Abstract
Brassinosteroids (BRs) are important plant growth regulators in multiple developmental processes. Previous studies have indicated that BR treatment enhanced auxin-related responses, but the underlying mechanisms remain unknown. Using 14C-labeled indole-3-acetic acid and Arabidopsis thaliana plants harboring an auxin-responsive reporter construct, we show that the BR brassinolide (BL) stimulates polar auxin transport capacities and modifies the distribution of endogenous auxin. In plants treated with BL or defective in BR biosynthesis or signaling, the transcription of PIN genes, which facilitate functional auxin transport in plants, was differentially regulated. In addition, BL enhanced plant tropistic responses by promoting the accumulation of the PIN2 protein from the root tip to the elongation zone and stimulating the expression and dispersed localization of ROP2 during tropistic responses. Constitutive overexpression of ROP2 results in enhanced polar accumulation of PIN2 protein in the root elongation region and increased gravitropism, which is significantly affected by latrunculin B, an inhibitor of F-actin assembly. The ROP2 dominant negative mutants (35S-ROP2-DA/DN) show delayed tropistic responses, and this delay cannot be reversed by BL addition, strongly supporting the idea that ROP2 modulates the functional localization of PIN2 through regulation of the assembly/reassembly of F-actins, thereby mediating the BR effects on polar auxin transport and tropistic responses.
INTRODUCTION
Since the identification of plant steroids more than two decades ago, brassinosteroids (BRs), such as brassinolide (BL), have turned out to be essential plant growth regulators in multiple developmental processes (Clouse and Sasse, 1998). Studies using BR synthesis– and signaling–deficient Arabidopsis thaliana mutants, such as de-etiolated2 (det2), diminuto1 (dim1), and BR-insensitive1 (bri1), that display altered photomorphogenesis (Chory et al., 1991; Takahashi et al., 1995; Clouse et al., 1996) have revealed profound effects of BRs on cell elongation, organogenesis, and reproductive growth (Clouse, 2002).
BR treatment leads to altered endogenous auxin levels and/or enhanced auxin sensitivity (Mandava, 1998; Sasse, 1999, Nakamura et al., 2003; Bao et al., 2004), suggesting potential crosstalk between BR and auxin signaling pathways. Studies on the transcriptional response of auxin-inducible IAA5 and IAA19 genes and the expression of a reporter gene containing a synthetic auxin response element fused to β-glucuronidase (DR5-GUS) in response to indole-3-acetic acid (IAA) or BR treatment suggested that BR and auxin signaling pathways both activated the transcription of IAA biosynthesis and auxin-responsive genes (Nakamura et al., 2003). Moreover, BRs might promote lateral root initiation/development by stimulating acropetal auxin transport (Bao et al., 2004).
Polar auxin transport (PAT) and the resulting auxin distribution are involved in almost all auxin-mediated growth responses (reviewed in Friml, 2003). Recent studies revealed several key components for PAT, such as the PIN proteins, which are encoded by a large gene family in Arabidopsis (Galweiler et al., 1998; Muller et al., 1998; Palme and Galweiler, 1999; Friml et al., 2002). In Arabidopsis, the PIN-FORMED1 (PIN1) protein is required for basipetal auxin movement and crucial for shoot vascular development, gravitropic responses, and photomorphogenesis (Galweiler et al., 1998; Rashotte et al., 2000; reviewed in Friml, 2003). PIN2 and PIN3 are essential for the root gravitropic response. pin2 mutant seedlings are agravitropic, and pin3 seedlings display decreased gravitropic and phototropic responses as well as inhibited hypocotyl and root growth (Muller et al., 1998; Friml et al., 2002). The observation that PIN protein positioning and cell polarity in Arabidopsis require STEROL METHYLTRANSFERASE1 function (Willemsen et al., 2003) suggests a role for sterols in PAT and PIN localization.
The regulation of auxin transport is essential for normal plant growth. Disruption of auxin efflux leads to defective or altered auxin transport, resulting in the redistribution of endogenous auxin and inhibited gravity responses (Geldner et al., 2001; Muday and Murphy, 2002). Recent studies revealed the important roles of the actin cytoskeleton in auxin transport and related growth (Geldner et al., 2001; Friml et al., 2002). Treatment with latrunculin B, an inhibitor of actin filament assembly, may enhance gravitropism through the alteration of auxin transport (Hou et al., 2003; Sun et al., 2004). The formation of cortical F-actin in Arabidopsis seedlings in turn is controlled by ROP (for Rho-of-plant) GTPases (Fu et al., 2002), and actin-depolymerizing factor mediates RAC/ROP GTPase–regulated pollen tube growth (Chen et al., 2003). Both constitutively active (CA-rop2) and dominant negative (DN-rop2) mutants of ROP2 GTPase alter auxin- and BR-related developmental processes, suggesting that ROP GTPases may participate in BR–auxin crosstalk (Li et al., 2001).
Here, we show that BRs play an important role in regulating PAT activity in Arabidopsis, thereby altering the distribution of endogenous auxin. The expression of PIN genes is differentially modified in BR-treated and in BR biosynthesis– and signaling–deficient mutants. Moreover, BR treatment dramatically promotes tropistic responses in reoriented plant seedlings, correlating with enhanced PIN2 longitudinal distribution from the root tip to the elongation zone. The expression and localization of ROP2 during tropistic responses suggest that ROP2 is involved in BR PAT regulation. Further investigation using the constitutive ROP2 overexpressor (35S-ROP2) and the dominant negative form (DA/DN-rop2) defined the ROP2-mediated BR modulation of PAT activity. Finally, the assembly/reassembly of F-actins may play a role during the process by modulating the membrane localization and cycling of PIN proteins.
RESULTS
BL Modifies PAT Activity and Auxin Distribution
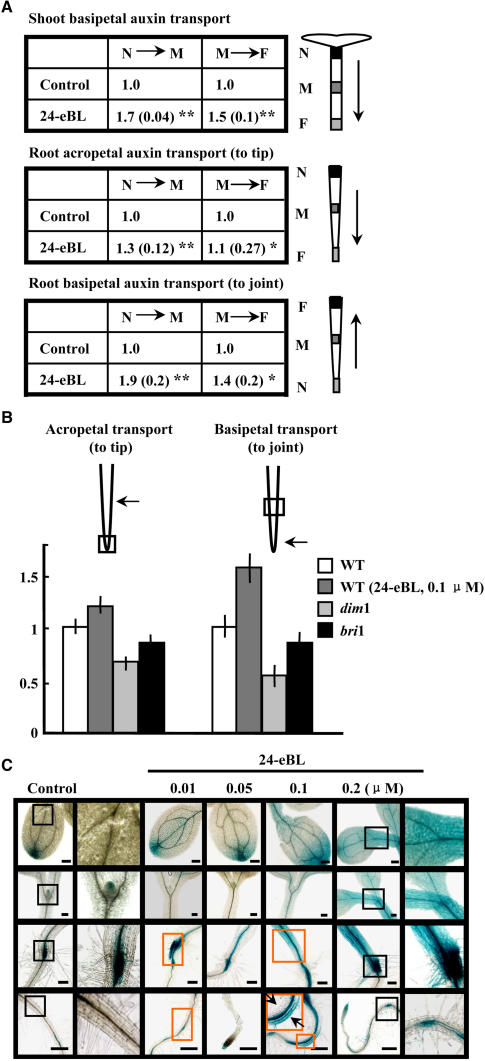
PAT in the hypocotyl occurs mainly from the apex to the base (shoot basipetal transport), and in roots, auxin is transported both from base to tip (acropetal transport) and from tip to base (basipetal transport). PAT capacities were analyzed in response to BL treatment to explore the potential interactions between them. Analysis of the PAT activities of 3-d-old Brassica napus seedlings using [14C]IAA showed that BL treatment (24-epi-BL [24-eBL]; 0.1 μM) strongly promoted shoot basipetal IAA transport, with up to a 70% increase from the shoot apex to the middle part (N to M) and an ∼50% increase from the middle part to the base (M to F) (Figure 1A, top). Root acropetal auxin transport was slightly enhanced from the middle region to the root tip (M to F) and increased by 30% from the base of the root to the middle part (elongation zone; N to M) (Figure 1A, middle). Root basipetal auxin transport was also stimulated, with up to a 40% increase from the root elongation zone to the shoot–root junction (M to F) and a 90% increase from the root tip to the middle region (Figure 1A, bottom), suggesting that exogenous BL effectively promotes auxin transport in both shoots and roots, particularly basipetal auxin transport in roots. In addition, PAT capacities were obviously reduced in the BR biosynthesis–defective mutant dim1 and the BR signaling–defective mutant bri1. In dim1 seedling roots, acropetal transport decreased by 32.8% compared with that in wild-type seedlings, and basipetal transport decreased by 59.7%. The bri1 mutant also exhibited a decrease in auxin transport compared with wild-type seedlings: root acropetal transport decreased by 13.4% and basipetal transport decreased by 17.7% (Figure 1B).
