Abstract
Long-term increases in the strength of excitatory transmission at Schaffer collateral-CA1 cell synapses of the hippocampus require the insertion of new α-amino-3-hydroxy-5-methyl-4-isoxazolepropionate receptors (AMPARs) into the synapse, but the kinetics of this process are not well established. Using microphotolysis of caged glutamate to activate receptors at single dendritic spines in hippocampal CA1 cells, we report the long-lasting potentiation of AMPAR-mediated currents with only a single pairing of photoreleased glutamate and brief postsynaptic depolarization. This potentiation was N-methyl-d-aspartate receptor (NMDAR)-dependent and was reversed with low-frequency photostimulation in an NMDAR-dependent manner, suggesting that it is mediated by the same mechanism(s) as conventional synaptic long-term potentiation. Potentiation of photolytic responses developed rapidly in a stepwise manner after a brief and variable delay (<60 s) at spines, but could not be induced at extrasynaptic sites on the dendritic shaft. Potentiation was accompanied by a concomitant decrease in postsynaptic, polyamine-dependent paired-pulse facilitation of the photolytic currents, indicating that a change in the subunit composition of the AMPARs underlying the response contributed to the potentiation. These changes are consistent with an increase in the proportion of GluR2-containing AMPARs in the spine head. These results demonstrate that activation of postsynaptic glutamate receptors by glutamate is not only necessary, but sufficient, for the induction of NMDAR-dependent long-term potentiation and reveal additional aspects of its expression.
Keywords: synaptic plasticity, hippocampus, plasticity
Long-term plasticity of excitatory synaptic transmission that depends on N-methyl-d-aspartate receptor (NMDAR) activation is likely to underlie learning and memory formation (1, 2). Despite an abundance of knowledge about long-term potentiation (LTP), considerable uncertainty and controversy about the mechanisms of LTP induction and expression persist. Some of these controversies are likely to stem from the use of conventional synaptic stimulation for studying LTP because the presynaptic nerve terminal is an unreliable source of glutamate. For example, several previous attempts to induce LTP with exogenous glutamate have not been successful (3, 4), raising the possibility that there is a factor other than glutamate that is coreleased from nerve terminals with synaptic stimulation and is required for LTP induction. Likewise, changes in paired-pulse ratio (PPR) have been reported to accompany LTP in some studies and have been interpreted as evidence of a change in presynaptic release probability (5, 6). Matsuzaki et al. (7) have demonstrated that potentiation of glutamate responses can be achieved with repeated photorelease from caged glutamate targeted to dendritic spines, suggesting that activation of synaptic glutamate receptors is critical.
The ability to study LTP with exogenous glutamate offers a powerful tool for answering fundamental questions about the mechanisms of its expression. First, can LTP be expressed at the dendritic shaft as well as the dendritic spine? With adequate spatial resolution, microphotolysis should allow for a direct comparison between spine head and dendritic shaft receptors, unlike conventional synaptic stimulation. Second, although conventional LTP is induced typically with multiple pairings of stimulation and postsynaptic depolarization, would a single pairing be sufficient if reliable postsynaptic receptor activation was ensured? Potentiation of a single test synaptic stimulus can be induced by pairing it with a tetanic conditioning stimulus train delivered to other synaptic inputs (8). Finally, what is the temporal profile of LTP expression? When LTP is induced synaptically, excitatory transmission is potentiated in a stepwise manner after a delay of several seconds (8, 9), yet there are conflicting reports about the time course of changes in postsynaptic α-amino-3-hydroxy-5-methyl-4-isoxazolepropionate receptor (AMPAR) sensitivity (10, 11).
Methods
Electrophysiology. Hippocampal slice cultures were prepared by using the roller tube method (12) (see Supporting Methods, which is published as supporting information on the PNAS web site). Cultures were placed in a 2-ml recording chamber with extracellular saline containing 137 mM NaCl, 2.8 mM KCl, 2 mM CaCl2, 2 mM MgCl2, 11.6 mM NaHCO3, 2 mM Hepes, 0.4 mM NaH2PO4, 0.02 mM phenol red, and 5.6 mM glucose, titrated to pH 7.4 by bubbling with 95% O2/5% CO2. Whole-cell patch-clamp recordings were made from CA1 pyramidal cell somata at room temperature (22-24°C). After obtaining stable whole-cell recordings, perfusion was stopped and 1 mM caged glutamate [N-(6-nitro-7-coumarylmethyl)-l-glutamate; synthesized by J.P.Y.K.] was added to the bath, along with 40 μM bicuculline and 1 μM tetrodotoxin to block fast GABAergic inhibition and action potentials. The presence of Hepes in the saline was sufficient to maintain a stable pH under these conditions. Experiments were stopped if the addition of caged glutamate caused an increase in holding current >50 pA. Because of the stability of this caging group, increases in holding current are likely to be caused by mechanical disturbances rather than spontaneous uncaging. All photolytic excitatory postsynaptic currents (phEPSCs) were elicited at -75 mV. Borosilicate patch pipettes (5-10 MΩ in the bath so as to slow intracellular dialysis) were filled with 90 mM CsCH3SO3, 50 mM CsCl, 0.4 mM Hepes, 1 mM MgCl2, 0.2 mM EGTA, and 0.1 mM Alexa 568 and titrated to pH 7.3 with 1 M CsOH. The presence of Cs+ in the pipette solution reduced potassium currents in the recorded cell. In the calcium imaging experiments, the EGTA was replaced with fluo-4 (300 μM). NMDARs were blocked in some experiments with 80 μM d,l-2-amino-5-phosphonopentanoic acid (AP5). In some experiments, neurons were transfected with 1-μm gold pellets coated with cDNA for enhanced GFP by using a Bio-Rad gene gun (13). Responses were considered potentiated when the increase in amplitude was >15% and maintained for >10 min.
