Abstract
A longstanding controversy in paleoanthropology surrounds the question of whether Neandertals shared the prolonged growth periods of modern humans. To address this question, this investigation compares the duration of enamel formation in Neandertals with that of three comparative modern human groups. Because dental and somatic growth are correlated with each other, dental growth periods are indicative of overall periods of growth. Growth increments on the anterior teeth of Neandertals, modern Inuit, and modern people from Newcastle and southern Africa were counted and their means compared. In addition, potential variation in the time spans represented by growth increments was considered and incorporated into the analysis of enamel formation times. These analyses show that Neandertal imbricational enamel formation times, although likely to have been faster than those of the Inuit, are not likely to have been faster than those of the Newcastle sample and for some teeth are clearly slower than those of the southern African sample. Thus, Neandertal tooth growth and, by extension, somatic growth, appears to be encompassed within the modern human range of interpopulation variation.
Keywords: perikymata, enamel, evolution, hominid
The prolonged period of infant and childhood growth in modern humans is unique among modern primates (1–3). Our extended growth periods appear to result from investment in rapid postnatal brain growth at the expense of somatic growth (1, 2, 4, 5) and the time required for extensive learning before attaining reproductive age (3, 6, 7). Large brains allow complex behavior, the selective advantages of which accrue over the extended human lifespan (8). Thus, a reduction of adult mortality rates may have been a precondition for the evolution of the human combination of prolonged childhood growth and large brains (1, 9, 10). While investigations into the evolutionary conditions and causes of the human life history pattern continue (1–3, 9), studies of dental development in fossil humans are providing insight into when this pattern emerged during human evolutionary history.
Across the primate order, aspects of dental development are highly correlated with the length of growth periods, as well as with brain size (11, 12). Dental and somatic development are closely linked, because teeth develop as part of growing organisms. For example, weaning cannot take place until teeth erupt, and molars can erupt only when the jaw has grown large enough to accommodate them (11, 12). Relative to other primates, modern humans erupt their first molars later, have larger brains, and experience extended periods of somatic growth (8, 11, 12). Given the relationship of somatic and dental development to brain size across the primate order, it is not surprising that the small-brained Plio-Pleistocene australopiths erupted their first molars 2.5–3 years earlier than modern humans and formed their anterior tooth crowns in significantly shorter periods of time (13).
Yet, the length of childhood growth periods in our more recent human ancestors and close relatives, whose brain sizes were comparable to those of modern humans, remains unclear. Trinkaus and Tompkins (10) suggested that Neandertals may have had high young adult mortality, which might suggest selection for more rapid growth relative to modern humans. However, Trinkaus (14) noted that the Neandertal mortality profile, which appears to be biased toward younger individuals, is an artifact of multiple factors, including population fluctuation. Most recently, Ramirez-Rozzi and Bermúdez de Castro (15) presented evidence that Neandertal anterior teeth grew in 15% less time than those of Upper Paleolithic–Mesolithic Homo sapiens, a finding they interpret to mean that Neandertals grew up significantly more quickly than modern humans. These authors (15) claim that this “surprisingly rapid growth” was an autapomorphic feature of Neandertals relative to modern humans. Clearly, however, to determine whether Neandertal dental growth periods were indeed abbreviated with respect to those of modern humans, the range of dental growth period variation in modern human populations must be known. To this end, the present investigation compares Neandertal anterior tooth growth with that of dental samples from three modern human populations from disparate regions (England, Alaska, and southern Africa).
Tooth Growth
Enamel grows in an incremental manner from the cusp of a tooth to its cervix (16, 17) (Fig. 1). These incremental growth layers are visible as dark lines, or striae of Retzius (more simply, striae), in transmitted light microscopy of thin sections (11, 16, 17). The exact period of growth represented by each stria can be determined by counting the daily growth increments, or cross striations, that lie between them (Fig. 1) (16–19). The number of days represented by each stria, its periodicity, is constant within the teeth of an individual (18). In the cuspal region of the tooth, the enamel growth layers cover each other in a series of domes. However, on the sides of the tooth, in the imbricational enamel, they outcrop onto the enamel surface as perikymata (Fig. 1). Because determining the formation of cuspal enamel requires sectioning teeth and is therefore rarely possible on fossil teeth, most studies of tooth formation in fossil humans focus only on imbricational enamel formation (12, 13, 19). Anterior teeth are preferred for these studies, because the majority of their enamel is imbricational rather than cuspal (15, 16).
Fig. 1.
Relationship between perikymata and striae of Retzius. Cross striations appear at higher magnifications as varicosities and constrictions along the enamel prisms. Images courtesy of Jay Kelley and Tanya Smith (35).
