Abstract
Since the catastrophic releases of CO2 in the 1980s, Lakes Nyos and Monoun in Cameroon experienced CO2 recharge at alarming rates of up to 80 mol/m2 per yr. Total gas pressures reached 8.3 and 15.6 bar in Monoun (2003) and Nyos (2001), respectively, resulting in gas saturation levels up to 97%. These natural hazards are distinguished by the potential for mitigation to prevent future disasters. Controlled degassing was initiated at Nyos (2001) and Monoun (2003) amid speculation it could inadvertently destabilize the lakes and trigger another gas burst. Our measurements indicate that water column structure has not been compromised by the degassing and local stability is increasing in the zones of degassing. Furthermore, gas content has been reduced in the lakes ≈12-14%. However, as gas is removed, the pressure at pipe inlets is reduced, and the removal rate will decrease over time. Based on 12 years of limnological measurements we developed a model of future removal rates and gas inventory, which predicts that in Monoun the current pipe will remove ≈30% of the gas remaining before the natural gas recharge balances the removal rate. In Nyos the single pipe will remove ≈25% of the gas remaining by 2015; this slow removal extends the present risk to local populations. More pipes and continued vigilance are required to reduce the risk of repeat disasters. Our model indicates that 75-99% of the gas remaining would be removed by 2010 with two pipes in Monoun and five pipes in Nyos, substantially reducing the risks.
Keywords: gas disaster, limnology, natural hazard
Volcanoes can release massive amounts of CO2 at the Earth's surface (1), and in the last 20 years natural lakes with CO2-rich waters have also proven to be highly dangerous (2-4). Before the nature of these gas-charged lakes was understood, sudden releases of large clouds of CO2 gas from Lakes Nyos and Monoun in Cameroon, in 1986 and 1984, respectively, claimed the lives of ≈1,800 people by asphyxiation (2, 3). The gas originates from magma at great depth, but dissolves into ground-water near the Earth's surface. The CO2-charged water enters the lake bottoms through springs (2, 4) and accumulates in the deep, stratified lakes. Although the timing of sudden releases may be modulated by climate (5), it is now clear that continuous gas recharge into the lakes ensures a natural cycle of repeating disasters (6-8).
Unlike most natural hazards, the certainty of future disasters at these lakes can be averted by directed mitigation. The solution is to degas the lakes through controlled piping of gas-rich bottom water to the lake surface where the gas is released harmlessly to the atmosphere in low concentrations. Once flow in a pipe is mechanically initiated, lift is provided by the buoyant rise of bubbly water and the process becomes spontaneous and self-sustaining. Theoretical models (9-11) indicated that this mitigation was feasible, and pumping began at Lake Nyos in 2001 and Lake Monoun in 2003 (12). However, questions still arose about the safety of this hazard mitigation, and it was unclear whether the degassing operation undertaken to prevent these disasters could instead disrupt the physical stability of the lakes and trigger another gas burst (13, 14). In this study, we report on measurements made over 12 years to estimate gas recharge and lake stability and show that (i) stability has been maintained while degassing has lowered gas content, but that (ii) additional measures are needed to reduce the dangerous amounts of gas remaining. Our model results of the future status of the lakes indicate that the presently deployed pipe in Lake Monoun will soon become ineffective and incapable of removing gas faster than the natural rate of recharge. In Lake Nyos the removal rate with one pipe will soon slow because of the lowered gas concentration at the pipe inlet, and the draw down to safe levels could take decades. During this time, however, the gas remaining will still be sufficient to result in loss of life if released into the surrounding area. Greater urgency in gas removal is warranted given that the survivors originally living near Nyos were evacuated after the disaster and already have been refugees for a generation. Our modeling indicates that additional pipes (one more in Monoun, four more in Nyos) will substantially increase the margin of safety near the lakes and reduce the time required for forced evacuation.