Photolysis. Photolysis of caged glutamate offers the ability to directly control the strength, timing, and location of glutamate reaching postsynaptic receptors (4, 14). In our experiments, an argon ion laser was fitted with UV optics to produce a continuous 300-mW beam of UV light at the 351- and 364-nm lines. The light was focused into a 25-μm multimode quartz fiber and delivered to the preparation by a dichroic mirror, so as to permit simultaneous wide-field excitation with an HBO lamp. Laser flash duration was controlled by a high-speed electromechanical shutter (NM Laser Products, Sunnyvale, CA). TTL signals used to trigger the shutter were digitized with the membrane current to indicate the time of flash. The proximal end of the fiber was focused by a relay lens assembly in a conjugate image plane with respect to the preparation and positioned with micromanipulators near the center of the field of view through the ×60 (1.0 numerical aperture) water immersion objective of an upright microscope (Nikon). The location and focus of the UV spot within the tissue were determined from excitation of a dye-filled dendritic shaft by an attenuated beam. The cell was then repositioned so that the UV spot was just distal to a spine head (Fig. 1b) on an oblique apical dendrite within 50 μm of the soma and near the top of the slice. Care was taken to ensure that no dendritic branches were close to the cone of illumination above or below the plane of focus. Photostimulation was delivered at 0.1 Hz or slower. Intracellular Alexa 568 or fluo-4 fluorescence was imaged with a charge-coupled device camera (Hamamatsu Orca ER II, effective pixel size = 0.012 μm2) controlled by simple pci software (Compix, Lake Oswego, OR).
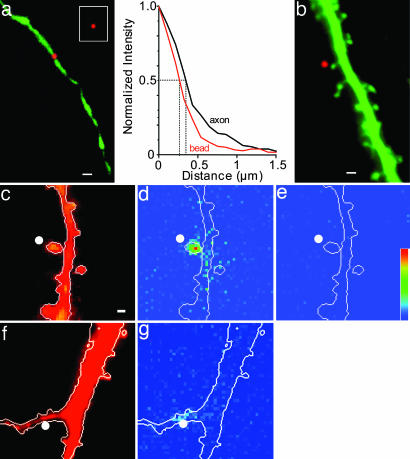
Fig. 1.
Stimulation of single dendritic spines with photolysis of caged glutamate. (a) The spatial resolution of the UV spot was determined by imaging GFP in the axon of a CA1 pyramidal cell in response to wide-field excitation (green channel) or excitation by a UV spot (red channel). (Inset) Wide-field excitation of a 0.2-μm-diameter fluorescent polystyrene micro-sphere (Molecular Probes) embedded 20 μm deep in a hippocampal culture. Plots of the normalized, background-subtracted fluorescence intensity profiles for the bead and the UV excited GFP spot in a, measured along the long axis of the axon, are shown. The widths of the intensity profiles at half maximal amplitude were 0.6 and 0.8 μm, respectively. (b) The UV spot (imaged in red channel) was targeted to a point just distal to the head of an individual dendritic spine, which was visualized with wide-field excitation of intracellular Alexa 568 (green channel). (c and f) Wide-field fluorescence image of a dendritic segment loaded with Alexa 568. The white spot marks the site of UV photolysis in c-g. (d) Image of the maximal change in fluo-4 fluorescence after the photolysis of caged glutamate by using a 1-ms UV pulse (Mg2+-free saline plus 6,7-dinitroquinoxaline-2,3-dione). Fluo-4 emission increased in the targeted dendritic spine, but not in adjacent spines or the underlying dendritic shaft. (e) Block of NMDARs with AP5 eliminated glutamate-induced fluo-4 emission. ΔF scale = 0-100 a.u. (also applies to d and g). (g) Image of the maximal change in fluo-4 fluorescence after the photolysis of caged glutamate by using a 1-ms UV pulse (Mg2+-free saline plus 6,7-dinitroquinoxaline-2,3-dione) directed to the dendritic shaft in f. (Scale bars: 1 μm.)
Results
Resolution of Glutamate Photolysis. Photolysis is spatially limited by the scattering of UV light in tissue. To achieve high spatial resolution, we used single-photon uncaging in organotypic hippocampal slice cultures prepared with the roller tube method (12) because they display conventional long-term synaptic plasticity (15) and because spines on intact dendrites can be photostimulated near the surface of the tissue. UV light from an argon ion laser was directed to the preparation via the microscope objective (16). We first determined the limits of the spatial resolution of the UV spots in these cultures. Excitation of GFP in superficial processes of GFP-transfected cells revealed that UV spots with diameters of <1 μm could be produced (Fig. 1a). For comparison, Fig. 1a Inset shows the fluorescence image of a subresolution (0.2 μm) fluorescent bead within the tissue culture excited by using wide-field illumination. The size of these UV spots was thus close to the limits of diffraction (estimated at 0.43 μm) and comparable to the size of dendritic spine heads.