In a thin section of a tooth, it is possible to determine the time taken for the tooth's imbricational enamel to form by multiplying the stria periodicity by the total number (or count) of striae on that tooth. However, because periodicities cannot be directly determined from enamel surfaces, the total length of time for the imbricational enamel to form in fossil humans is usually estimated by counting the total number of perikymata on a tooth and multiplying by a periodicity of 9 (13, 15, 20), because this was the mean and modal periodicity found in a combined sample of 184 African apes and humans (20). More recently, Smith et al. (21) found a mean and modal periodicity of 8 in 365 modern human teeth (in a combined sample of diverse origins). Reid and Ferrell (22) found a modal periodicity of 8 and a mean periodicity of 9 in a sample of 49 Danish canines. Continuing histological work on a small sample of fossil human specimens indicates that they also had mean and modal periodicities of 8 or 9 (23). D.G.-S. has observed a histological section from the Tabun II Neandertal (courtesy of M. C. Dean and the Natural History Museum of London), which appears to have a periodicity of 8. The totality of this evidence suggests that Neandertals would have had mean and modal periodicities similar to those of modern and fossil humans.
However, periodicities within modern human populations are highly variable, exhibiting a range of 6–12 days (present study and refs. 20–22 and 24). The greatest source of variation in periodicities in two different hominin samples is therefore expected to come from variation introduced by sampling error rather than from any fundamental difference in mean periodicities between populations or species. Because of the wide variation in periodicities within human populations, any analysis of crown formation times based solely on perikymata counts with unknown periodicities must consider the potential range of periodicities that might exist in the sample. Unlike any previous study of crown formation times in hominin fossils, our analysis considers this range by taking advantage of a recently discovered relationship between total perikymata counts and periodicity (22, 24), as we describe below.
Samples
Table 1 lists the Neandertal teeth and sample sizes. The Neandertal sample spans ≈150,000–40,000 years. The sample from England derives from a single living population from Newcastle-upon-Tyne. The southern African sample derives from several indigenous populations, with a mixture of ethnic backgrounds. The Alaskan sample is an archaeological one of Point Hope Inuit, spanning six culture periods: the Near Ipiutak (500–100 B.C.), Ipiutak (100 B.C. to A.D. 500), Birnirk (A.D. 500–900), Western Thule (A.D. 900–1300), Tigara (A.D. 1300–1700), and Recent (A.D. 1700–present) (25).
Table 1. Sample composition.
| Tooth type | Neandertal specimens | Neandertal, n* | Inuit, n | Southern African, n | Newcastle, n |
|---|---|---|---|---|---|
| UI1 | Krapina 91, 93, 94, 123, 126, 155, 194, 195; Devil's Tower 1, Le Moustier 1 | 10 | 10 | 20 | 19 |
| UI2 | Krapina 122, 128, 130, 131, 148, 156, 160, 196; Le Moustier 1 | 9 | 10 | 21 | 16 |
| UC | Krapina Maxilla E, 37, 56, 76, 102, 103, 141, 142, 144, 146, 191; Kůlna, Le Moustier 1, La Quina 5 | 14 | 9 | 26 | 39 |
| LI1 | Krapina Mandible E, 73; Ochoz, Tabun II, Le Moustier 1 | 5 | 12 | 20 | 15 |
| LI2 | Krapina Mandible C, Mandible D, Mandible E, 71, 90; Ochoz, Tabun II, Le Moustier 1 | 8 | 14 | 23 | 13 |
| LC | Krapina Mandible D, Mandible E, 75, 119, 120, 121, 145; Ochoz, Tabun II, Le Moustier 1 | 10 | 10 | 24 | 13 |
| Total teeth | 55 | 65 | 134 | 115 | |
| Total individuals | 30 | 17 | 114 | 115 |
Total number of Krapina Neandertals based on the designation of associated teeth as “Krapina Dental People” (34)
Materials and Methods
In this study, only one tooth (right or left) was used from each individual for each tooth type. The choice of right or left teeth was made on the basis of which antimere was most complete. Only teeth estimated to have 80% or more of their crown heights intact (i.e., minimally worn teeth) were selected for analysis. Crown heights were measured by using a reticule calibrated to a magnification of ×50. Original crown heights were reconstructed by following the contour of each side of the tooth cusp and projecting it until the sides meet. Both measured and reconstructed crown heights were recorded.