Materials and Methods
Temperature and conductivity profiles were measured by using recently calibrated conductivity-temperature-depth instruments; frequent surveys have found no horizontal gradients in the lake, and profiles taken at fixed locations are representative of the entire lake. Raft-mounted thermistor chains measured temperature each minute and recorded averages hourly at 14 depths in Nyos (1, 10, 20, 30, 40, 60, 80, 110, 140, 160, 170, 180, 190, and 200 m) and 12 depths in Monoun (1, 3, 5, 10, 15, 25, 45, 55, 65, 75, 85, and 90 m). In situ gas pressures were measured by using a gas-permeable probe responsive to all dissolved gas species; in these lakes CO2 and CH4 dominate the total gas pressure, with N2 contributing slightly in surface waters. Total error of the pressure probe is ≈0.15 bar (7). Gas saturation is calculated as the total gas pressure divided by the total system pressure (hydrostatic plus atmospheric; 100% saturation = bubble-point pressure). Concentrations of dissolved CO2 and CH4 were measured by using three different methods: (i) CO2 in surface waters (≈0-50 m in Nyos and ≈0-20 m in Monoun) was calculated by using pH and alkalinity data (7); (ii) CO2 and CH4 in deep-water samples were analyzed by gas chromatography after collection in situ with preevacuated stainless-steel cylinders (7); and (iii) CO2 in deep-water samples was also analyzed by titration of total CO2 after collection in situ with syringes (9).
Stability Calculations. Overall lake stability represents the energy required to mix the lake to uniform density. Stability was calculated by using temperature, dissolved salts, and gas content to determine the water density profile (following refs. 15 and 16). Temperature and gas content data are taken from Figs. 1 and 2 and Tables 1 and 2; dissolved salt data for recent years are unpublished, but are similar to previous reports (7, 15, 17). Dissolved CO2 has a strong, positive effect on water density, but when saturation is reached the formation of bubbles destroys nearby water column stability regardless of the density structure caused by temperature and dissolved salts. Because of this unusual behavior, a modified local stability parameter, E* = [(1/ρ)(Δρ/ΔZ)] × [(PAMB/PGAS) - 1] × (1/PGAS), was derived (8) to include the effects of dissolved gas and estimate the local resistance to mixing between two vertically adjacent, horizontally homogenous water layers. The first term is the standard oceanographic definition of local stability where ρ is potential density of the water and Z is depth. Inclusion of the second and third terms, where PAMB is the hydrostatic pressure and PGAS is the total in situ gas pressure, is required to account for the nonlinear effects of dissolved gas on lake water density and to distinguish between conditions where gas saturation may be reached in a layer of low absolute gas pressure (e.g., near the lake surface). E* thus combines the effects of density stratification and percent of gas saturation into a single parameter. No energy units can be assigned, but as PGAS approaches PAMB the stability E* goes to zero. Negative values indicate instability and can be caused by more dense water overlying less dense water or to oversaturation of gas pressures (PGAS > PAMB). Both causes result in overturning of the local water column, by gravity currents in the first case and rising bubbles in the second case.
Fig. 1.
Depth distribution of total in situ gas pressure (A and B) and conductivity (C and D) in Lakes Nyos (A and C) and Monoun (B and D) over time [1992 = black squares; 1998 = green diamonds; 2001 (Nyos) and 2003 (Monoun) = just before degassing, red triangles; 2004 = postdegassing, blue circles]. The gas saturation line (total hydrostatic plus atmospheric pressure) indicates the bubble-point pressure at which dissolved gas will form bubbles.
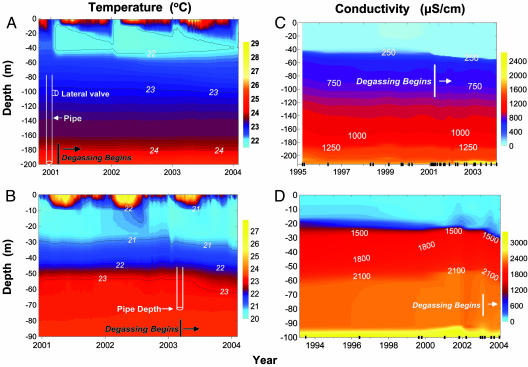
Fig. 2.
Time course of changes in temperature (A and B) in Lake Nyos (A) and Lake Monoun (B) and in conductivity (C and D) in Lake Nyos (C) and Lake Monoun (D), showing the depth of degassing pipe openings and the start of degassing. Marks on x axis of C and D indicate sampling dates; sampling in A and B was hourly.
