eLife 11, e74581 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang J., Yu Q., Yu J., Kang L., Guo X., 4-Vinylanisole promotes conspecific interaction and acquisition of gregarious behavior in the migratory locust. Proc. Natl. Acad. Sci. U.S.A 120, e2306659120 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li Y., Zhang J., Chen D. F., Yang P. C., Jiang F., Wang X. H., Kang L., CRISPR/Cas9 in locusts: Successful establishment of an olfactory deficiency line by targeting the mutagenesis of an odorant receptor co-receptor (Orco). Insect Biochem. Mol. Biol. 79, 27–35 (2016). [DOI] [PubMed] [Google Scholar]
- 18.Cui W., Ge J., Chen D., Nie X., Dong L., Wang X., Kang L., Dibutyl phthalate released by solitary female locusts mediates sexual communication at low density. Proc. Natl. Acad. Sci. U.S.A. 121, e2401926121 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mappin F., Bellantuono A. J., Ebrahimi B., DeGennaro M., Odor-evoked transcriptomics of Aedes aegypti mosquitoes. PLOS ONE 18, e0293018 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koerte S., Keesey I. W., Khallaf M. A., Cortes Llorca L., Grosse-Wilde E., Hansson B. S., Knaden M., Evaluation of the DREAM technique for a high-throughput deorphanization of chemosensory receptors in Drosophila. Front. Mol. Neurosci. 11, 366 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morris G. M., Goodsell D. S., Halliday R. S., Huey R., Hart W. E., Belew R. K., Olson A. J., Automated docking using a Lamarckian genetic algorithm and an empirical binding free energy function. J. Comput. Chem. 19, 1639–1662 (1998). [Google Scholar]
- 22.Bender B. J., Gahbauer S., Luttens A., Lyu J. K., Webb C. M., Stein R. M., Fink E. A., Balius T. E., Carlsson J., Irwin J. J., Shoichet B. K., A practical guide to large-scale docking. Nat. Protoc. 16, 4799–4832 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bankaitis V. A., Mousley C. J., Schaaf G., The Sec14 superfamily and mechanisms for crosstalk between lipid metabolism and lipid signaling. Trends Biochem. Sci. 35, 150–160 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saito K., Tautz L., Mustelin T., The lipid-binding SEC14 domain. Biochim. Biophys. Acta 1771, 719–726 (2007). [DOI] [PubMed] [Google Scholar]
- 25.Schaaf G., Ortlund E. A., Tyeryar K. R., Mousley C. J., Ile K. E., Garrett T. A., Ren J., Woolls M. J., Raetz C. R., Redinbo M. R., Bankaitis V. A., Functional anatomy of phospholipid binding and regulation of phosphoinositide homeostasis by proteins of the sec14 superfamily. Mol. Cell 29, 191–206 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rebecchi M. J., Pentyala S. N., Structure, function, and control of phosphoinositide-specific phospholipase C. Physiol. Rev. 80, 1291–1335 (2000). [DOI] [PubMed] [Google Scholar]
- 27.Rhee S. G., Regulation of phosphoinositide-specific phospholipase C. Annu. Rev. Biochem. 70, 281–312 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saleem H., Tovey S. C., Molinski T. F., Taylor C. W., Interactions of antagonists with subtypes of inositol 1,4,5-trisphosphate (IP3) receptor. Br. J. Pharmacol. 171, 3298–3312 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wei J., Shao W., Cao M., Ge J., Yang P., Chen L., Wang X., Kang L., Phenylacetonitrile in locusts facilitates an antipredator defense by acting as an olfactory aposematic signal and cyanide precursor. Sci. Adv. 5, eaav5495 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guo W., Ren D., Zhao L., Jiang F., Song J., Wang X., Kang L., Identification of odorant-binding proteins (OBPs) and functional analysis of phase-related OBPs in the migratory locust. Front. Physiol. 9, 984 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu N. Y., Yang K., Liu Y., Xu W., Anderson A., Dong S. L., Two general-odorant binding proteins in Spodoptera litura are differentially tuned to sex pheromones and plant odorants. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 180, 23–31 (2015). [DOI] [PubMed] [Google Scholar]