More important than the size of the UV spot, however, is the spatial extent of the response of the postsynaptic cell to photoreleased glutamate. We targeted the UV spots just distal to the heads of individual apical dendritic spines near the surface of the culture (Fig. 1b) to minimize diffusion of photoreleased glutamate to the dendritic shaft and took advantage of the high affinity for glutamate and Ca2+ permeability of the NMDAR to determine the spatial boundaries of the photoreleased glutamate. CA1 pyramidal cells were loaded with 300 μM fluo-4 from whole-cell recording pipettes, and experiments were conducted by using 1 mM caged glutamate in Mg2+-free extracellular saline with AMPARs blocked by 40 μM 6,7-dinitroquinoxaline-2,3-dione. Under these conditions, a 1-ms pulse of UV light at 1-3 mW produced a large increase in fluo-4 fluorescence in the spine head (ΔF/F = 60 ± 3%, n = 4), considerably larger than the change in adjacent spines or in the dendritic shaft underlying the stimulated spine (12 ± 4%) (Fig. 1 c-e). Glutamate-induced Ca2+ responses were completely eliminated by the NMDAR antagonists AP5 (80 μM) or MK-801 (20 μM) (n = 4 cells). The corresponding NMDAR-mediated inward current had an amplitude of -7.7 ± 1.0 pA at -75 mV and decayed with a time constant of 79.4 ± 7.6 ms (n = 5). The failure to detect Ca2+ influx in the underlying dendritic shaft did not result from a lack of NMDARs because directing the photorelease of glutamate onto shaft sites produced significant AP5-sensitive Ca2+ influx (20 ± 5%, n = 6) (Fig. 1 f and g). These results thus provide positive evidence that photolysis of caged glutamate under our conditions activates a high proportion of glutamate receptors in the spine, many of which are likely to be the same ones activated by synaptically released glutamate.
AMPAR-mediated currents, elicited at a holding potential of -75 mV with 1-ms UV pulses in the presence of AP5 and recorded with whole-cell pipettes at the cell soma, had amplitudes and decay time constants (15.0 ± 4.1 pA; 16.2 ± 1.3 ms, n = 6 cells) that were not significantly different (P > 0.5; unpaired t test) from mean uniquantal spontaneous miniature EPSCs in the same cells in the presence of tetrodotoxin (12.5 ± 1.5 pA; 13.1 ± 1.6 ms). The 10-90% rise time of the phEPSCs was significantly slower than for miniature EPSCs (4.7 ± 0.4 ms vs. 3.2 ± 0.3 ms; P < 0.05), however. These data further support the conclusion that these responses, which we term phEPSCs, result from the activation of AMPARs at single dendritic spines.
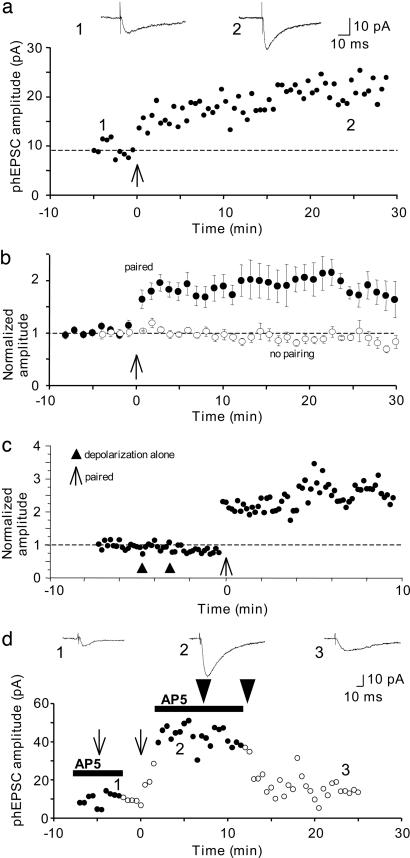
LTP of Photolytic Responses. phEPSC amplitudes were stable during prolonged recordings (Fig. 2b), provided the series resistance was stable. However, pairing a single phEPSC with one depolarizing voltage step (200 ms to -10 mV) to relieve the block of NMDAR-gated channels by Mg2+ resulted in a persistent, significant increase in the amplitude of subsequent phEPSCs (n = 13 cells) (Fig. 2 a and b). On average, the phEPSC was potentiated to 183 ± 6% of the control amplitude in five cells surviving >20 min after pairing (P < 0.01). Potentiation was not caused by nonspecific effects of UV light or the release of the “spent” caging group because it was fully and reversibly prevented by application of AP5 (Fig. 2d) (n = 3), establishing that it was NMDAR-dependent. In addition, depolarizing steps alone, unpaired with a phEPSC, did not induce potentiation (Fig. 2c) (n = 5), indicating that the potentiation was not caused by tonic levels of extracellular glutamate. Furthermore, potentiated phEPSCs could be depotentiated in an AP5-sensitive manner by delivering 100 UV pulses at 10 Hz while holding the cell at -75 mV (Fig. 2d) (mean amplitude after depotentiation = 50 ± 4% of the potentiated phEPSC amplitude, n = 3). These results demonstrate that a single pairing of exogenous glutamate and postsynaptic depolarization is sufficient for LTP induction, and that this LTP shares many of the key features of conventional, synaptically induced LTP.
Fig. 2.