Figure 1.
Effects of BL on PAT Capacity and Endogenous Auxin Distribution.
(A) Exogenous BL promotes PAT capacity in different tissues. Hypocotyls and primary roots of 3-d-old B. napus seedlings, in the absence or presence of 24-eBL (0.1 μM), were treated with [14C]IAA at the apex or the base, and radioactivity was measured by monitoring [14C]IAA in 3-mm segments from the apex, middle, and base of hypocotyls and roots. Averaged data (n = 20; se in parentheses) indicate relative transport capacities compared with the untreated control, which was set to 1.0. Arrows indicate the PAT orientation. For each measurement, the parts of samples incubated in half-strength MS medium containing radioactive IAA are marked as N (near), M (middle), and F (far). ** P < 0.01 as determined by the one-tailed Student's t test; * P < 0.05.
(B) Deficiency of BR biosynthesis and signaling affects root bidirectional PAT capacities. Seedling roots of Arabidopsis wild-type (with or without 0.1 μM 24-eBL treatment), BR biosynthesis–deficient mutant (dim1), and BR-insensitive mutant (bri1) plants were tested as described for (A). Arrows indicate the regions of sample incubation in the half-strength MS medium containing radioactive IAA, and squares indicate the segments in which transported IAA was detected. Averaged data (n = 15; error bars indicate se) show relative transport activities compared with the untreated control, which was set to 1.0. Six-day-old roots of the dim1 mutant (average root length of 13.2 mm), the bri1 mutant (average root length of 14.8 mm), or wild-type (average root length of 16.4 mm) seedlings were used for measurements. The one-tailed Student's t test indicated significant differences (P < 0.01) among the treatments.
(C) BL caused altered distribution of endogenous auxin, as revealed by GUS activity in DR5-GUS Arabidopsis seedlings. Four-day-old seedlings growing in medium supplemented with gradients of 24-eBL (0 to 0.2 μM) were analyzed. Brown frames highlight significantly enhanced GUS expression. Regions in black frames are enlarged in the adjacent images to the right. Bars = 1 mm.
We next analyzed the concentrations of endogenous IAA in BL-treated B. napus seedlings. The results showed that exogenous BL treatment (0.01 to 0.1 μM) dramatically changed the IAA concentrations in different organs. After 0.01 μM BL treatment, the concentration of free IAA in shoots (including the cotyledons; 3-d-old seedlings) was increased from 1772.11 ± 130.65 (sd) pmol/g to 3849.16 ± 132.83 pmol/g, whereas in roots it was decreased from 3633.65 ± 224.08 pmol/g to 2272.11 ± 146.02 pmol/g. Similar effects were observed after treatment with 0.1 μM BL. Analysis of Arabidopsis seedlings harboring the auxin-responsive reporter DR5-GUS (Sabatini et al., 1999) confirmed the modulation of BL on auxin transport (Figure 1C). Consistent with a recent report by Bao et al. (2004), BL-treated seedlings showed higher levels of and broader GUS expression from the root tip to the elongation zone, and the GUS expression was dispersed throughout the stele and strong at the root–shoot junction (Figure 1C, highlighted by brown frames). In addition, GUS expression was particularly prevalent in primary roots after BR treatment (0.01 to 0.2 μM) and was asymmetrically distributed in the elongation zone (0.1 μM; brown frame, enlarged); a similar distribution was observed in the gravistimulated seedlings and those treated with BL (see Figure 4C below). The altered DR5-GUS expression patterns caused by BL treatment imply that BL affects auxin accumulation and distribution in Arabidopsis seedlings. Together, these results suggest that BRs regulate auxin accumulation and distribution by adjusting its transport. We then focused our studies on the underlying molecular mechanisms and potential mediators of BR effects.
Figure 4.
Regulation of Plant Tropism by BL.
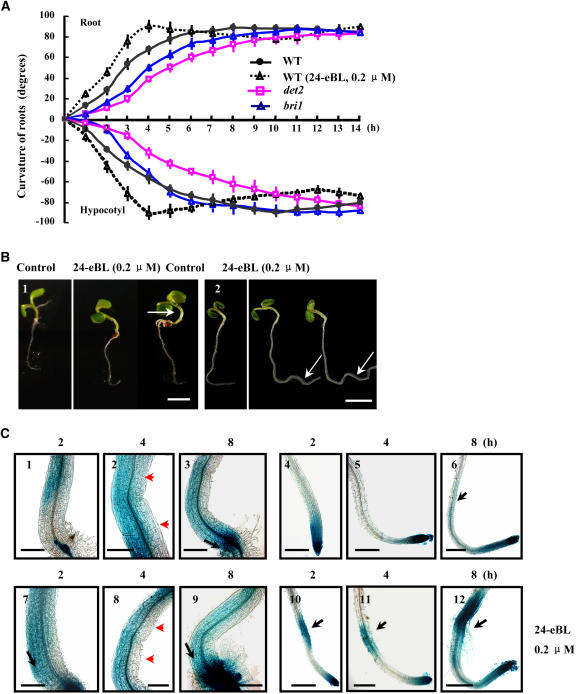
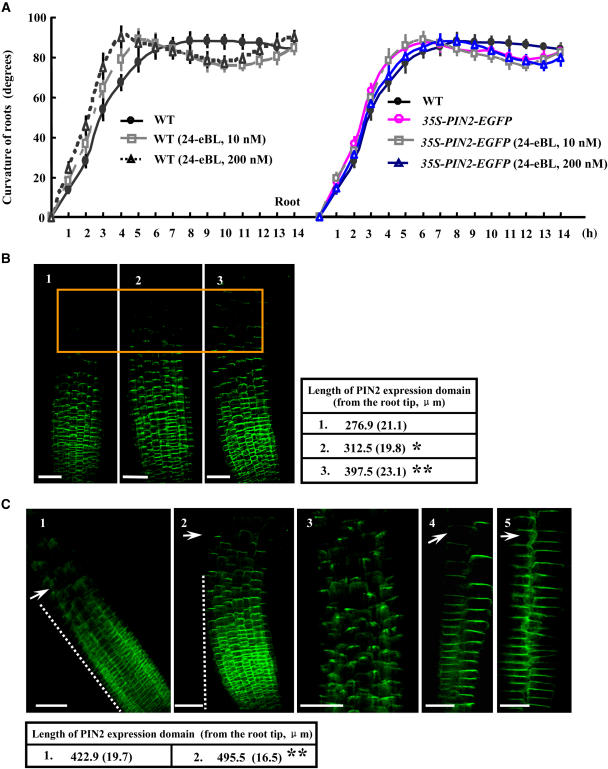
(A) Time course of curvature in seedling tropistic responses. Four-day-old Arabidopsis wild-type (in the absence or presence of 0.2 μM 24-eBL) or BR-related mutant (det2 and bri1) seedlings, treated with a 90° horizontal reorientation and unilateral topside light stimulus, were used for measurement. The curvature of seedlings was calculated every 60 min after the reorientation and analyzed statistically. n > 20 for each treatment, and error bars show sd. P < 0.05 determined by the one-tailed Student's t test. Please note that symbols may cover some bars.
(B) BR caused the enhancement of hypocotyl bending and root curving during tropistic responses. Four-day-old Arabidopsis seedlings were treated with DMSO (Control; left) or 24-eBL (0.2 μM; right) after a 90° horizontal reorientation for 8 h (1) and 14 h (2) after treatment. Arrows indicate the bent hypocotyls (1) and the second curves of roots (2) in the BR-treated seedlings. Bars = 4 mm.
(C) Altered auxin distribution in plant shoots (1 to 3 and 7 to 9) and roots (4 to 6 and 10 to 12) in response to gravity. Four-day-old DR5-GUS–harboring Arabidopsis seedlings were treated with a 90° horizontal reorientation, and GUS activities were detected after 2 h (1, 4, 7, and 10), 4 h (2, 5, 8, and 11), and 8 h (3, 6, 9, and 12). 24-eBL (0.2 μM) was added during the process (7 to 12). Statistical analysis indicated that the proportions of seedlings showing asymmetric expression of GUS were ∼67.7% (2 h), 63% (4 h), and 58.5% (8 h). n > 100 for each treatment, repeated in triplicate. Black arrows highlight the enhanced GUS expression downward to the shoot–root junction, and red arrowheads indicate the obviously asymmetric auxin distribution. Bars = 200 μm.