Table 1. Concentrations of dissolved CO2 and CH4 in Lake Nyos.
| Concentration
|
||||||
|---|---|---|---|---|---|---|
| Date | Gas, μmol/kg | 170.7 m | 182.9 m | 190.5 m | 198.2 m | 205.8 m |
| April 1992 | CH4 | 564 | 704 | 864 | 1,064 | 1,591 |
| May 1998 | CH4 | 847 | 1,230 | 1,941 | 1,829 | 2,520 |
| Nov. 1999 | CH4 | 1,442 | 2,024 | 1,976 | 2,084 | |
| Jan. 2001 | CH4 | 928 | 1,514 | 2,578 | 1,748 | |
| Oct. 2001 | CH4 | 1,526 | 2,062 | 2,344 | ||
| Jan. 2003 | CH4 | 1,051 | 1,542 | 2,459 | 2,516 | |
| Jan. 2004 | CH4 | 1,134 | 1,600 | 2,257 | 2,252 | 2,418 |
| April 1992 | CO2 | 144,300 | 154,600 | 208,800 | 288,600 | 317,133 |
| May 1998 | CO2 | 141,600 | 173,000 | 342,600 | 340,500 | 349,000 |
| Nov. 1999 | CO2 | 210,600 | 343,500 | 341,600 | 338,300 | |
| Jan. 2001 | CO2 | 145,400 | 250,400 | 375,000 | 300,500 | |
| Oct. 2001 | CO2 | 205,100 | 350,100 | 351,400 | ||
| Jan. 2003 | CO2 | 141,400 | 181,100 | 365,400 | 354,800 | |
| Jan. 2004 | CO2 | 138,000 | 166,200 | 345,700 | 353,700 | 363,800 |
All samples were collected in situ with stainless steel cylinders. Dates in italics are postdegassing.
Table 2. Concentrations of dissolved CO2 and CH4 in Lake Monoun.
| Concentration
|
|||||
|---|---|---|---|---|---|
| Date | Gas, μmol/kg | 61 m | 68.6 m | 79.3 m | 95.4 m |
| March 1992 | CH4 | 2,118 | 2,166 | 2,724 | |
| May 1998 | CH4 | 2,213 | 2,803 | 2,227 | 3,385 |
| Nov. 1999 | CH4 | 2,384 | 2,520 | 2,496 | 3,449 |
| Jan. 2003 | CH4 | 2,462 | 2,857 | 2,474 | 3,682 |
| Jan. 2004 | CH4 | 2,622 | 2,745 | 2,885 | 3,948 |
| March 1992 | CO2 | 130,600 | 145,300 | 147,900 | 149,800 |
| May 1998 | CO2 | 143,200 | 157,700 | 150,600 | 152,800 |
| Nov. 1999 | CO2 | 141,700 | 150,900 | 153,600 | 146,300 |
| Jan. 2003 | CO2 | 148,500 | 157,700 | 155,500 | 148,900 |
| Jan. 2004 | CO2 | 140,200 | 148,600 | 156,900 | 157,200 |
All samples were collected in situ with stainless steel cylinders. Dates in italics are postdegassing.
Gas Recharge and Removal. Gas recharge rates into the lakes were calculated as the slope of gas content versus time below 50 m in Nyos and below 20 m in Monoun (Table 3). The variance of these recharge rates is taken as the standard error of the slope of the line best fitting changes in gas content over time. Surface-water CO2 losses include stream outflow and ventilation to the atmosphere and are calculated as the difference between changes in total CO2 content and changes in CO2 content below 50 m in Nyos or below 20 m in Monoun. These depths correspond to the uppermost sharp density and salt gradient (chemocline) in each lake (Fig. 1 C and D), which best separates the effects of piping from surface losses because the chemocline boundary retards mixing and separates gas-rich lower layers from surface waters. In Lake Nyos, the removal of gas through pipe discharge was calculated from gas concentrations in Table 1 and a water flow from 203-m depth of 2 × 106 m3/yr (12). In Lake Monoun, gas removal through pipe discharge was calculated from gas concentrations in Table 2 and a water flow from 73-m depth of 9 × 105 m3/yr (12).
Table 3.