- 32.Huang G. Z., Liu J. T., Zhou J. J., Wang Q., Dong J. Z., Zhang Y. J., Li X. C., Li J., Gu S. H., Expressional and functional comparisons of two general odorant binding proteins in Agrotis ipsilon. Insect Biochem. Mol. Biol. 98, 34–47 (2018). [DOI] [PubMed] [Google Scholar]
- 33.Maida R., Mameli M., Müller B., Krieger J., Steinbrecht R. A., The expression pattern of four odorant-binding proteins in male and female silk moths, Bombyx mori. J. Neurocytol. 34, 149–163 (2005). [DOI] [PubMed] [Google Scholar]
- 34.Zhou J.-J., Robertson G., He X., Dufour S., Hooper A. M., Pickett J. A., Keep N. H., Field L. M., Characterisation of Bombyx mori odorant-binding proteins reveals that a general odorant-binding protein discriminates between sex pheromone components. J. Mol. Biol. 389, 529–545 (2009). [DOI] [PubMed] [Google Scholar]
- 35.Phillips S. E., Sha B., Topalof L., Xie Z., Alb J. G., Klenchin V. A., Swigart P., Cockcroft S., Martin T. F., Luo M., Bankaitis V. A., Yeast Sec14p deficient in phosphatidylinositol transfer activity is functional in vivo. Mol. Cell 4, 187–197 (1999). [DOI] [PubMed] [Google Scholar]
- 36.Patton-Vogt J. L., Griac P., Sreenivas A., Bruno V., Dowd S., Swede M. J., Henry S. A., Role of the yeast phosphatidylinositol/phosphatidylcholine transfer protein (Sec14p) in phosphatidylcholine turnover and INO1 regulation. J. Biol. Chem. 272, 20873–20883 (1997). [DOI] [PubMed] [Google Scholar]
- 37.Lipp N.-F., Ikhlef S., Milanini J., Drin G., Lipid exchangers: Cellular functions and mechanistic links with phosphoinositide metabolism. Front. Cell Dev. Biol. 8, 663 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peterman T. K., Ohol Y. M., McReynolds L. J., Luna E. J., Patellin1, a novel Sec14-like protein, localizes to the cell plate and binds phosphoinositides. Plant Physiol. 136, 3080–3094 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ognibene M., Vanni C., Blengio F., Segalerba D., Mancini P., De Marco P., Torrisi M. R., Bosco M. C., Varesio L., Eva A., Identification of a novel mouse Dbl proto-oncogene splice variant: Evidence that SEC14 domain is involved in GEF activity regulation. Gene 537, 220–229 (2014). [DOI] [PubMed] [Google Scholar]
- 40.Barash Y., Calarco J. A., Gao W., Pan Q., Wang X., Shai O., Blencowe B. J., Frey B. J., Deciphering the splicing code. Nature 465, 53–59 (2010). [DOI] [PubMed] [Google Scholar]
- 41.Nilsen T. W., Graveley B. R., Expansion of the eukaryotic proteome by alternative splicing. Nature 463, 457–463 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hnisz D., Shrinivas K., Young R. A., Chakraborty A. K., Sharp P. A., A phase separation model for transcriptional control. Cell 169, 13–23 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Razin S. V., Ulianov S. V., Divide and rule: Phase separation in eukaryotic genome functioning. Cells 9, 2480 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Krol J., Loedige I., Filipowicz W., The widespread regulation of microRNA biogenesis, function and decay. Nat. Rev. Genet. 11, 597–610 (2010). [DOI] [PubMed] [Google Scholar]
- 45.Hansen T. B., Jensen T. I., Clausen B. H., Bramsen J. B., Finsen B., Damgaard C. K., Kjems J., Natural RNA circles function as efficient microRNA sponges. Nature 495, 384–388 (2013). [DOI] [PubMed] [Google Scholar]