Differential Expression of Arabidopsis PIN Genes after BR Treatment
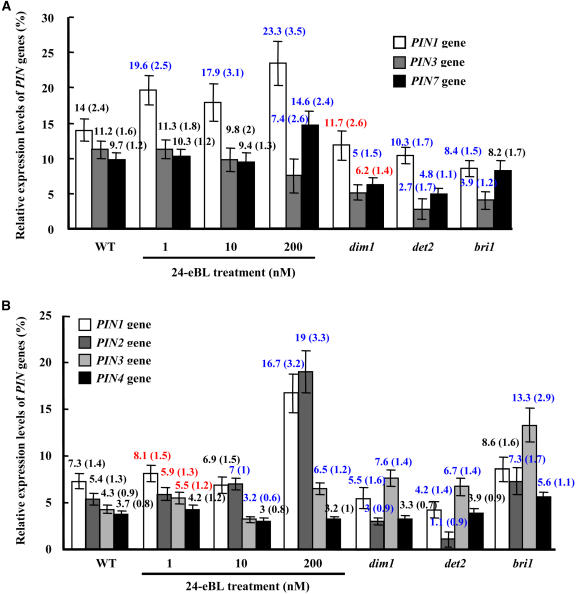
To test the effect of BRs on PIN gene expression, the modulation by BL of the expression of PIN1, PIN2, PIN3, PIN4, and PIN7 in Arabidopsis was studied through real-time quantitative RT-PCR (RT-qPCR). Relative transcript levels of PIN1, PIN3, and PIN7 were analyzed in 7-d-old seedling shoots (including cotyledons and hypocotyls) of wild-type (with or without BL treatment; 0.001, 0.01, or 0.2 μM), BR biosynthesis–deficient (dim1 and det2), and BR-insensitive (bri1) mutants (Figure 2A). Considering the BR effects on hypocotyl elongation (Clouse, 2002), root tropisms (Kim et al., 2000), and lateral root initiation (Bao et al., 2004), exogenous BL was applied for 7 d (with final concentrations of 1 or 10 nM) or 12 h (0.2 μM). In the upper part of the seedlings, PIN1 expression was highly enhanced by BL treatment; it was suppressed in the bri1 mutant and in BL-untreated dim1 and det2 mutants. In addition, the expression of both PIN3 and PIN7 was suppressed in these mutants.
Figure 2.
Altered Expression of PIN Genes in BL-Treated or BR Biosynthesis/Signaling Mutant Plants.
(A) BR-regulated PIN gene expression in shoots. Transcripts of PIN1, PIN3, and PIN7 in shoots (including cotyledons and hypocotyls) of wild-type seedlings with or without BR treatment (0.001 and 0.01 μM for 7d, or 0.2 μM for 12 h), BR biosynthesis–deficient mutant (dim1 and det2), or BR-insensitive mutant (bri1) seedlings were analyzed by real-time RT-qPCR. One-week-old Arabidopsis seedlings were used for total RNA extraction. Relative gene expression levels of PIN genes compared with ACTIN2 are shown as percentages (as for [B]). Statistical analysis by the one-tailed Student's t test showed significant differences, indicated in red (P < 0.01) or blue (P < 0.05) (as for Figure 4B). Error bars indicate sd (also in parentheses).
(B) BR-regulated PIN gene expression in roots. RT-qPCR analysis of the transcripts of PIN1, PIN2, PIN3, and PIN4 was performed in root tissues of wild-type seedlings under BL treatment or in BR-related mutant seedlings (as described for [A]). One-week-old Arabidopsis seedling roots were used for total RNA extraction. Error bars indicate sd (also in parentheses).
On the other hand, the regulation of PIN genes by BRs appears to be more complicated in roots. The expression of PIN 1, PIN2, PIN3, and PIN4 was differentially modulated after BL treatment (Figure 2B), suggesting distinct regulatory effects of BRs on different PIN genes. PIN1 plays an important role in auxin basipetal transport in dicots, and the combination of pin mutations reduces root growth (Blilou et al., 2005). Compared with that in control roots, PIN1 transcript was increased slightly in response to exogenous BL (1 nM for 7 d) and obviously stimulated at 0.2 μM (12-h treatment), but it was decreased in the BR biosynthesis–deficient mutants dim1 and det2. bri1 mutants, on the other hand, revealed no changes in PIN1 expression. PIN2, which is specifically expressed in roots and facilitates auxin basipetal transport, is critical for gravitropism. The transcription of PIN2 in bri1 was induced by BL treatment, whereas it was suppressed to very low levels in dim1 and was even lower in det2. The PIN3 transcript was upregulated under a low (1 nM) or high (0.2 μM) concentration of BL and in BR biosynthesis–deficient or –insensitive mutants. PIN4 transcription was increased only in the BR-insensitive mutants; it was unaltered under BL treatment, consistent with BR's effects on the accumulation of endogenous auxin described previously (i.e., through at least partial regulation of diverse PIN genes).
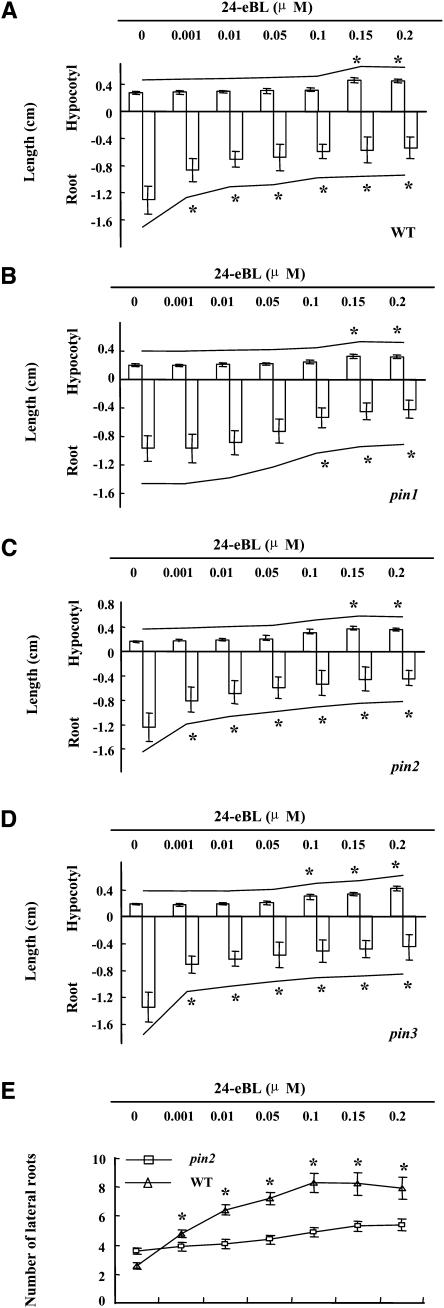
We next investigated how hypocotyl growth and primary root development respond to BR treatment in PIN1-, PIN2-, and PIN3-deficient mutants. In wild-type seedlings, 24-eBL (0 to 0.2 μM) treatments inhibited primary root growth but slightly promoted hypocotyl elongation (Figure 3A). The sensitivity for BR-dependent inhibition of root growth was reduced in the pin1 mutant (Figure 3B). Whereas 1 nM BR caused obvious inhibition of primary root growth in wild-type seedlings, similar effects were detected in the pin1 mutant only at 0.1 μM. pin2 and pin3 seedlings had normal responses to BL (Figures 3C and 3D), but BL promotion of lateral root initiation as seen in wild-type plants was barely detectable in pin2 (Figure 3E), suggesting that excess BL-induced PIN2 expression might promote plant lateral root initiation and the gravitropic response.
Figure 3.
Altered Responses of Arabidopsis PIN-Deficient Mutants to BL.
(A) to (D) Hypocotyl and primary root growth of PIN-deficient mutants (pin1, pin2, and pin3) under BL treatment. Averaged lengths of hypocotyls (shown as positive numbers) and primary roots (shown as negative numbers) of 1-week-old Arabidopsis seedlings of wild-type (A), pin1 (B), pin2 (C), and pin3 (D) seedlings after treatments with 24-eBL gradients (0 to 0.2 μM) were measured (n > 60). Error bars indicate sd, and the curves above and below the data bars indicate tendency. Asterisks indicate significant changes (P < 0.05) induced by BL compared with the untreated control.
(E) Lateral root growth of pin2 seedlings under BL treatment. Numbers of lateral roots and visible lateral root primordia per centimeter of primary root were counted in 1-week-old seedlings (n > 60) in the presence of a 24-eBL gradient (0 to 0.2 μM). Error bars indicate sd. Asterisks indicate significant changes (P < 0.05) induced by BL compared with the untreated control.