Overall lake stability (S) and CO2 content in Lake Nyos and Lake Monoun
| Date | Lake | S, Joule/m2 | Total CO2† | CO2 at depth‡ |
|---|---|---|---|---|
| March–April 1992 | Nyos | 65,570 | 1.343 | 1.325 |
| May 1998 | Nyos | 69,720 | 1.375 ± 0.021 | 1.355 ± 0.023 |
| Nov. 1999 | Nyos | 75,250 | 1.438 ± 0.014 | 1.410 ± 0.010 |
| Jan. 2001 | Nyos | 71,970 | 1.504 ± 0.061 | 1.452 ± 0.064 |
| Jan. 2003 | Nyos | 71,110 | 1.390 ± 0.001 | 1.362 ± 0.036 |
| Jan. 2004 | Nyos | 67,980 | 1.305 ± 0.009 | 1.302 ± 0.009 |
| March–April 1992 | Monoun | 6,500 | 5.362 | 5.328 |
| Nov. 1999 | Monoun | 6,700 | 5.849 ± 0.114 | 5.831 ± 0.115 |
| Jan. 2003 | Monoun | 6,420 | 6.284 ± 0.011 | 6.250 ± 0.020 |
| Jan. 2004 | Monoun | 7,130 | 5.393 ± 0.065 | 5.365 ± 0.066 |
CO2 content is in mol × 1010 for Lake Nyos and mol × 108 for Lake Monoun (main basin only)
CO2 at depth gives content from 50- to 210-m depth (Nyos) and 20- to 100-m depth (Monoun). CO2 content of surface waters is equal to (total CO2 – CO2 at depth), and changes over time of this difference indicate surface water losses of CO2
Total gas content in the lakes was calculated by using gas concentrations and bathymetric data (Fig. 5, which is published as supporting information on the PNAS web site). Briefly, Lake Nyos has a maximum area of 1.58 × 106 m2 and a maximum volume of 1.794 × 108 m3. Lake Monoun consists of three basins; the main basin is the largest and deepest, contains the high gas concentrations, and has a maximum area of 3.13 × 105 m2 and a maximum volume of 1.104 × 107 m3. The reported error in measures of CO2 content is the standard deviation of average gas content calculated with two different methods, the in situ collection of water in stainless steel cylinders and the in situ collection of water in syringes followed by titration to determine total CO2 (see above).
Modeling. Our model of changes in CO2 content over time uses a mass balance approach defined by [CO2_Total] = [CO2_Initial] + [CO2_Recharge] - [CO2_Pipe] - [CO2_Loss], where [CO2_Total] is the current total mass of CO2 in the lake, [CO2_Initial] is the total mass of CO2 at the previous time step in the model, [CO2_Recharge] is the addition of CO2 to the lake by natural recharge into bottom waters, [CO2_Pipe] is the mass of CO2 removed from bottom waters by the degassing pipe, and [CO2_Loss] includes removal from the lake in surface stream outflow and ventilation to the atmosphere. Note that CO2_Recharge is a net value and includes the dominant term of gas input and the much smaller but unknown amount of gas leakage from the bottom of the lake into groundwater. In the model, CO2_Recharge is initially distributed evenly below 60 m in Monoun and 170 m in Nyos. Gas removal through the pipe is driven by the gas concentration at the inlet, and the CO2_Pipe term was estimated by using the measured relationship between the current, maximum pipe flow rates in both lakes (12), and the gas pressures at the pipe inlets (Fig. 1 A and B). This empirical relationship produces a mean water flow of ≈4.6 ± 0.2 liter/s per bar total pressure (or ≈185 liter/s per mol CO2 per kg) and includes the variation caused by pipe length and friction. Ventilation plus outflow losses (CO2_Loss) are taken as the average rates from 2001-2004 in Lake Nyos and from 1999-2004 in Lake Monoun and are assumed to remain constant into the future.
Degassing rate will be a nonlinear function of time because as gas is removed the chemoclines subside and a new, lower gas pressure drives flow at the pipe inlet. Changes in gas pressures over time at the pipe inlets are constrained by the pressure profiles in the lakes (Fig. 1 A and B). At each time step in the model CO2 concentration at the pipe inlet was adjusted to reflect the CO2 removed by the pipe plus surface-water losses and that gained by natural recharge. The water brought to the surface from depth is assumed to be completely degassed into the atmosphere; the small amount of CO2 that actually remains dissolved in the water is accounted for in the changes in surface water CO2 content. The model uses the mass balance of water pumped through the pipe, and that entering the lake in the recharge fluid, to determine the change in lake structure, layering, and resulting CO2 concentrations at any depth. We set the CO2 concentration in recharge fluid at the highest levels observed in our measurements (0.375 mol/kg in Nyos and 0.159 mol/kg in Monoun) and used the observed recharge rate of CO2 (mol/yr) to determine the flow rate of incoming water (liter/yr). Water is removed from depth by the pipe and returned to the surface, and the lake level remains constant. The net amount of water removed from depth in each time step was compared with an equal volume in a layer X m thick at the pipe inlet, where X represents the depth of chemocline lowering and thus the change in lake structure. The range of gas removal estimates was generated from the error estimates in recharge rates (given below).