Potentiation of photolysis-induced responses. (a) The amplitude of single spine phEPSCs is plotted as a function of time. After 5 min of baseline recording, a single 200-ms depolarizing voltage step to -10 mV was paired with one phEPSC (time indicated by arrow), resulting in an increase in response amplitude. Averaged single spine phEPSCs from indicated times before and after the pairing are shown at the top. Vertical deflections indicate time of laser pulse. (b) Shown are pooled data demonstrating that phEPSC amplitude is stable when not paired with a depolarizing current pulse (n = 5 cells; open symbols), and pooled data showing the mean potentiation (±SEM) of phEPSCs in 13 cells (filled symbols). (c) A depolarizing voltage step to -10 mV that is not paired with photolysis (delivered at the time indicated by ▴) did not induce phEPSC potentiation, whereas an identical step combined with uncaging of glutamate did induce potentiation at the same spine in the same cell (arrow). (d) Block of NMDARs with AP5 (black line) prevented potentiation of phEPSCs reversibly (thin arrows) and also reversibly prevented depotentiation of responses produced by 100 UV pulses delivered at 10 Hz (thick arrows). Averaged phEPSCs from the indicated times are shown at the top.
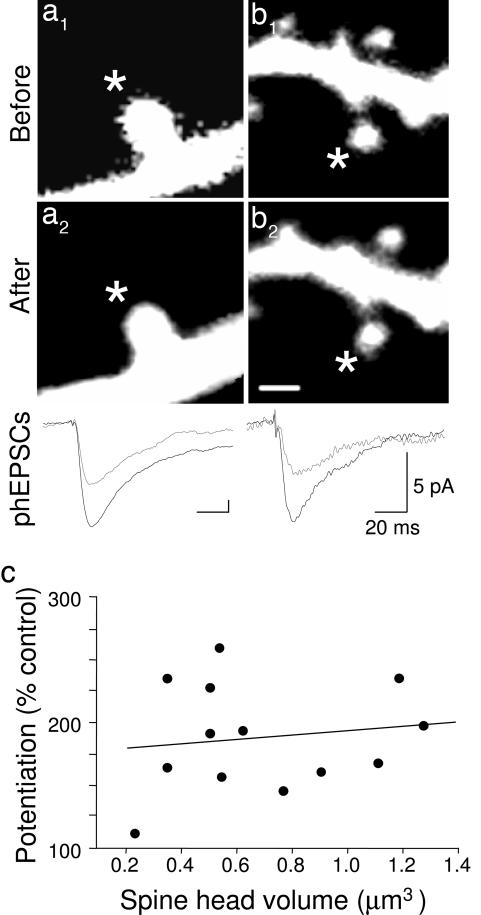
LTP induction was not accompanied by a statistically significant change in the volume of the stimulated spine (mean volume 5 min after LTP induction = 93 ± 5% of the control volume; n = 13) (Fig. 3 a and b) (cf. refs. 7 and 17), suggesting that such changes are not obligatory for LTP expression. We also found no correlation between the amount of potentiation and the volume of the spine head over a large range (r = 0.15) (Fig. 3c) (cf. ref. 7).
Fig. 3.
Both large and small spines express LTP. (a and b) Images of stimulated large (a) and small (b) dendritic spines (indicated by asterisks) are shown 1 min before (a1 and b1) and 5 min after the pairing procedure (a2 and b2), i.e., the peak of swelling reported by Matsuzaki et al. (7). (Scale bar: 1 μm.) The volume of the spine in a was 1.27 μm3 before and 1.13 μm3 after LTP induction, whereas the spine in b was 0.35 μm3 before and 0.36 μm3 after LTP. Corresponding phEPSCs before (gray) and 5 min after pairing (black) are shown superimposed at the bottom. Calibration bars apply to all phEPSCs. (c) Plot of phEPSC potentiation, measured 5 min after pairing, as a function of the volume of the head of the stimulated spine. We observed no correlation (r = 0.14) between early LTP and the volume of the spine head over a large range (cf. ref. 7). We conclude that changes in spine volume need not accompany LTP.
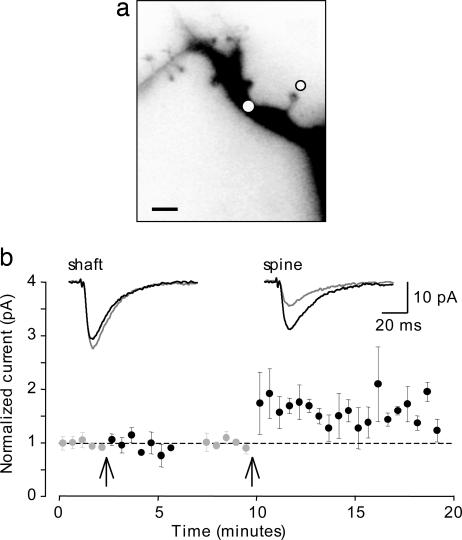
Selective Potentiation of Spine Responses. Why have previous investigations failed to elicit or detect potentiation of responses to exogenous glutamate (3, 4)? One possibility is that the techniques available were too coarse and that potentiation can only be induced or expressed at glutamate receptors in the spine head. We tested this hypothesis by directing the photolysis of caged glutamate to dendritic shafts by using identical UV spots. Remarkably, single pairings of phEPSCs at dendritic shafts with depolarizing steps (200 ms to -10 mV) never induced any significant change in the amplitude of the phEPSCs (mean amplitude 5 min after pairing = 96 ± 5% of control; n = 6 cells). If the UV spot was subsequently redirected a few microns laterally to the head of a nearby spine, then the identical pairing procedure resulted in a potentiation of the phEPSC to 163 ± 6% of the control amplitude (n = 3 cells) (Fig. 4). The failure to induce potentiation at sites on the dendritic shaft cannot be attributed to a lack of plasticity in the recorded cell, but rather suggests that the spine head is uniquely capable of expressing LTP. The failure to express LTP cannot be attributed to a lack of NMDAR expression at shaft sites, because Ca2+ influx was readily detected as an increase in fluo-4 emission when photostimulation was directed onto the shaft (n = 6) (Fig. 1 f and g). The change in fluorescence in response to stimulation of shaft sites was, however, significantly smaller than that observed with stimulation of spine heads (ΔF/F = 20 ± 5% vs. 60 ± 3%) (compare Fig. 1 d and g).