For the Neandertal and Inuit samples, high-resolution polyvinyl siloxanes (Coltene's President Jet and Struer's RepliSet) were used to make dental impressions from the buccal surfaces of anterior teeth. These were cast in high-resolution epoxy (Struer's Epofix) and were coated with a gold–palladium alloy. Perikymata were counted under a light microscope, and a scanning electron microscope was used to create a micrographic record of tooth surfaces. Each replica was oriented orthogonally to the microscope's optical axis.
The samples from Newcastle and southern Africa are thin sections on which striae of Retzius were counted under transmitted light microscopy. Only striae clearly outcropping onto the surface as perikymata were counted. For this reason, we refer to the striae counts in our histological sample as perikymata counts. For the Newcastle and southern African samples, it was possible to count cross striations to determine the periodicity for each tooth. Hence the number of days it took to form the imbricational enamel in each of these teeth could be calculated directly by multiplying the tooth's periodicity by the total number of perikymata on the tooth.
Each tooth was divided into 10% increments (deciles) of the reconstructed crown height, and perikymata were counted within the increments. For teeth missing up to 20% of their crowns because of wear, estimates of perikymata were made for the first two deciles. Perikymata counts within the first two deciles of complete crowns used in this study have very low standard deviations, ranging from one to two perikymata for each tooth type within each population. Such low variation within the first two deciles of growth makes it possible to accurately estimate growth in slightly worn teeth based on the perikymata counts in the first and second deciles of complete crowns for each tooth type and each population sample. Teeth were excluded from the study if more than one decile beyond the first two deciles contained indistinct perikymata. For teeth in which a single decile contained indistinct perikymata, counts were estimated from adjacent deciles. To eliminate interob-server error, only counts by D.J.R. were used in the statistical analysis. Intraobserver error for D.J.R. has been calculated at <5% for perikymata counts (13).
We test the hypothesis that Neandertals grew their teeth in shorter time periods than the modern human comparative samples in two ways. First, using the same method of Ramirez-Rozzi and Bermúdez de Castro (15), we compare the means of the total perikymata counts per tooth of the Neandertal sample with each of the comparative samples. Under the assumption that the mean periodicity of the Neandertal sample is equivalent to that of the modern human comparative samples, Neandertals can be inferred to be forming their imbricational enamel in shorter periods of time only if they have significantly lower mean perikymata counts than the comparative samples. We first analyze differences in perikymata count means across population samples for all anterior teeth combined, so that our results can be compared with those of Ramirez-Rozzi and Bermúdez de Castro (15). On the combined sample, we used a general linear model and performed an ANOVA in which the factors were tooth type, population, and the interaction of tooth type and population. We conducted an additional ANOVA using reconstructed crown height as a covariate, because we found that, within populations, crown heights are generally positively correlated with the total number of perikymata on a tooth. We performed pair-wise contrasts of total perikymata count means between Neandertals and each comparative sample using Dunnett's simultaneous t tests for significant differences (26). Then, for each tooth type, we conducted one-way ANOVAs of total perikymata counts and Dunnett's simultaneous t tests to determine whether there were significant differences in mean perikymata counts between Neandertals and each comparative sample.
The second way we test the hypothesis of abbreviated growth in Neandertal teeth takes into account the unknown periodicities in our Neandertal sample. For each tooth type from a given population, there is a strong negative correlation (r from -0.90 to -0.99) between total perikymata counts on teeth and their periodicities (22, 24). Because the time it takes for imbricational enamel to form is equal to the total number of perikymata on a tooth multiplied by its periodicity, the strong negative correlation between periodicity and total perikymata counts means that crown formation times are fairly constant for each tooth type in a population, with only small standard deviations from the mean (22, 24). Regression equations of the following form can be used to describe this relationship for each tooth type in the Newcastle and southern African samples: Periodicity = α + β(perikymata count). For each population (Newcastle and southern Africa), every point on the regression line for a particular tooth type represents a value for periodicity and total perikymata count, which, when multiplied together, result in that tooth type's average crown formation time. The r2 values for these 12 regression equations (two populations, each with six tooth types) range from 0.828 to 0.949, all with P values = <0.001. They are therefore highly predictive of the relationship between the total number of perikymata and periodicity for a given tooth type, a relationship that is determined by the time it takes for a particular tooth type to form (22, 24).
As shown in Results, perikymata counts in our Neandertal sample are higher than those of the southern African sample; nevertheless, it could be argued that if the Neandertals in our sample had lower periodicities than southern Africans, they might be growing their teeth in equivalent periods of time. It could further be argued that the Neandertals in our sample might be forming their teeth in shorter periods than southern Africans, even 15% shorter, if the Neandertals in our sample had still lower periodicities. Therefore, we use the southern African regression equations for the six anterior teeth relating periodicity to perikymata counts to determine what the periodicities in our Neandertal samples would have to be if their imbricational enamel formation times were equivalent to or 15% shorter than those of southern Africans. We refer to these conditional periodicities as “hypothetical periodicities.”