BRs Stimulate Plant Tropistic Responses by Promoting PIN2 Activity
Exogenous BR increased gravitropic curvature in the maize (Zea mays) primary root (Kim et al., 2000), providing the first evidence for a connection between BRs and gravitropism. To determine whether BRs affect Arabidopsis tropism and how this relates to their modulatory effects on PAT activities, we first treated 4-d-old wild-type seedlings with 0.2 μM 24-eBL and reoriented them by 90° for 0 to 14 h to examine their tropistic responses. Time-course curvature analysis indicated that BL enhanced the tropistic responses to gravity (Figure 4A). In BL-treated seedlings, hypocotyl curvature reached its first maximum at 4 h after reorientation, compared with 6 h in untreated control seedlings (Figure 4B, left). With regard to root curvature, the second root curve appeared in BL-treated seedlings at 14 h after the induction of tropistic growth, which was much enhanced compared with that in untreated control seedlings (Figure 4B, right). Neither BR biosynthesis– nor BR signaling–deficient mutants displayed defects in tropisms when grown without reorientation (Chory et al., 1991; Clouse et al., 1996). However, decreased curvature of the mutant seedlings was observed after 90° reorientation, especially within the first 6 h. The most obvious delay in the tropistic response occurred in the BR biosynthesis–deficient mutant det2, but not in the BR-insensitive mutant bri1, indicating that the amounts of BR might play a key role in promoting plant tropistic responses.
As auxin movement in seedlings is crucial in plant growth and tropisms, we then examined the distribution of auxin during tropistic responses in Arabidopsis plants carrying the DR5-GUS reporter (Figure 4C). In normally growing seedlings, GUS activity was present in hypocotyls and primary roots, accumulated at the root-shoot junction, and was abundant at the root tip (Figure 1C, control). At 2 h after 90° reorientation, asymmetric expression of GUS in hypocotyls was detected, and GUS activity accumulated at the root-shoot junction after 8 h of gravity stimulus (Figure 4C, 1 to 6, arrows). Although diffuse GUS expression was observed in curving roots, treatment with BL (0.2 μM; seedlings here were exposed to both BL and gravity stimulation) enhanced the gravity-induced redistribution of GUS expression (Figure 4C, 7 to 12, arrows). Activation of asymmetric GUS expression in both hypocotyls and the bottom flank of the root elongation zone could be observed 8 h after the 90° reorientation in control seedlings, whereas such asymmetric activation upon BR treatment was detected only 4 h after the gravity stimulus. Similarly, strongly enhanced GUS staining at the shoot-root junction appeared in BR-treated seedlings after 2 h of gravity stimulus, whereas in control seedlings it occurred 8 h after reorientation, indicating that BR accelerates the tropism-related asymmetric distribution of auxin.
The tropistic response was enhanced in seedlings overexpressing PIN2 (35S-PIN2-EGFP; Figure 5A); however, the PIN2 overexpressor did not show a response to externally added BL, although the curvature apex occurred sooner than in the wild type. Whether the expression/distribution of the PIN2 protein was modified under BR treatment during the process was then examined. Indeed, a 90° reorientation of seedlings promoted the expression and polar localization of PIN2 (from the root tip) to the root elongation zone within 2 h (Figure 5B, 2; compare with the normal PIN2 protein localization in Figure 5B, 1); this was even more obvious with additional BL (0.2 μM; Figure 5B, 3). PIN2 expression was also more enhanced when the seedlings were reoriented by 90° for 6 h (Figure 5C, 1). Additional BL significantly induced PIN2 basipetal accumulation in the upper parts of the roots (Figure 5C, 2 and 3). Detailed analysis of PIN2 expression in the epidermis and cortex confirmed the effect of BR on the polar distribution of PIN2 in these longitudinal cell arrays, especially in the cortex (Figure 5C, 4 and 5). In addition, statistical analysis showed significant differences in the lengths of PIN2-expressing domains, further supporting the notion that BRs stimulate the extended distribution of PIN2 into the root elongation zone in response to gravity.
Figure 5.
PIN2 Responses to Both BL Treatment and Gravity.
(A) Altered gravitropic responses to BL treatment of PIN2-overexpressing plants. Measurements were performed as described for Figure 4A using 35S-PIN2-EGFP lines. n > 20 for each treatment. Error bars indicate sd. P < 0.05 compared with wild-type seedlings, determined by the one-tailed Student's t test. Please note that symbols may cover some bars.
(B) Expression and localization of PIN2 in 2-h reoriented primary roots. Four-day-old Arabidopsis seedlings with the PIN2-EGFP construct were planted under normal conditions (1) or with 90° horizontal reorientation for 2 h in the absence (2) or presence (3) of supplemental 0.2 μM 24-eBL and observed with a confocal microscope. The orange frame highlights the segments of the root tip near the elongation zone, where the differential expression of PIN2 appeared. n > 20 for each experiment, repeated in triplicate (as for [C]). Bars = 50 μm. Seedlings with similar root length were selected and observed with a confocal microscope (as for [C] and Figure 6). Lengths of the PIN2 expression domain (from the root tip) exposed to the various conditions are shown in the table (sd in parentheses). Statistical analysis indicated significant differences (* P < 0.05, ** P < 0.01) compared with the untreated control.
(C) PIN2 expression and localization in primary roots of 6-h reoriented 4-d-old Arabidopsis seedlings treated as described for (B). PIN2 expression and localization were observed in reoriented control seedlings (1) and seedlings treated with 0.2 μM 24-eBL (2), whereas diffused PIN2 expression was detected in the upper belt of the elongation zone (3). Magnification of epidermis and cortex cells revealed basipetal localization of PIN2 in control seedlings (4) or with 0.2 μM 24-eBL (5). Arrows indicate stimulated PIN2 polar accumulation. Bars = 50 μm (1 to 3) or 20 μm (4 and 5). The lengths of the PIN2 expression domain (from the root tip) are shown in the table (sd in parentheses). Statistical analysis indicated significant differences (** P < 0.01) compared with the control.
Given the previous observation that BL did not stimulate the gravitropic response of the PIN2 overexpressors although it highly promoted that of the wild type, it was hypothesized that PIN2 accumulation upward to the root elongation zone might be activated in the early phases of root tropisms, which could be promoted by exogenous BL, whereas high levels of PIN2 in the overexpressors may not be enhanced further, perhaps because of some kind of feedback regulation during this process.
ROP2 Is Stimulated by BR and Mediates BR PAT Modulation in Tropistic Responses
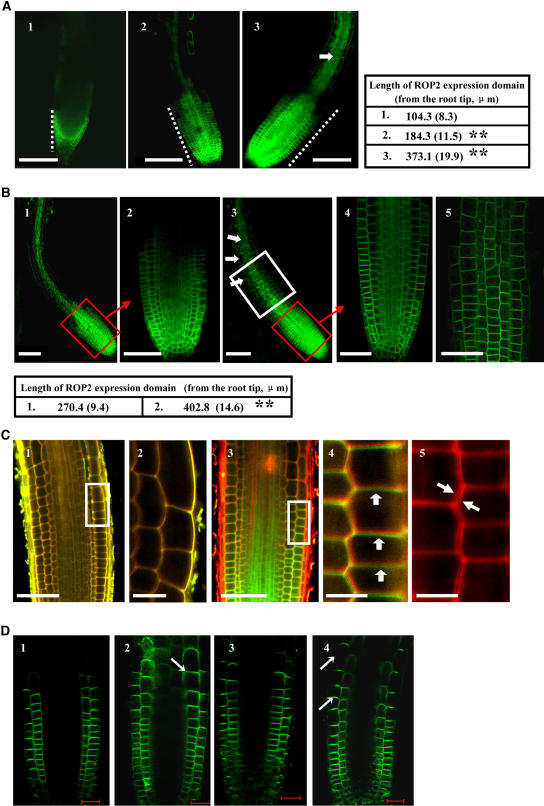
Previous studies have revealed that ROP GTPase controls F-actin accumulation, suggesting a possible role of ROP GTPase in auxin transport regulation (such as actin-dependent PIN1 cycling) and hence the tropistic responses (Geldner et al., 2001; Friml et al., 2002; Fu et al., 2002). ROP2 accumulates in growing root hairs (Jones et al., 2002) and in columella cells and controls the formation of cortical fine F-actin and directional cell expansion at an early phase of organogenesis (Fu et al., 2002). The expression and localization of ROP2 in Arabidopsis seedlings expressing a ROP2-EYFP fusion protein were investigated, and the results showed that in normally growing wild-type seedling roots, ROP2 accumulation was detected mainly in the columella of the root tip (Figure 6A, 1), which was enhanced at the root meristems 2 h after a 90° reorientation (Figure 6A, 2). Application of exogenous BR further increased the accumulation during the reorientation (Figure 6A, 3). Such enhancement was most remarkable 2 h later; by then, ROP2 had accumulated from the root tip upward to the elongation zone (Figure 6B, frames) and was polarly localized (Figure 6C).
Figure 6.
Promotion by BL of ROP2 Expression and Polar Accumulation during Gravitropic Responses.
(A) Expression and localization of ROP2 in response to a 2-h gravity stimulus. Roots of 4-d-old Arabidopsis seedlings with the ROP2-EYFP construct in normal growing conditions (1) or after a 90° horizontal reorientation for 2 h in the absence (2) or presence (3) of 0.2 μM 24-eBL were observed with a confocal microscope. The arrow shows the ROP2 localization in the elongation zone, which was detected only in the presence of exogenous 24-eBL (3). Bars = 100 μm. n > 20 for each experiment, repeated in triplicate. Images were captured and analyzed under the same laser conditions (as for [B] to [D]). The lengths of the ROP2 expression domain (from the root tip) exposed to the various conditions are shown in the table (sd in parentheses). Statistical analysis indicated significant differences (** P < 0.01) compared with the untreated control.