Fig. 4.
Potentiation of phEPSCs at the spine head but not at the dendritic shaft. (a) Negative image of an Alexa 568-filled dendritic segment showing two sites of photolysis; on the dendritic shaft (white spot) and at an adjacent spine head (black circle). (Scale bar: 2 μm.) (b) Pooled data (n = 3 cells) showing mean phEPSC amplitude (±SEM) for responses elicited from the dendritic shaft and after repositioning the UV spot to the spine head. Pairing a phEPSC with a single depolarizing pulse (at times indicated by arrow) failed to potentiate shaft responses, but did potentiate spine responses. In these cells, phEPSCs at the shaft and spine head had amplitudes of 13 ± 5 and 7 ± 2 pA, respectively, and showed comparable kinetics. Representative shaft (Left Inset) and spine (Right Inset) phEPSCs before (gray) and after pairing (black) for the cell illustrated in a are shown.
Potentiation Was Accompanied by a Delayed, Stepwise Change in AMPAR Subunit Composition. We used the potentiation of phEPSCs to address several questions concerning LTP expression. Because the phEPSC was independent of the release of glutamate from presynaptic nerve terminals, its potentiation must have been expressed as an increase in the sensitivity of the postsynaptic cell to glutamate. The insertion of new AMPARs is likely to underlie LTP (18-21). Studies of hippocampal cells from mice lacking the GluR1 AMPAR subunit (22) and WT cells expressing recombinant AMPARs (18) have indicated that GluR1 is necessary for activity-dependent insertion of AMPARs. AMPARs form heteromultimers, however, and it is not known what other subunits are part of the endogenous AMPARs inserted by LTP-inducing stimuli.
AMPARs lacking GluR2 subunits are partially blocked by intracellular polyamines (23, 24). Paired stimuli relieve the polyamine block of GluR2-lacking AMPARs, resulting in postsynaptic paired-pulse facilitation. The facilitation is greater at depolarized potentials because polyamine block is increased. We observed that phEPSCs elicited with pairs of glutamate pulses at an interstimulus interval of 10 ms with NMDARs blocked had a PPR of 1.65 ± 0.09 (n = 7), i.e., the amplitude of the second response was 65% larger than the amplitude of the first response. The PPR was significantly greater at +80 mV (2.34 ± 0.12; n = 4; P < 0.01) and in cells recorded with pipette solutions containing the polyamine spermidine (10 μM; 2.48 ± 0.17; n = 3; P < 0.05). We conclude that some spine AMPARs lack GluR2 subunits in hippocampal slice cultures at rest.
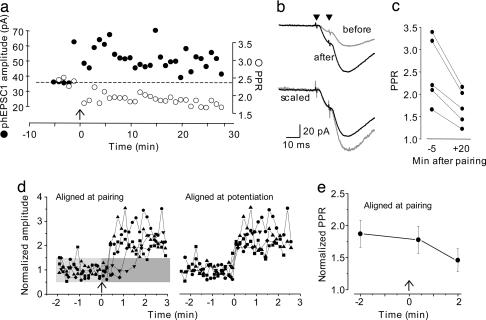
If insertion of new AMPARs having a different proportion of GluR2 subunits than the original AMPARs underlies LTP expression, then a change in PPR should be apparent. Indeed, when pairs of UV pulses were delivered before and after potentiation of phEPSCs, there was a rapid decrease in the amount of facilitation. The decrease in facilitation occurred concurrently with the potentiation and persisted for as long as the responses were monitored (Fig. 5 a-c). On average, the PPR was reduced from 2.37 ± 0.31 before to 1.72 ± 0.23 after potentiation (P < 0.05; n = 5 cells). These data are consistent with an increase in the proportion of AMPARs containing GluR2 subunits in the dendritic spine after potentiation of AMPAR-mediated phEPSCs.
Fig. 5.
Potentiation of phEPSCs is accompanied by a decrease in postsynaptic paired-pulse facilitation. (a) At the time indicated by the arrow, potentiation of phEPSC amplitude (•) occurred simultaneously with a decrease in the PPR of the phEPSCs (○) elicited with a pair of pulses delivered 10 ms apart. (b) Representative averaged traces elicited with pairs of UV pulses (indicated by black triangles) from the experiment in a before (gray) and 20 min after (black) pairing are shown superimposed (upper traces). The decrease in the relative amplitude of the second response is readily apparent in the lower pair of traces, in which the responses have been scaled so that the amplitudes of the first phEPSCs in the pair are the same. (c) Summary plot indicating the decreased PPR before and 20-25 min after induction of potentiation. (d) Potentiation of phEPSCs occurred in a delayed, stepwise manner, as indicated by the graph (Left), in which the amplitudes of phEPSCs in five cells are plotted superimposed as a function of time relative to the pairing pulse (indicated by arrow). The gray box indicates 2 SDs from the control amplitude. (Right) The stepwise nature of the amplitude transition is more readily apparent after the data are aligned by the time of the transition and replotted. (e) Although less well resolved than the change in phEPSC amplitude, the change in PPR also occurred in a delayed stepwise manner, as shown in this plot of mean PPR (±SEM) 2 min before, 25 s after, and 2 min after the pairing pulse (indicated by arrow) for the same five cells as in d. The control PPR was significantly different from the PPR at 2 min after pairing (P < 0.05), but not the PPR immediately after pairing (ANOVA, Scheffé's test).