Results
Gas Concentration and Pressure. Before degassing, the concentrations of CO2 and CH4 increased in both lakes, especially in deeper waters (Tables 1 and 2). However, maximum CO2 concentrations appear to have leveled off after reaching values of 350-375 mmol/kg near the bottom of Lake Nyos, whereas concentrations increased gradually up to 157 mmol/kg in the lowest levels of Lake Monoun (since 1992, the time of last reported values, ref. 8). In both lakes CH4 concentrations increased much more rapidly than CO2. These CH4 increases were caused mainly by biological methanogenesis (2, 18), as opposed to the CO2 increases that result mainly from inputs of CO2-charged, slightly thermal groundwater (2, 7). Maximum CH4 concentrations reached 7.33 mmol/kg at 208.7-m depth in Lake Nyos in January 2004 and 3.95 mmol/kg at 95.4-m depth in Lake Monoun.
Total gas pressures also increased before degassing in both lakes, most dramatically below 180-m depth in Lake Nyos and below 65-m depth in Lake Monoun, and reaching maximums of 15.6 bar in Nyos in 2001 and 8.3 bar in Monoun in 2003 (Fig. 1 A and B). At Nyos the CH4 pressure has increased alarmingly to 5.5 bar at 208.7 m, which is more than one-third of the total gas pressure at lake bottom (Fig. 1 A and Table 1). In January 2001 we suspended an inverted 2,000-cm2 funnel at the upper chemocline and collected ≈1 cm3/hr of rising gas bubbles that were 47% CH4 (data not shown). These rising bubbles at ≈50-m depth indicate that CH4 saturation may be reached at the sediment-water interface.
Rates of Gas Recharge and Removal. Changes in gas content below the upper chemoclines since 1992 were used to calculate an average CO2 recharge rate of 1.26 ± 0.48 × 108 mol/yr into Nyos and 8.2 ± 1.5 × 106 mol/yr into Monoun (Table 3). This recharge led to a maximum CO2 content of 1.50 ± 0.06 × 1010 mol in 2001 at Lake Nyos and 6.28 ± 0.01 × 108 mol in 2003 at Lake Monoun, the last assessments performed before piping (Table 3). The pipe-degassing operation is described in detail elsewhere (12), and the effects are easily seen in our measurements (Fig. 1). The pipe installed in Lake Nyos operates with a maximum CO2 removal rate of ≈7.50 × 108 mol/yr when withdrawing from 203-m depth, which is similar to the 8 × 108 mol CO2/yr reported with different methods of estimating gas concentrations (12). The degassing noticeably lowered total gas pressures, especially between 45- and 65-m depths and between 170- and 185-m depths in Lake Nyos (Fig. 1 A). At 185 m the total gas pressure was reduced by 3.6 bar from January 2001 to January 2003. The pipe in Lake Monoun has an opening at 73-m depth and a maximum gas removal rate of 1.4 × 108 mol CO2/yr. From January 2003 to January 2004 the degassing lowered gas pressures between 24- and 34-m depths and between 45- and 65-m depths in Lake Monoun (Fig. 1B), with a maximum reduction of 1.99 bar at 56 m.
Lake Structure and Stability. Thermal structure (Fig. 2 A and B) and especially the distribution of electrical conductivity (a measure of dissolved salts; Fig. 2 C and D) in both lakes has been relatively consistent over time. Changes in temperature and conductivity in the surface layers (<50 m in Nyos, <20 m in Monoun) are driven by seasonal changes in climate (5, 11, 16). In Lake Monoun, subsidence of the 1,500 and 2,100 μS/cm conductivity isoclines and 23°C isotherm after degassing began (Fig. 2 B and D) is caused by the removal of high-density and gas-rich water from 73-m depth. The detailed change in physical structure of Lake Monoun is highlighted in Fig. 1D, which shows the lowering of the main chemoclines at 25- and 55-m depth. This chemocline lowering, as opposed to vertical mixing and weakening of density gradients, was predicted before degassing based on theoretical considerations (8-11). A similar effect occurred in Lake Nyos, although to a lesser degree (Figs. 1C and 2 A and C). In both lakes these changes have had a relatively minor impact on stability (Table 3).