(B) Expression and polar accumulation of ROP2 in roots in response to gravity (90° reorientation) for 4 h (1) or to treatment with 0.2 μM 24-eBL (3). Magnification of the cell layers in the root meristem (red frames) shows the localization of ROP2 under the gravity stimulus in the presence (4) or absence (2) of 0.2 μM 24-eBL. ROP2 distribution in the elongation zone, which was detected only in the presence of exogenous 24-eBL, is highlighted with a white frame and arrows (3) and enlarged (5). Bars = 100 μm. Lengths of the ROP2 expression domain (from the root tip) are shown in the table (sd in parentheses). Statistical analysis indicated significant differences (** P < 0.01) compared with the control.
(C) ROP2 polar localization occurred 2 h after the gravity stimulus in seedling roots. Roots of the control seedlings were stained with propidium iodide after a 2-h reorientation (1; white frame magnified in 2), and plasmolyzed cells were observed after being detached slightly from the cell wall (3; white frame magnified in 4). Diminished polar distribution of ROP2 was also observed in roots treated with 2 μM latrunculin B for 15 min (5). Bars = 20 μm (1 and 3) or 10 μm (2, 4, and 5).
(D) Expression and localization of PIN2 in 4-d-old Arabidopsis seedlings with the PIN2-EGFP construct in normal growing conditions (1) or with a 90° reorientation for 4 h (2); those with enhanced expression of ROP2 (Arabidopsis seedlings with both 35S-ROP2 and PIN2-EGFP) were observed (3, normal growth; 4, 90° reorientation for 4 h). Arrows highlight the enhanced accumulation of PIN2 at the basal cell side under enhanced ROP2, orientating toward the elongation zone. n > 60 for each treatment. Bars = 20 μm.
We observed enhanced expression and basipetal localization at the plasma membrane of ROP2-EYFP in response to gravity (Figure 6), contrary to a previous observation of such expression at the both the apical and basal sides (Xu and Scheres, 2005). Extension of ROP2 expression domains into the root elongation zone cells was also significantly stimulated by BR treatment: the length of the expression domain increased from 104.3 ± 8.3 μm to 373.1 ± 19.9 μm within 2 h with BL treatment and 90° reorientation, and it continued to increase to 402.8 ± 14.6 μm 2 h later. This result and previous studies of similar promotion of basipetal accumulation of PIN2 during the gravitropic response (longitudinally along the root axis within 2 h of the gravity stimulus) encouraged us to further explore the role of ROP2 in the BR PAT regulation of the plant tropistic response.
To investigate whether ROP2 polarity was modified under the gravity stimulus, root tip cells were plasmolyzed (the cell wall was stained with 10 μg/mL propidium iodide before plasmolysis; Figure 6C, 1 and 2) and the basipetal localization of ROP2 along the root axes was detected (Figure 6C, 3 and 4). Also, latrunculin B treatment (2 μM, 15 min) could disrupt the polar localization of the ROP2 protein (Figure 6C, 5), suggesting that the ROP2 polar localization was dependent on F-actin filament dynamics. Furthermore, the expression and distribution of PIN2 in ROP2-overexpressing plants (35S-ROP2) were analyzed to investigate whether ROP2 GTPase itself plays a role in the modulation of BR on PAT activities. Compared with the polar localization of PIN2 in the cortex and epidermis of seedling root meristems (Figure 6D, 1), a 90° reorientation for 4 h induced PIN2 accumulation up to the elongation zone (Figure 6D, 2). Enhanced basipetal accumulation of PIN2 was observed in the ROP2-overexpressing plants under normal growth conditions (Figure 6D, 3) and was most significant after the reorientation (4 h; Figure 6D, 4).
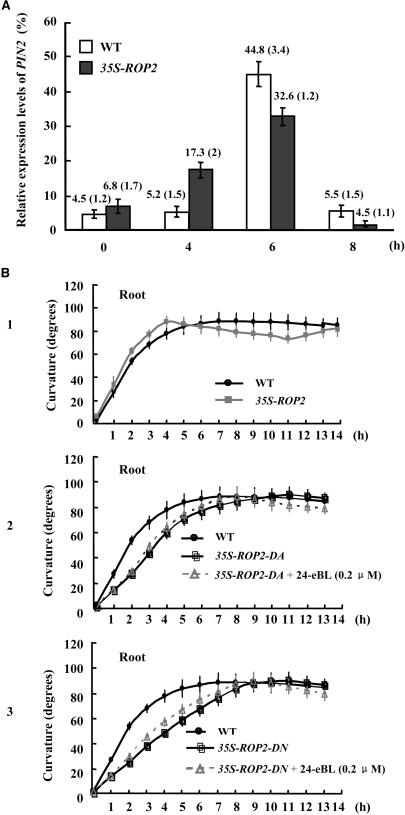
Thus, considering the crucial role of PIN2 in root gravitropism, it was conjectured that BR stimulation of tropism formation might occur through its modulation of PAT activities, especially PIN2. Analysis by RT-qPCR indicated that PIN2 gene expression was altered in response to 90° reorientation. The PIN2 transcript in wild-type seedling roots was detected at the highest level at 6 h after the gravity stimulus and diminished 2 h later (Figure 7A). Most interestingly, compared with wild-type seedlings, ROP2-EYFP seedlings had peak accumulation of PIN2 transcripts at 4 h after the gravity stimulus, indicating that overexpressing ROP2 may accelerate the transcription and accumulation of PIN2 in gravistimulated primary roots, consistent with the previous hypothesis that ROP2 mediates BR PAT modulation during the gravitropic response.
Figure 7.
ROP2 Mediates the BL Modulation of PAT Activities.
(A) Relative PIN2 expression in gravistimulated primary roots. The time-course analysis of PIN2 expression by RT-qPCR was performed using 7-d-old Arabidopsis seedlings of wild-type or 35S-ROP2 plants with 90° reorientation. Transcript levels of PIN2 were determined at 0, 4, 6, and 8 h after reorientation and are indicated as percentages of the ACTIN2 level. Error bars indicate sd (in parentheses). P < 0.05 for each treatment, compared with the wild-type seedlings, determined by the one-tailed Student's t test.
(B) Time-course analysis of curvature in gravitropic responses of the 35S-ROP2-YFP line (1) and the dominant negative ROP2 plants 35S-ROP2-DA (2) and 35S-ROP2-DN (3). The same measurements were made as described for Figure 4A. n > 20 for each treatment, and error bars indicate sd. P < 0.05 for 35S-ROP2-DA/DN and P < 0.01 for the 35S-ROP2 line, compared with the wild-type seedlings (without BR treatment), determined by the one-tailed Student's t test. Please note that symbols may cover some bars.
Seedlings constitutively expressing ROP2 (35S-ROP2) showed increased gravitropic responses (Figure 7B, 1), suggesting that ROP2 may function in the tropistic response by stimulating PIN2 activity. Compared with previous findings that BR could induce ROP2 accumulation, ROP2 dominant negative (DA/DN-rop2) mutants showed decreased gravitropic responses, which were not recovered by supplemented BL (Figure 7B, 2 and 3).
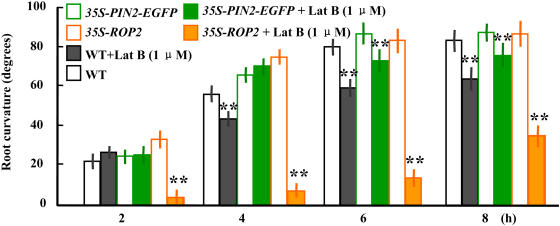
Latrunculin B (1 μM) was applied to clarify whether the assembly of F-actins influenced the plant tropisms. Our preliminary studies showed that treatment with latrunculin B had identical effects on the root growth of all seedlings (with different backgrounds) tested (data not shown), and after a 4-h treatment with latrunculin B, plant tropism was suppressed. Plants overexpressing PIN2, however, did not show the suppression of tropistic responses by latrunculin B. By contrast, the tropism of ROP2-EYFP seedlings was suppressed by latrunculin B within the first 2 h, and it did not resume even after 6 h (Figure 8), confirming that F-actin assembly is important for plant tropism. Compared with wild-type seedlings, those overexpressing ROP2 are highly sensitive to the latrunculin B treatment (latrunculin B affected the gravitropic response more obviously), and those overexpressing PIN2 show reduced sensitivity (the overexpressors were more resistant), suggesting that F-actin accumulation (i.e., the assembly/reassembly of F-actins) might participate in BR-enhanced plant tropism responses.
Figure 8.
Effects of Latrunculin B on Root Tropistic Responses.