There are conflicting data about how rapidly new AMPARs are inserted after LTP induction (10, 11). Because exogenous glutamate responses can be potentiated by a single pairing step, our protocol offers the possibility of defining the time course of LTP expression with greater resolution than is possible with conventional synaptic LTP. UV pulses were delivered every 10 s before and after the induction of LTP, as above. Comparison of response amplitudes revealed that the onset of the transition from the control amplitude to the potentiated amplitude was delayed from the delivery of the paired stimulus (mean delay = 38 ± 10 s, n = 5) (Fig. 5d). Although the delay varied between cells, it was nevertheless apparent that the change from the control to the potentiated amplitude occurred rapidly (<10 s, the interstimulus interval) in a stepwise fashion, as opposed to increasing gradually. The change in PPR also occurred in a delayed, stepwise manner that was synchronous with the change in phEPSC amplitude (Fig. 5e). These data are remarkably similar to the 22-s delay reported by Petersen et al. (9) using minimal synaptic stimulation, and they establish that the delay can be entirely accounted for by postsynaptic phenomena.
Discussion
We have developed techniques that allow us to photorelease glutamate in a small volume having dimensions comparable to those of the head of a single dendritic spine on hippocampal pyramidal cells in organotypic slice cultures. Using small-diameter quartz fibers and relay lenses to deliver laser-generated UV light by the microscope objective, we could record postsynaptic response electrophysiologically while simultaneously performing wide-field fluorescence microscopy. We used the Ca2+ conductance and high glutamate affinity of the NMDAR to demonstrate that the photoreleased glutamate activated AMPARs that were confined to single spines. This resolution is at least an order of magnitude better than can be achieved in ex vivo hippocampal slices (3, 4). Consistent with our conclusion, photolytically evoked EPSCs had amplitudes and kinetics comparable to those of miniature EPSCs generated by activation of single spines by a quantum of synaptically released glutamate.
Surprisingly, we observed that photolytically induced EPSCs displayed voltage- and polyamine-sensitive paired-pulse facilitation. These are properties of AMPARs lacking the GluR2 subunit (23, 24). CA1 pyramidal cells in hippocampal slice cultures express high levels of GluR2 mRNA (25), unlike other cell types with numerous GluR2-lacking AMPARs (26), and express only low levels of GluR3 and GluR4. There are conflicting accounts of the presence or absence of GluR2-lacking AMPARs in hippocampal pyramidal cells. Biochemical studies of AMPAR subunit composition (27), immunocytochemical studies of GluR2 localization (28), and some electrophysiological examinations of the polyamine sensitivity of Schaffer collateral-CA1 cell EPSCs in mice (29) suggest that most AMPARs contain GluR2s. Nevertheless, there are other studies suggesting that some AMPARs may lack GluR2s and be Ca2+-permeable (30-33) at these synapses. We suggest that some of the AMPARs at naïve Schaffer collateral-CA1 cell synapses in hippocampal slice cultures are GluR1 homomers or GluR1-3 or GluR1-4 heteromers, although additional study is needed to clarify this issue.
We used glutamate microphotolysis to demonstrate that a long-lasting potentiation of postsynaptic AMPAR-mediated responses can be elicited when photolytic application of exogenous glutamate is paired with postsynaptic depolarization. This potentiation shared many features of conventional LTP induced with synaptic stimulation, such as a dependence on NMDAR activation and the ability to be reversed, or depotentiated, in an NMDAR-dependent manner by low-frequency stimulus trains. Because potentiation of AMPAR responses can be elicited with exogenous glutamate, we conclude that no additional synaptically released factor is required for the induction of LTP. That is, activation of glutamate receptors is not only necessary, but sufficient for the induction of LTP. Matzusaki et al. have suggested that dendritic spine head size is positively correlated with AMPAR number (14) and that spine head size increases with LTP induction in an NMDAR-dependent manner (7). We have observed, however, that a change in spine head size is not an obligatory concomitant of LTP expression, suggesting that the two phenomena are not causally linked. We also find that small and large spines display comparable capacities to become potentiated (cf. ref. 7).
We found that LTP could be induced only when photoreleased glutamate was directed to dendritic spines, and not dendritic shafts. There are several possible explanations for a failure to induce LTP at extrasynaptic sites. First, extrasynaptic receptors lack the concentration of protein kinases and other signaling and trafficking molecules necessary for LTP induction and expression that are found at the spine head (19, 20). Whole-cell recordings may exaggerate this difference if there is a more rapid or thorough dialysis of the dendritic shaft. Second, we observed that influx of Ca2+ through NMDARs produced a higher intracellular [Ca2+] in the confined, diffusionally restricted space of the spine head (34, 35) as compared with dendritic shafts. Our ability to elicit potentiation with photolytic glutamate release, in contrast to previous attempts in ex vivo hippocampal slices (3, 4), is thus likely to be caused by the better spatial resolution that can be achieved in cultured hippocampal slices. Larger, more diffuse sites of glutamate uncaging in ex vivo slices are likely to activate a much higher percentage of extrasynaptic shaft AMPARs.