From 1992 to the initiation of degassing, E* generally decreased in both lakes because of the increasing gas pressures over time, most notably at ≈200-m depth in Nyos and 40-70 m in Monoun (Fig. 3). Since degassing began, however, E* has on average increased in both lakes (Fig. 3). This increase in stability has been most notable from 180-200 m and above 90-m depth in Lake Nyos and above ≈60-m depth in Lake Monoun.
Fig. 3.
Depth distribution of local water column stability modified for the effects of dissolved gas (E*, see text) in Lake Nyos (A) and Lake Monoun (B). Values from 1992 (black circles) are compared with values before degassing (red squares, 2001 in Nyos, 2003 in Monoun) and after degassing (green triangles, 2004).
Modeling of Future Status. To predict the future state of the lakes we modeled the changes in gas content over time. As a check on the model, results were compared with actual measurements between the time that degassing began and our sample collection in 2003 for Nyos and 2004 for Monoun when one pipe had been operating in each lake. The model performed well; the predicted CO2 removal was within 0.3% of the measured values in both lakes.
With the single pipe now operating in Monoun, the model predicts that within the next 2 years the rate of CO2 removal and recharge will become essentially equal (Fig. 4). At this point, the piping becomes ineffective, and the gas content will actually increase slightly because of greater recharge gain than pumping removal (Fig. 4). This situation arises because, first, as the chemoclines drop the maximum gas pressure is decreased in the layer around the pipe inlet. Second, the gas recharge is distributed relatively evenly in the lower water column [based on the observed rise of CO2 over previous years (refs. 8 and 9, Table 3, and Fig. 1)] rather than concentrated into a high-pressure layer at the bottom of the lake. In Nyos the model shows that gas removal by the single pipe will be slow, and by 2015 ≈75% of the current gas content will still remain (Fig. 4).
Fig. 4.
Model predictions of CO2 content in Lake Nyos (A) below 50 m and Lake Monoun (B) below 20 m with one or more degassing pipes in operation. The dashed line shows the CO2 content at which the average gas pressure in the entire lake is equal to atmospheric pressure (0.9 bar) at the lake surface. For Nyos, minimum content is set at the recharge level, and the “min released” bar shows the gas content equal to the minimum estimate of gas released during the disaster in 1986.
To evaluate the effectiveness of other degassing configurations, we modeled the impact of adding more pipes to the lakes. For Lake Nyos the model included one additional pipe installed each year starting in 2005 for a total of five pipes in the lake by 2008, and the inlet depth for all pipes was at 206 m. For Lake Monoun one additional pipe was incorporated into the model in 2005 with the pipe inlet depth at 97 m, and the existing pipe inlet was lowered from 73 to 97 m. Both configurations resulted in a much greater and faster reduction in gas content than the currently operating single pipes (Fig. 4). The two pipes in Monoun will remove ≈75% of the CO2 remaining, and the five pipes in Nyos will remove >90% of the gas remaining by 2010 (Fig. 4). Average gas pressures attributable to the dissolved CO2 remaining in both lakes would be <0.9 bar if the lake were well mixed, which is the atmospheric pressure at the lake surface (Fig. 4, dashed lines). Thus, complete mixing of the water column, either intentionally or otherwise, would result in only diffusive escape of CO2 to the air with a very low hazard potential.
Discussion
The ratio between in situ gas pressure and hydrostatic pressure at any depth determines the gas saturation, which is an important indicator of the potential for spontaneous bubble formation that could trigger a massive gas burst. Before the initiation of degassing, gas saturation reached the critical level of 97% at 56-m depth in Lake Monoun, and Lake Nyos water was up to 73% saturated at 206 m. Although degassing operations have reduced the dangers, gas saturation values still reach 80-90% in Monoun (Fig. 1B) and >70% at the bottom of Lake Nyos (Fig. 1 A). These levels highlight the need for continued gas removal because (i) even at lower saturation levels a landslide or earthquake could trigger another gas burst, and (ii) there is still more gas in the lakes than was likely released during the 1980s disasters (3, 4).