Latrunculin B (Lat B; 1 μM) was applied during root gravitropic responses (as described for Figure 4A) to the seedlings of wild-type, PIN2-EGFP, and 35S-ROP2 plants. The curvatures were calculated at 2, 4, 6, and 8 h after the horizontal reorientation. n > 20. Error bars indicate sd. Double asterisks indicate significant differences (P < 0.01) determined by the one-tailed Student's t test.
DISCUSSION
Recent reports have revealed interactions among various phytohormones, such as auxin, gibberellin, ethylene, and BRs (Fu and Harberd, 2003; Bao et al., 2004; Souter et al., 2004). Through analysis of gene expression in various mutants or hormone-treated plants, it has been recognized that BRs, auxin, and other plant hormones influence each other during plant development, although the mechanisms remain poorly understood. Here, we provide novel insights into the interactions between BRs and auxin and propose ROP2 as an important mediator in BR PAT modulation of plant tropism.
We show that exogenous BL promotes both plant acropetal and basipetal auxin transport and modifies the accumulation pattern of endogenous auxin. These effects are potentially modulated through regulation of the expression of PIN genes, especially PIN1 and PIN2, although it is not known whether the regulation is at the transcriptional or posttranscriptional level. A model of the effect of BRs on PAT activity in dicots was proposed based on these observations (Figure 9). As auxin transport naturally proceeds through several distinct pathways that regulate plant development, this modulation may affect multiple growth responses, such as organ formation, root gravitropism, and lateral root development. For example, BR deficiency results in reduced PIN1 transcript levels and basipetally transported IAA in the shoot (Figures 1 and 2); consistently, BR biosynthesis– and signaling–deficient plants display obviously inhibited shoot growth. Exogenous BL enhances lateral root initiation and development related to acropetal auxin transport (Bao et al., 2004), and pin2 mutant plants display reduced sensitivity to BL in lateral root development, supporting the idea that PIN2 is required for this regulation (Figure 3E). In addition, analysis of the root lengths of pin1, pin2, and pin3 knockout mutants in response to BL showed that only pin1 displayed reduced sensitivity (Figure 3B), suggesting that the BR modulation of primary root growth was mainly facilitated through PIN1 actions. However, further studies are required to determine how other PIN proteins are involved in BR PAT regulation.
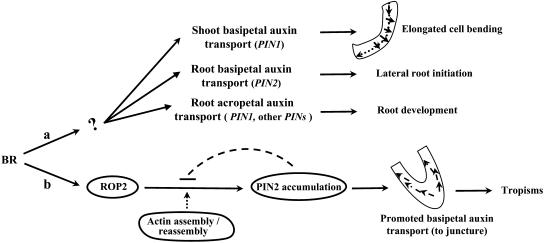
Figure 9.
Hypothetical Model of BR-Mediated Regulation of PAT Activities during Plant Tropisms and Multiple Developmental Processes.
The regulation of diverse PIN genes by BRs suggests potential modulatory effects of BR on plant developmental processes, including hypocotyl elongation and bending, lateral root initiation, and primary root growth, through their effects on PAT activities (a). In addition, ROP2 and actin assembly/reassembly may mediate BR PAT modulation of plant tropisms through the regulation of PIN2 accumulation and distribution (b).
RT-qPCR analysis showed that, in response to the altered BR levels in seedlings, either through the application of exogenous BL or using BR biosynthesis–defective plants, transcript levels of PIN1 and PIN2 were stimulated after exogenous BL treatment and were suppressed when endogenous BL was reduced. However, it is worth noting that the expression of PIN1 and PIN2 in bri1 did not support this trend. Furthermore, PIN2 and PIN3 were more stimulated by BL in bri1 than in det2 or wild-type seedlings. These surprising results may be explained by the fact that biologically active BRs accumulate in bri1 as a result of this mutant's inability to regulate BR biosynthesis (Noguchi et al., 1999). Because of the difficulty of measuring the exact levels of endogenous BRs in different parts of young seedlings, whether and how BRs modify auxin transport through its signaling pathway is still mysterious. It is possible that higher levels of selected PIN gene transcripts were present in bri1 mutant roots than in the wild type as a result of an alternative non-BRI1 signaling pathway that responded to the higher BR levels in the bri1 mutant.
Muessig et al. (2003) reported that low levels (0.05 and 0.1 nM) of exogenous BRs stimulate root growth. In those studies, exogenous BRs were applied to 10-d-old Arabidopsis seedlings and root length was measured 10 d later, or the BR-regulated genes were profiled from seedling roots treated with a higher concentration of BR (300 nM) for 1 h. More recently, Bao et al. (2004) analyzed BR stimulation of lateral root initiation at concentrations of 0 to 10 nM. We studied the phenotypes of 3- to 10-d-old seedlings with BR concentration gradients (0 to 500 nM), in which low concentrations of BL (1 or 10 nM) were applied for 7 d and a high concentration (200 nM) was applied for a short period (12 h). However, the longer treatment did not allow the accurate determination of BR effects on PIN transcription, and the primary versus secondary effects of BR treatment on PIN gene expression remain to be addressed.
The actin cytoskeleton in plants is essential for plant growth, and the assembly/reorganization of F-actins in plants may control the targeting of auxin efflux–related proteins to specific membrane domains (Muday et al., 2000), including the actin-dependant PIN1 cycling (Geldner et al., 2001). Furthermore, actin-dependent vesicle sorting of carrier proteins to membrane surfaces plays a critical role in establishing auxin transport polarity and the redirection of auxin flow after environmental stimuli (Steinmann et al., 1999; Muday and Murphy, 2002). Recently, cytoskeleton inhibitors, such as latrunculin B, have been used to determine the roles of F-actin integrity and dynamics in gravitropism. Blancaflor et al. (2003) showed that at low concentration (100 nM), latrunculin B promotes maize root gravitropism, consistent with the result that application of latrunculin B to root caps results in strong curvature responses (Hou et al., 2003). Our study demonstrated that 1 μM latrunculin B prevented Arabidopsis root gravitropic responses. The opposite effect on maize root gravitropism may be attributable to the different treatment. In the study by Blancaflor et al. (2003), the seedlings were actually rotated after a brief horizontal stimulus and treated with latrunculin B for 1 h.
Together with the fact that actin accumulation is under the control of ROP GTPase signaling (Fu et al., 2002), the involvement of the assembly/reorganization of F-actins in BR PAT regulation (activated ROP2 was required) is further suggested. However, among all of the membrane-localized PIN proteins, only PIN1 and PIN3 have been proven to be associated with actin-dependent cycling (Geldner et al., 2001; Friml et al., 2002). Whether a similar cycling of PIN2 exists in plants, or whether F-actin accumulation affects PIN2 actions through other mediators, are still unknown.
ROP2 is essential in multiple growth processes, especially in root development and lateral root initiation (Li et al., 2001). ROP2 is transcribed in seedling roots and accumulated during rapid cell growth and cell elongation in the epidermis and cortex. BR treatment promotes ROP2 accumulation from the stele toward the elongation zone of roots in response to gravity (Figures 6A and 6B) and enhances the polar distribution of ROP2, suggesting the possible roles of ROP2 in BR promotion of plant tropisms. Bao et al. (2004) reported the BR regulation of lateral root development through the promotion of acropetal auxin transport in Arabidopsis roots and suggested the possibility of ROP GTPase action in auxin/BR signaling, or the interaction of the two. Indeed, the ROP2 dominant negative (DN-rop2) mutant displayed phenotypes similar to BR-insensitive/synthetic mutants (i.e., dwarfism, reduced leaf size, and dramatically decreased sensitivity of lateral root formation to IAA stimulation) (Li et al., 2001). Our results provide evidence that ROP2 responds to BR in tropistic responses and contributes to root gravitropism. It is worth noting that BR-enhanced ROP2 expression appeared more obvious than BR-induced PIN2 expression (Figures 5B and 6A) after 90° reorientation for 2 h and that BR-stimulated polar accumulation of ROP2 diminished earlier than BR-induced longitudinal PIN2 distribution (data not shown). These findings are consistent with the supposition that exogenous BR activates ROP2 in response to gravity (with assembly/reorganization of F-actins occurring), resulting in altered auxin transport and distribution in seedlings and finally affecting plant growth.
In summary, we propose that BRs enhance the polar accumulation of PIN2 in root meristems in response to gravity, resulting in the redistribution of auxin from the root tip toward the elongation zones and promoting plant tropism (Figure 9). ROP2 acts as an important factor in this gravitropic response by regulating PIN2 actions through F-actin assembly/reorganization.
METHODS
Enzymes and Chemicals
DNA primers for the PCR and Taq DNA polymerase were purchased from Genecore. IAA (I-2886), 24-epi-BL (E-1641), and radioactive IAA (31464-1) were purchased from Sigma-Aldrich. Latrunculin B (76343-94-7) was purchased from Biomol.