Potentiation of the photolytic AMPAR-mediated responses was found to be accompanied by a decrease in postsynaptic, polyamine-dependent paired-pulse facilitation. Decreased AMPAR facilitation is likely to be caused by an increase in the proportion of AMPARs that contain GluR2 subunits because polyamine block depends solely on the subunit composition of the AMPARs (36). Synaptic LTP expression requires GluR1 subunits and the interaction of their C termini with PDZ proteins (22, 37). Assuming the expression of LTP under our conditions also depends on GluR1-mediated interactions, then our observation of changes in GluR2 content of AMPARs would suggest that the insertion of new endogenous GluR1-GluR2 heteromeric AMPARs underlies LTP expression in these experiments, as suggested previously for insertion of recombinant AMPARs in synaptic LTP (18). Our observation also offers a postsynaptic explanation for the decrease in PPR seen in some LTP studies and attributed to changes in presynaptic release probability (5, 6).
Because we could induce LTP with a single pairing, we were able to determine the dynamics of AMPAR insertion with high temporal resolution. We observed that the expression of LTP was delayed by ≈38 s after induction, but that when the potentiation occurred, it was expressed in a stepwise, all-or-none manner in <10 s. This type of expression mechanism seems to be shared with conventional, synaptically induced LTP because it is consistent with previous observations of a delayed (8) and quantized (9) increase in synaptic response amplitude. Our data allow us to attribute these steps to the time required for the appearance of new AMPARs in the postsynaptic membrane. That is, we suggest that LTP expression under our conditions is mediated by the quantized insertion of a cluster of new AMPARs into the dendritic spine head. Such a quantized process might be expected if LTP is mediated by the fusion of an intracellular vesicle containing AMPARs with the subsynaptic plasma membrane (38-43). Although triggered by Ca2+ and dependent on SNARE proteins (39, 43), like synaptic vesicle fusion, the fusion of AMPAR-containing vesicles is delayed from the time of Ca2+ influx. It will be of considerable interest to determine the biochemical and enzymatic steps accounting for this delay.
Supplementary Material
Acknowledgments
We thank Drs. B. Alger and T. Abrams for their comments on the manuscript; S. Muralidharan for synthesis of caged glutamate; and Ms. S. Nauman for the preparation and care of the slice cultures. This work was supported by grants from the National Institute of Mental Health (to S.M.T.), National Institute of Neurological Disorders and Stroke (to S.M.T.), National Institute of General Medical Sciences (to J.P.Y.K.), and Department of Veterans Affairs (to C.-M.T.).
Author contributions: A.A.B. and S.M.T. designed research; A.A.B. performed research; J.P.K. and C.-M.T. contributed new reagents/analytic tools; A.A.B. analyzed data; and A.A.B., J.P.K., C.-M.T., and S.M.T. wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: AMPAR, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionate receptor; AP5, d,l-2-amino-5-phosphonopentanoic acid; LTP, long-term potentiation; NMDAR, N-methyl-d-aspartate receptor; EPSC, excitatory postsynaptic current; phEPSC, photolytic EPSC; PPR, paired-pulse ratio.
References
- 1.Malenka, R. C. & Nicoll, R. A. (1999) Science 285, 1870-1874. [DOI] [PubMed] [Google Scholar]
- 2.Morris, R. G., Moser, E. I., Riedel, G., Martin, S. J., Sandin, J., Day, M. & O'Carroll, C. (2003) Philos. Trans. R. Soc. London B 358, 773-786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kandler, K., Katz, L. C. & Kauer, J. A. (1998) Nat. Neurosci. 1, 119-123. [DOI] [PubMed] [Google Scholar]
- 4.Eder, M., Zieglgänsberger, W. & Dodt, H. U. (2002) J. Neurosci. 22, 7558-7568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schulz, P. E., Cook, E. P. & Johnston, D. (1994) J. Neurosci. 14, 5325-5337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kleschevnikov, A. M., Sokolov, M. V., Kuhnt, U., Dawe, G. S., Stephenson, J. D. & Voronin, L. L. (1997) Neuroscience 76, 829-843. [DOI] [PubMed] [Google Scholar]
- 7.Matsuzaki, M., Honkura, N., Ellis-Davies, G. C. & Kasai, H. (2004) Nature 429, 761-766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gustafsson, B., Asztely, F. & Wigstrom, H. (1989) Eur. J. Neurosci. 1, 382-394. [DOI] [PubMed] [Google Scholar]
- 9.Petersen, C. C., Malenka, R. C., Nicoll, R. A. & Hopfield, J. J. (1998) Proc. Natl. Acad. Sci. USA 95, 4732-4737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davies, S. N., Lester, R. A., Reymann, K. G. & Collingridge, G. L. (1989) Nature 338, 500-503. [DOI] [PubMed] [Google Scholar]
- 11.Montgomery, J. M., Pavlidis, P. & Madison, D. V. (2001) Neuron 29, 691-701. [DOI] [PubMed] [Google Scholar]
- 12.Gähwiler, B. H., Thompson, S. M., McKinney, R. A., Debanne, D. & Robertson, R. T. (1989) in Culturing Nerve Cells, eds. Banker, G. & Goslin, K. (MIT Press, Cambridge, MA), 2nd ed., pp. 379-411.