The total CO2 content in Lake Nyos declined by ≈1.99 × 109 mol from 2001 to 2004, and of that amount, the part attributable to the piping operation was ≈1.50 × 109 mol (Table 3). The difference between the total CO2 change and that caused by piping (0.49 × 109 mol) was the net amount lost through surface flushing and ventilation. Since 1992 this surface loss term in the lake CO2 budget has been much smaller, averaging only 3.9 × 107 mol/yr (Table 3). It is likely that the larger loss term from 2001 to 2004 (1.6 × 108 mol/yr) was caused in part by enhanced erosion of the upper chemocline and some mixing of gas-rich water to the surface. The enhanced erosion is clearly seen in Figs. 1C and 2C, where the depth of the upper mixing layer increased from ≈38 to 51 m. Although some part of this change in mixing depth occurred because of chemocline lowering as bottom water was piped to the surface, two other processes also are responsible. First, average surface water conductivity increased from 61 μS/cm in January 2001 to 87 μS/cm in January 2004 mainly because of high-conductivity bottom water released at the surface from the degassing pipe. Second, surface water cooling was particularly strong at the beginning of 2002, as seen when the 22°C isotherm intersected the surface of the lake and penetrated to ≈50-m depth (Fig. 2 A). These two changes reduced the density gradient across the upper pycnocline and permitted gassy water between ≈40 and 50 m to be mixed upward and ventilated to the atmosphere or removed in the lake outlet stream. In Lake Monoun, a total of 8.91 × 107 mol CO2 was removed from the lake during 2003-2004, with a negligible change in surface waters above 20-m depth (+0.05 × 107 mol). Thus it is unlikely that the same chemocline erosion and vertical transport of gas toward the surface that occurred in Lake Nyos also occurred in Lake Monoun. In both lakes the total amount of gas lost since degassing started is less than the maximum removal rate of the pipes over that period because of pipe stoppages.
A critical assumption of this method of hazard reduction is that instabilities caused directly by the degassing operation will be minimal (8). Our measurements clearly show that the overall structure of the lakes (Fig. 2) and stability have been minimally disrupted by the degassing operations (Table 3). There is no strong trend in either lake of decreasing stability since degassing began, and in fact stability increased in Lake Monoun after the start of degassing, as previously predicted (8). A second, independent measure of the impact of degassing on the lake is the local stability at specific depths as represented by E*. E* has increased at most depths since the degassing began in these lakes (Fig. 3), indicating that the degassing process is not destabilizing the lakes as feared (13, 14). Still, it is critical to note that current values of E* remain very low below the main chemoclines in both lakes because of high gas content and low density gradient. In addition, the values will decrease further through time at depths below the intake of the pipes because of natural recharge. Although E* defines whether two adjacent horizontal layers will mix or overturn, it is only a relative measure of the potential for violent degassing. For example, E* may be slightly negative, indicating unstable conditions and local mixing, but these conditions do not necessitate a large release of gas if the mixing fails to lift a water parcel far enough to become oversaturated. E* may also be negative because PGAS > PAMB and bubbles form, but if the effect is localized the bubbles may redissolve before triggering a gas burst. More negative values of E* indicate greater potential for violent degassing, and thus E* must be frequently assessed as the degassing proceeds.
The current mitigation strategies have reduced the potential for a hazardous gas burst, but dangerous levels of gas and the possibility of its release with lethal consequences still linger. Future hazards depend on the balance between controlled gas removal and natural recharge. Our model of future status predicts that the current gas inventory (2004) will be reduced only slightly in Lake Monoun before the degassing becomes ineffective. In part, this situation is caused by the depth of the pipe inlet that physically limits total gas removal; the current pipe cannot remove the ≈2 × 108 mol of CO2 contained below the 73-m intake (≈37% of the total gas content). In Lake Nyos with the single pipe in operation gas content will be reduced slowly, and only ≈32% will be removed in the next 10 years. During this time, with the current degassing configuration the lakes would still contain dangerous amounts of gas (relative to the 1980s releases), and thus still pose grave dangers to local populations. These model results argue for at a minimum immediately lowering the pipe inlet in Lake Monoun and installing additional pipes in Lake Nyos to increase the degassing rate.
The effectiveness of adding one pipe to Monoun and four pipes to Nyos, and lowering the pipe inlets, was tested by the model and shown to greatly increase the rate and amount of gas that could be withdrawn from the lakes (Fig. 4). In this scenario the amount of CO2 remaining in Lake Nyos below 50 m by 2010 would be ≈0.003 km3 (at standard temperature and pressure), which is more than an order of magnitude smaller than the minimum gas cloud thought to have been released in 1986 (3, 19). By 2010 only ≈0.002 km3 of CO2 would remain below 20 m in Lake Monoun. Although the amount of gas released from Monoun in 1984 is unknown, it is unlikely that a substantial release could occur given the low gas pressures that would exist in the lake (<2.5 bar). Thus the model verifies the need for additional pipes to achieve a safe solution in a prudent amount of time.