Materials and Growth Conditions
Arabidopsis thaliana (Columbia ecotype) marker lines harboring construct DR5-GUS and knockout mutant lines pin1-3, BR biosynthesis–defective mutants det2 and dim1, and BR-insensitive mutant bri1 were plated on half-strength Murashige and Skoog (MS) medium (Murashige and Skoog, 1962) at 4°C in darkness for 2 d of vernalization, then transferred to a phytotron under conditions of 20 to 22°C with a 16-h light/8-h dark cycle. Brassica napus ecotype Huyou 15 was cultured on the same medium at 25°C under normal light conditions. Gradients of 24-eBL were added into the culture medium. Seedlings were grown in the light for <1 week. PIN2-EGFP and ROP2-EYFP were reported by Xu and Scheres (2005); 35S-PIN2-EGFP was generated from PIN2-EGFP by exchanging the PIN2 promoter for the 35S promoter. At least 20 seedlings of two independent lines were used in the experiments, and the experiments were repeated twice.
Observation of Plants
Seedlings of the wild type, the DR5-GUS marker line, and mutants were photographed using a Nikon (SMZ 800) digital camera, and the lengths of primary roots and hypocotyls were measured and analyzed using MetaMorph version 5.0 software (Universal Imaging). The numbers of lateral roots were analyzed with the Interference Discrepancy Contrast Microscope system (Leica) for the visible lateral root primordia to determine root initiation or elongation. Detection of GUS activities was performed as described by Jefferson et al. (1987).
Measurement of Free IAA Concentration
BR-treated and untreated control B. napus seedlings were grown on plates for 3 d. The upper parts (hypocotyls and cotyledons) and seedling roots (>0.1 mg per sample) were used for ELISA measurement of free IAA concentration, as described by Liang and Yin (1994).
Measurement of IAA Transport Activity
Measurement of auxin transport activity was conducted using primary roots and hypocotyls of 2.5-d-old B. napus seedlings (24-eBL–treated and untreated) or Arabidopsis mutant seedlings. In the assay, 2.5-cm-long root/hypocotyl axis segments were incubated (in both normal and inverted orientations) in half-strength MS liquid medium containing 1.45 pM (0.08 pCi/mL final concentration) [14C]IAA for 18 h. After incubation, samples were washed at least three times in half-strength MS medium, and 5-mm-long pieces from the upper, middle, and lower portions of each segment were placed in scintillation fluid to measure the continuous pulse of radioactive auxin using a liquid scintillation counter (Beckman LS650) in 3 mL of counting solution, within 24 h after incubation.
The primary roots of Arabidopsis mutant seedlings were cut into 10-mm segments for the acropetal auxin transport test. [14C]IAA was added to a final concentration of 1.45 pM (as described above) to the half-strength MS solution (with 0.8% [w/v] agar) and was dropped at the root 10 mm back from the root tip just before agar solidification. After incubation for 8 h, samples were washed at least three times in half-strength MS medium, and 3-mm segments either of the root tip or the place where radioactive IAA was applied were used for measurement of the continuous pulse of radioactive auxin. To test basipetal auxin transport, [14C]IAA was added at the root tip, and 2-mm segments were cut either at the root tip or 7 mm back from the root tip (based on the measurement reported by Rashotte et al. [2000]). Fifteen seedlings were used for each assay, and relevant statistical analysis was performed. The measurements were repeated three times.
Real-Time RT-qPCR Analysis
RT-qPCR analyses were performed to study the transcription of PIN family genes in Arabidopsis seedlings after BR treatment, with or without a 4-h gravity stimulus. Total RNA was isolated from Arabidopsis shoot or root tissue using Trizol reagent (Huashun) and reverse-transcribed according to the manufacturer's instructions (SuperScript preamplification system; Promega). Reverse transcription was performed in a total volume of 40 μL using 4 μg of total RNA as template incubated at 42°C for 60 min. RT-PCR was performed, and the open reading frame sequence of Arabidopsis ACTIN2 (locus number At3g18780) was used as a positive internal control with primers 5′-CCTTCGTCTTGATCTTGCGG-3′ (forward) and 5′-AGCGATGGCTGGAACAGAAC-3′ (reverse). The primers used for PCR amplification were as follows: PIN1 (forward, 5′-TTGCTGAGCTCCTACTTAAG-3′; reverse, 5′-GGCATGGCTATGTTCAGTCT-3′); PIN2 (forward, 5′-AAGTCACGTACATGCATGTG-3′; reverse, 5′-AGATGCCAACGATAATGAGTG-3′); PIN3 (forward, 5′-GAGTTACCCGAACCTAATCA-3′; reverse, 5′-TTACTGCGTGTCGCTATAGT-3′); PIN4 (forward, 5′-ACCACTTAACTAGAAACTTCA-3′; reverse, 5′-TCATTGCTTGTGGGAACTCT-3′); PIN7 (forward, 5′-TCTAGTTGCGTTCCACTAATC-3′; reverse, 5′-CGGTAAAACATATGCCACCA-3′); and ROP2 (forward, 5′-TGTTTGTTTCCGATCTTGCG-3′; reverse, 5′-CATCAGCACCACGGTAACTCA-3′).
RT-qPCR was then performed on a RotorGene 3000 system (Corbett Research) using a SYBR green detection protocol according to the manufacturer's instructions (SYBR Premix Ex Taq system; TaKaRa). The amount of product was determined at the end of each cycle using RotorGene software. The differences in cycle number during the linear amplification phase between diverse samples were used to examine differential gene expression, which was indicated as relative expression levels (percentage) compared with the transcript of ACTIN2.
Analysis of Tropism Responses
Four-day-old dark-grown Arabidopsis seedlings with similar root lengths were selected and transferred to fresh half-strength MS medium supplemented with or without 24-eBL (0.2 μM). 24-eBL was dissolved in DMSO as stock (10 mM). The same amount of DMSO was added in the medium and used as the solvent control in each test. Seedlings were reoriented by 90°, cultured under unilateral (top only) light, and observed with a stereoscope (SMZ 800; Nikon) every 60 min. Seedling lengths and angles throughout the tropistic stimulation were measured from digitized images using MetaMorph 5.0 software (as described previously by Hou et al. [2003]). Experiments were repeated three times (n > 60) and statistically analyzed.
The PIN2-EGFP– or ROP2-EYFP–containing plants, constitutive PIN2/ROP2 overexpressors (35S-PIN2/ROP2), and the ROP2 dominant negative mutant plants (35S-ROP2-DA/DN) were treated with 24-eBL (0.2 μM) according to the measurements described previously and the observations of gravitropic response. Expression of the PIN2-EGFP fusion protein, which indicated PIN2 localization in tropistic responses, and ROP2-EYFP, which was used as a marker for ROP2 localization and potential actin accumulation in Arabidopsis root sections, was imaged by confocal laser scanning microscopy (Radiance 2100; Bio-Rad). For ROP2-EYFP analysis, roots (subjected to the gravity stimulus) were counterstained with 10 μg/mL propidium iodide (Sigma-Aldrich) and placed on slides in drops of 0.5 M mannitol solution, and the images were scanned every 5 min until cell plasmolyzation was obtained. Latrunculin B (2 μM) was applied for 15 min after cell plasmolyzation (>20 seedlings). Images showing PIN2 and ROP2 distribution were captured and analyzed with MetaMorph version 5.0 software. The lengths of the expression domains showing detectable fluorescence signals were calculated and analyzed statistically using confocal laser scanning microscopy with the same capture conditions.
Accession Numbers
Sequence data from this article can be found in the EMBL/GenBank data libraries under the following accession numbers: Arabidopsis PINI (locus number At1g73590), PIN2 (locus number At5g57090), PIN3 (locus number At1g70940), PIN4 (locus number At2g01420), PIN7 (locus number At1g23080), ROP2 (locus number At1g20090), DET2 (locus number At2g38050), and DIMI (locus number At3g19820).
Acknowledgments
This study was supported by the Chinese Academy of Sciences and by Grants 30070073 and 30421001 from the National Natural Sciences Foundation of China. We thank Kang Chong (Institute of Botany, Chinese Academy of Sciences) for kindly providing Arabidopsis bri1 seeds and Zu-Hua He (Shanghai Institute of Plant Physiology and Ecology, Chinese Academy of Sciences) for Arabidopsis det2 mutant seeds. We thank Hanma Zhang (Center for Plant Sciences, School of Biology, University of Leeds) for helpful discussions. We especially thank Ben Scheres (Department of Molecular Genetics, Utrecht University), Hong Ma (Pennsylvania State University), and Xiao-Ya Chen (Shanghai Institute of Plant Physiology and Ecology) for reading and critical comments on the manuscript.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantcell.org) is: Hong-Wei Xue (hwxue@sibs.ac.cn).
Article, publication date, and citation information can be found at www.plantcell.org/cgi/doi/10.1105/tpc.105.034397.