- 46.G. Antunes, F. M. Simoes de Souza, “Chapter 7 - Olfactory receptor signaling” in Methods in Cell Biology, A. K. Shukla, Ed. (Academic Press, 2016), vol. 132, pp. 127–145. [DOI] [PubMed] [Google Scholar]
- 47.Boekhoff I., Raming K., Breer H., Pheromone-induced stimulation of inositol-trisphosphate formation in insect antennae is mediated by G-proteins. J. Comp. Physiol. B 160, 99–103 (1990). [Google Scholar]
- 48.Stengl M., Pheromone transduction in moths. Front. Cell. Neurosci. 4, 133 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Krannich S., Stengl M., Cyclic nucleotide-activated currents in cultured olfactory receptor neurons of the hawkmoth Manduca sexta. J. Neurophysiol. 100, 2866–2877 (2008). [DOI] [PubMed] [Google Scholar]
- 50.Murmu M. S., Martin J.-R., Interaction between cAMP and intracellular Ca2+-signaling pathways during odor-perception and adaptation in Drosophila. Biochim. Biophys. Acta 1863, 2156–2174 (2016). [DOI] [PubMed] [Google Scholar]
- 51.Fleischer J., Krieger J., Insect pheromone receptors – Key elments in sensing intraspecific chemical signals. Front. Cell. Neurosci. 12, 425 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Murmu M. S., Stinnakre J., Réal E., Martin J.-R., Calcium-stores mediate adaptation in axon terminals of Olfactory Receptor Neurons in Drosophila. BMC Neurosci. 12, 105 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jumper J., Evans R., Pritzel A., Green T., Figurnov M., Ronneberger O., Tunyasuvunakool K., Bates R., Žídek A., Potapenko A., Bridgland A., Meyer C., Kohl S. A. A., Ballard A. J., Cowie A., Romera-Paredes B., Nikolov S., Jain R., Adler J., Back T., Petersen S., Reiman D., Clancy E., Zielinski M., Steinegger M., Pacholska M., Berghammer T., Bodenstein S., Silver D., Vinyals O., Senior A. W., Kavukcuoglu K., Kohli P., Hassabis D., Highly accurate protein structure prediction with AlphaFold. Nature 596, 583–589 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Varadi M., Anyango S., Deshpande M., Nair S., Natassia C., Yordanova G., Yuan D., Stroe O., Wood G., Laydon A., AlphaFold Protein Structure Database: Massively expanding the structural coverage of protein-sequence space with high-accuracy models. Nucleic Acids Res. 50, D439–D444 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang Y., Forli S., Omelchenko A., Sanner M. F., AutoGridFR: Improvements on AutoDock affinity maps and associated software tools. J. Comput. Chem. 40, 2882–2886 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.O'Boyle N. M., Banck M., James C. A., Morley C., Vandermeersch T., Hutchison G. R., Open Babel: An open chemical toolbox. J. Chem. 3, 14 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Eberhardt J., Santos-Martins D., Tillack A. F., Forli S., AutoDock Vina 1.2.0: New docking methods, expanded force field, and python bindings. J. Chem. Inf. Model. 61, 3891–3898 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sun Y. F., De Biasio F., Qiao H. L., Iovinella I., Yang S. X., Ling Y., Riviello L., Battaglia D., Falabella P., Yang X. L., Pelosi P., Two odorant-binding proteins mediate the behavioural response of aphids to the alarm pheromone (E)-β-farnesene and structural analogues. PLOS ONE 7, e32759 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ma Y., Lu H., Wang W., Zhu J., Zhao W., Cui F., Membrane association of importin alpha facilitates viral entry into salivary gland cells of vector insects. Proc. Natl. Acad. Sci. U.S.A. 118, e2103393118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figs. S1 to S10
Tables S1 to S4