Supplementary Material
Acknowledgments
We thank the Cameroon Ministries of Scientific and Technical Research and Mines, Water, and Power for their cooperation and financial support in this research and Karen Riseng, Ibrahim Issa, Aka Festus, Nia Paul, Chris Wallace, Mark Brahce, and Keisuke Nagao for field or laboratory help. This work was also supported by U.S. Office of Foreign Disaster Assistance Grant AOTA-00-99-00223-00, the U.S. Geological Survey, the American and French embassies in Cameroon, and Japan Society for the Promotion of Science Grant 13573013 (to M.K.).
Author contributions: G.W.K., W.C.E., G.T., and M.K. designed research; G.W.K., W.C.E., G.T., M.K., Y.Y., and J.V.H. performed research; G.W.K., W.C.E., G.T., M.K., and T.O. analyzed data; and G.W.K. wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Allard, P., Carbonnelle, J., Dajlevic, D., Le Bronec, J., Morel, P., Robe, M. C., Maurenas, J. M., Faivre-Pierret, R., Martin, D., Sabroux, J. C. & Zettwoog, P. (1991) Nature 351, 387-391. [Google Scholar]
- 2.Kling, G. W., Clark, M., Compton, H. R., Devine, J. D., Evans, W. C., Humphrey, A. M., Lockwood, J. P. & Tuttle, M. L. (1987) Science 236, 169-175. [DOI] [PubMed] [Google Scholar]
- 3.Sigurdsson, H., Devine, J. D., Tchoua, F. M., Presser, T. S., Pringle, M. K. W. & Evans, W. C. (1987) J. Volcanol. Geotherm. Res. 31, 1-16. [Google Scholar]
- 4.Le Guern F. & Sigvaldason, G. E., eds. (1989) J. Volcanol. Geotherm. Res. 39, 97-265. [Google Scholar]
- 5.Kling, G. W. (1987) Science 237, 1022-1024. [DOI] [PubMed] [Google Scholar]
- 6.Nojiri, Y., Kusakabe, M., Hirabayashi, J., Sato, H., Shinohara, H., Njine, T. & Tanyileke, G. (1990) Nature 346, 322-323. [Google Scholar]
- 7.Evans, W. C., Kling, G. W., Tuttle, M. L., Tanyileke, G. & White, L. D. (1993) Appl. Geochem. 8, 207-221. [Google Scholar]
- 8.Kling, G. W., Evans, W. C., Tuttle, M. L. & Tanyileke, G. (1994) Nature 368, 405-406. [Google Scholar]
- 9.Kusakabe, M., Tanyileke, G., McCord, S. & Schladow, G. (2000) J. Volcanol. Geotherm. Res. 97, 241-260. [Google Scholar]
- 10.McCord, S. A. & Schladow, S. G. (1998) J. Geophys. Res. 103, 12355-12364. [Google Scholar]
- 11.Schmid, M., Lorke, A., Wuest, A., Halbwachs, M. & Tanyileke, G. (2003) Ocean Dynamics 53, 288-301. [Google Scholar]
- 12.Halbwachs, M., Sabroux, J.-C., Grangeon, J., Kayser, G., Touchon-Danguy, J.-C., Felix, A., Béard, J.-C., Villevieille, A., Vitter, G., Richon, P., et. al. (2004) EOS 85, 281-285. [Google Scholar]
- 13.Pickrell, J. (2001) Science 291, 965-967. [DOI] [PubMed] [Google Scholar]
- 14.Krajick, K. (2003) Science 299, 805. [DOI] [PubMed] [Google Scholar]
- 15.Kling, G. W., Tuttle, M. L. & Evans, W. C. (1989) J. Volcanol. Geotherm. Res. 39, 151-166. [Google Scholar]
- 16.Kling, G. W. (1988) Limnol. Oceanogr. 33, 27-40. [Google Scholar]
- 17.Kusakabe, M., Ohsumi, T. & Aramaki, S. (1989) J. Volcanol. Geotherm. Res. 39, 167-185. [Google Scholar]
- 18.Kling, G. W., Evans, W. C. & Tuttle, M. L. (1991) Vereinigung Int. Limnol. 24, 1102-1105. [Google Scholar]
- 19.Giggenbach, W. F. (1990) J. Volcanol. Geotherm. Res. 42, 337-362. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.