References
- Bao, F., Shen, J., Brady, S.R., Muday, G.K., Asami, T., and Yang, Z. (2004). Brassinosteroids interact with auxin to promote lateral root development in A. thaliana. Plant Physiol. 134, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blancaflor, E.B., Hou, G.C., and Mohamalawari, D.R. (2003). The promotive effect of latrunculin B on maize root gravitropism is concentration dependent. Adv. Space Res. 31, 2215–2220. [DOI] [PubMed] [Google Scholar]
- Blilou, I., Xu, J., Wildwater, M., Willemsen, V., Paponov, I., Friml, J., Heidstra, R., Aida, M., Palme, K., and Scheres, B. (2005). The PIN auxin efflux facilitator network controls growth and patterning in Arabidopsis roots. Nature 433, 39–44. [DOI] [PubMed] [Google Scholar]
- Chen, C.Y., Cheung, A.Y., and Wu, H.M. (2003). Actin-depolymerizing factor mediates Rac/Rop GTPase-regulated pollen tube growth. Plant Cell 15, 237–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chory, J., Nagpal, P., and Peto, C.A. (1991). Phenotypic and genetic analysis of det2, a new mutant that affects light-regulated seedling development in Arabidopsis. Plant Cell 3, 445–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clouse, S.D. (2002). Arabidopsis mutants reveal multiple roles for sterols in plant development. Plant Cell 14, 1995–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clouse, S.D., Langford, M., and McMorris, T.C. (1996). A brassinosteroid-insensitive mutant in Arabidopsis thaliana exhibits multiple defects in growth and development. Plant Physiol. 111, 671–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clouse, S.D., and Sasse, J.M. (1998). Brassinosteroids: Essential regulators of plant growth and development. Annu. Rev. Plant Physiol. Plant Mol. Biol. 49, 427–451. [DOI] [PubMed] [Google Scholar]
- Friml, J. (2003). Auxin transport: Shaping the plant. Curr. Opin. Plant Biol. 6, 7–12. [DOI] [PubMed] [Google Scholar]
- Friml, J., Wisniewska, J., Benkova, E., Mendgen, K., and Palme, K. (2002). Lateral relocation of auxin efflux regulator PIN3 mediates tropism in Arabidopsis. Nature 415, 806–809. [DOI] [PubMed] [Google Scholar]
- Fu, X., and Harberd, N.P. (2003). Auxin promotes Arabidopsis root growth by modulating gibberellin response. Nature 421, 740–743. [DOI] [PubMed] [Google Scholar]
- Fu, Y., Li, H., and Yang, Z. (2002). The ROP2 GTPase controls the formation of cortical fine F-actin and the early phase of directional cell expansion during Arabidopsis organogenesis. Plant Cell 14, 777–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galweiler, L., Guan, C., Muller, A., Wisman, E., Mendgen, K., Yephremov, A., and Palme, K. (1998). Regulation of polar auxin transport by AtPIN1 in Arabidopsis vascular tissue. Science 282, 2226–2230. [DOI] [PubMed] [Google Scholar]
- Geldner, N., Friml, J., Stierhof, Y.D., Juergens, G., and Palme, K. (2001). Auxin transport inhibitors block PIN1 cycling and vesicle trafficking. Nature 413, 425–428. [DOI] [PubMed] [Google Scholar]
- Hou, G., Mohamalawari, D.R., and Blancaflor, E.B. (2003). Enhanced gravitropism of roots with a disrupted cap actin cytoskeleton. Plant Physiol. 131, 1360–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, H., Shen, J., Zheng, Z., Lin, Y., and Yang, Z. (2001). The Rop GTPase switch controls multiple developmental processes in Arabidopsis. Plant Physiol. 126, 670–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang, H.Y., and Yin, W.L. (1994). Assay of exogenous IAA, ABA and GA1–3 of shoots of leaf and flower in Metasequoia glyptostroboides Hu et Cheng. For. Res. 11, 13–15. [Google Scholar]
- Jefferson, R.A., Kavanagh, T.A., and Bevan, M.W. (1987). GUS fusions: β-Glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J. 6, 3901–3907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, M.A., Shen, J.J., Fu, Y., Li, H., Yang, Z., and Geiserson, C.S. (2002). The Arabidopsis Rop2 GTPase is a positive regulator of both root hair initiation and tip growth. Plant Cell 14, 763–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, S.K., Chang, S.C., Lee, E.J., Chung, W.S., Kim, Y.S., Hwang, S., and Lee, J.S. (2000). Involvement of brassinosteroids in the gravitropic response of primary root of maize. Plant Physiol. 123, 997–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandava, N.B. (1998). Plant growth promoting brassinosteroids. Annu. Rev. Plant Physiol. Plant Mol. Biol. 39, 23–52. [Google Scholar]
- Muday, G.K., Hu, S., and Brady, R. (2000). The actin cytoskeleton may control the polar distribution of an auxin transport protein. Gravit. Space Biol. Bull. 13, 75–83. [PubMed] [Google Scholar]
- Muday, G.K., and Murphy, A.S. (2002). An emerging model of auxin transport regulation. Plant Cell 14, 293–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muessig, C., Shin, G.H., and Altmann, T. (2003). Brassinosteroids promote root growth in Arabidopsis. Plant Physiol. 133, 1261–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller, A., Guan, C., Galweiler, L., Tanzler, P., Huijser, P., Marchant, A., Parry, G., Bennett, M., and Wisman, E. (1998). AtPIN2 defines a locus of Arabidopsis for root gravitropism control. EMBO J. 17, 6903–6911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murashige, T., and Skoog, F. (1962). A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 15, 473–497. [Google Scholar]
- Nakamura, A., Higuchi, K., Goda, H., Fujiwara, M.T., Sawa, S., Koshiba, T., Shimada, Y., and Yoshida, S. (2003). Brassinolide induces IAA5, IAA19, and DR5, a synthetic auxin response element in Arabidopsis, implying a cross talk point of brassinosteroid and auxin signaling. Plant Physiol. 133, 1843–1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi, T., Fujioka, S., Choe, S., Takatsuto, S., Yoshida, S., Yuan, H., Feldmann, K.A., and Tax, F.E. (1999). Brassinosteroid-insensitive dwarf mutants of Arabidopsis accumulate brassinosteroids. Plant Physiol. 121, 743–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palme, K., and Galweiler, L. (1999). PIN-pointing the molecular basis of auxin transport. Curr. Opin. Plant Biol. 2, 357–381. [DOI] [PubMed] [Google Scholar]
- Rashotte, A.M., Brady, S.R., Reed, R.C., Ante, S.J., and Muday, G.K. (2000). Basipetal auxin transport is required for gravitropism in roots of Arabidopsis. Plant Physiol. 122, 481–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatini, S., Beis, D., Wolkenfelth, H., Murfett, J., Guilfoyle, T., Malamy, J., Benfey, P., Leyser, O., Bechtold, N., Weisbeek, P., and Scheres, B. (1999). An auxin-dependent distal organizer of pattern and polarity in the Arabidopsis root. Cell 99, 463–472. [DOI] [PubMed] [Google Scholar]
- Sasse, J. (1999). Physiological actions of brassinosteroids. In Brassinosteroids: Steroidal Plant Hormones, A. Sakurai, T. Yokota, and S.D. Clouse, eds (Tokyo: Springer-Verlag), pp. 137–161.
- Steinmann, T., Geldner, N., Grebe, M., Mangold, S., Jackson, C.L., Paris, S., Galweiler, L., Palme, K., and Jurgens, S. (1999). Coordinated polar localization of auxin efflux carrier PIN1 by GNOM ARF GEF. Science 286, 316–318. [DOI] [PubMed] [Google Scholar]
- Souter, M.A., Pullen, M.L., Topping, J.F., Zhang, X., and Lindsey, K. (2004). Rescue of defective auxin-mediated gene expression and root meristem function by inhibition of ethylene signaling in steroid biosynthesis mutants of Arabidopsis. Planta 219, 773–783. [DOI] [PubMed] [Google Scholar]
- Sun, H., Basu, S., Brady, S.R., Luciano, R.L., and Muday, G.K. (2004). Interactions between auxin transport and the actin cytoskeleton in developmental polarity of Fucus distichus embryos in response to light and gravity. Plant Physiol. 135, 266–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi, T., Gasch, A., Nishizawa, N., and Chua, N.H. (1995). The DIMINUTO gene of Arabidopsis is involved in regulating cell elongation. Genes Dev. 9, 97–107. [DOI] [PubMed] [Google Scholar]
- Willemsen, V., Friml, J., Grebe, M., van den Toorn, A., Palme, K., and Scheres, B. (2003). Cell polarity and PIN protein positioning in Arabidopsis require STEROL METHYLTRANSFERASE1 function. Plant Cell 15, 612–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, J., and Scheres, B. (2005). Dissection of Arabidopsis ADP-RIBOSYLATION FACTOR1 function in epidermal cell polarity. Plant Cell 17, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]