- 13.McAllister, A. K. (2000) Science STKE 2000, 10.1126/stke.2000.51.pl1. [DOI]
- 14.Matsuzaki, M., Honkura, N., Ellis-Davies, G. C. & Kasai, H. (2001) Nat. Neurosci. 4, 1086-1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Debanne, D., Gähwiler, B. H. & Thompson, S. M. (1994) Proc. Natl. Acad. Sci. USA 91, 1148-1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wei, D. S., Mei, Y. A., Bagal, A., Kao, J. P., Thompson, S. M. & Tang, C. M. (2001) Science 293, 2272-2275. [DOI] [PubMed] [Google Scholar]
- 17.Lang, C., Barco, A., Zablow, L., Kandel, E. R., Siegelbaum, S. A. & Zakharenko, S. S. (2004) Proc. Natl. Acad. Sci. USA 101, 16665-16670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shi, S. H., Hayashi, Y., Esteban, J. A. & Malinow, R. (1999) Science 284, 1811-1816. [DOI] [PubMed] [Google Scholar]
- 19.Malinow, R. & Malenka, R.C. (2002) Annu. Rev. Neurosci. 25, 103-126. [DOI] [PubMed] [Google Scholar]
- 20.Sheng, M. & Kim, M. J. (2002) Science 298, 776-780. [DOI] [PubMed] [Google Scholar]
- 21.Song, I. & Huganir, R. L. (2002) Trends Neurosci. 25, 578-588. [DOI] [PubMed] [Google Scholar]
- 22.Zamanillo, D., Sprengel, R., Hvalby, O., Jensen, V., Burnashev, N., Rozov, A., Kaiser, K. M., Koster, H. J., Borchardt, T., Worley, P., et al. (1999) Science 284, 1805-1811. [DOI] [PubMed] [Google Scholar]
- 23.Rozov, A., Zilberter, Y., Wollmuth, L. P. & Burnashev, N. (1998) J. Physiol. (London) 511, 361-377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rozov, A. & Burnashev, N. (1999) Nature 401, 594-598. [DOI] [PubMed] [Google Scholar]
- 25.Gerfin-Moser, A., Grogg, F., Rietschin, L., Thompson, S. M. & Streit, P. (1995) Neuroscience 67, 849-865. [DOI] [PubMed] [Google Scholar]
- 26.Geiger, J. R., Melcher, T., Koh, D. S., Sakmann, B., Seeburg, P. H., Jonas, P. & Monyer, H. (1995) Neuron 15, 193-204. [DOI] [PubMed] [Google Scholar]
- 27.Wenthold, R. J., Petralia, R. S., Blahos, J. & Niedzielski, A. S. (1996) J. Neurosci. 16, 1982-1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Petralia, R. S., Wang, Y. X., Mayat, E. & Wenthold, R. J. (1997) J. Comp. Neurol. 385, 456-476. [DOI] [PubMed] [Google Scholar]
- 29.Mainen, Z. F., Jia, Z., Roder, J. & Malinow, R. (1998) Nat. Neurosci. 1, 579-586. [DOI] [PubMed] [Google Scholar]
- 30.Lerma, J., Morales, M., Ibarz, J. M. & Somohano, F. (1994) Eur. J. Neurosci. 6, 1080-1088. [DOI] [PubMed] [Google Scholar]
- 31.Tsubokawa, H., Oguro, K., Masuzawa, T., Nakaima, T. & Kawai, N. (1995) J. Neurophysiol. 74, 218-225. [DOI] [PubMed] [Google Scholar]
- 32.Toomim, C. S. & Millington, W. R. (1998) J. Comp. Neurol. 402, 141-154. [PubMed] [Google Scholar]
- 33.Yin, H. Z., Sensi, S. L., Carriedo, S. G. & Weiss, J. H. (1999) J. Comp. Neurol. 409, 250-260. [PubMed] [Google Scholar]
- 34.Koch, C. & Zador, A. (1993) J. Neurosci. 13, 413-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yuste, R., Majewska, A. & Holthoff, K. (2000) Nat. Neurosci. 3, 653-659. [DOI] [PubMed] [Google Scholar]
- 36.Panchenko, V. A., Glasser, C. R., Partin, K. M. & Mayer, M. L. (1999) J. Physiol. (London) 520, 337-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hayashi, Y., Shi, S. H., Esteban, J. A., Piccini, A., Poncer, J. C. & Malinow, R. (2000) Science 287, 2262-2267. [DOI] [PubMed] [Google Scholar]
- 38.Spacek, J. & Harris, K. M. (1997) J. Neurosci. 17, 190-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lledo, P. M., Zhang, X., Sudhof, T. C., Malenka, R. C. & Nicoll, R. A. (1998) Science 279, 399-403. [DOI] [PubMed] [Google Scholar]
- 40.Maletic-Savatic, M., Koothan, T. & Malinow, R. (1998) J. Neurosci. 18, 6814-6821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Broutman, G. & Baudry, M. (2001) J. Neurosci. 21, 27-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lu, W., Man, H., Ju, W., Trimble, W. S., MacDonald, J. F. & Wang, Y. T. (2001) Neuron 29, 243-254. [DOI] [PubMed] [Google Scholar]
- 43.Park, M., Penick, E. C., Edwards, J. G., Kauer, J. A. & Ehlers, M. D. (2004) Science 305, 1972-1975. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.