The key to our analysis is that the known lower limit of periodicities in modern humans and African apes is 6. In fact, there are only 2 individuals of 184 in Dean and Reid's (20) combined African ape and human sample, exhibiting periodicities of 6. In the combined Newcastle and southern African samples used in our study, there is only one case of 249 teeth in which the periodicity was 6. Smith et al. (21) have found no chimpanzee with periodicities of <6. Thus we reject the hypothesis that Neandertals grew their teeth in the same period, or in 15% less time, than southern Africans if the only way for these hypotheses to be true is to assume that any of our Neandertal specimens had periodicities <6.
We obtain hypothetical periodicities for Neandertals under the assumption of equivalence to southern African crown formation times by inserting Neandertal perikymata counts into the southern African regression equations. We determine what the periodicities for the Neandertals would be if their teeth grew in 15% less time than those of the southern African sample in the following way. We first multiply the hypothetical Neandertal periodicities based on southern African regression equations by the number of perikymata. This gives an approximate time in days for Neandertal enamel formation that is comparable to that of a southern African tooth with the same number of perikymata. We then multiply this approximate enamel formation time by 0.85 and divide it by the number of perikymata for each Neandertal tooth to obtain a second set of hypothetical periodicities for Neandertals.
Results
Table 2 contains basic descriptive statistics on periodicity for the southern African and Newcastle samples, the two samples for which we could directly determine periodicity. Note that when rounded to the nearest whole number, mean, and modal periodicities for both samples are nine.
Table 2. Descriptive statistics for periodicities: Range, mode, and mean for two population samples.
| Population | Range | Mode | Mean | SD |
|---|---|---|---|---|
| Southern African | 6–12 | 9 | 9.097 | 1.207 |
| Newcastle | 7–11 | 9 | 8.748 | 1.042 |
Table 3 contains the results of two ANOVAs of perikymata counts in the combined sample of anterior teeth, one ANOVA without and one with reconstructed crown height as a covariate. The population factor has four levels: Neandertal, Inuit, Newcastle, and southern African. The tooth type factor has six levels: upper and lower first incisors, second incisors, and canines. Although the inclusion of crown height as a covariate causes the F value for the population main effect to increase and the F value for the tooth type main effect to decrease, both main effects and the interaction between them remain as significant sources of variation. Fig. 2 shows the graphs of the interaction plots from the first ANOVA (without adjustment for crown height). Fig. 2 shows that Neandertal mean perikymata counts are generally lower than the mean perikymata counts of the Inuit, variable with respect to those of the sample from Newcastle, and generally higher than those of southern Africans.
Table 3. ANOVA results for total perikymata counts.
| Without crown height as covariate, R2 = 66.39%
|
With crown height as covariate, R2 = 70.82%
|
|||||
|---|---|---|---|---|---|---|
| Factors | df | F | P | df | F | P |
| Population | 3 | 78.74 | 0.000 | 3 | 90.59 | 0.000 |
| Tooth type | 5 | 62.19 | 0.000 | 5 | 31.20 | 0.000 |
| Population by tooth type | 15 | 5.14 | 0.000 | 15 | 5.46 | 0.000 |
| Crown height | 1 | 52.08 | 0.000 | |||
Fig. 2.
Plot of the interaction of tooth type by population for total perikymata counts.
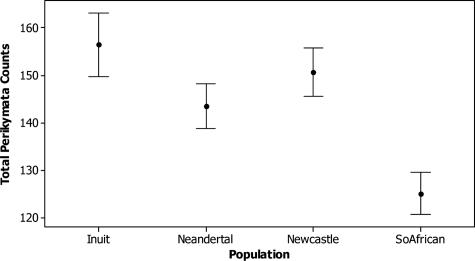
Fig. 3 gives 95% confidence intervals of the means for all anterior teeth combined, and Table 4 contains Dunnett's simultaneous t test results of mean differences in perikymata counts between Neandertals and each comparative modern human sample. Both the 95% confidence intervals and Dunnett's simultaneous t tests show that Neandertals have lower means than the Inuit, higher means than the southern Africans, and means that are not significantly different from those of the sample from Newcastle. Thus, assuming equivalent mean and modal periodicities across the samples, Neandertals form their teeth more quickly than the Inuit and more slowly than southern Africans and cannot be distinguished in their imbricational enamel formation times from the Newcastle sample.
Fig. 3.
Ninety-five percent confidence intervals for mean perikymata counts (all anterior teeth combined).
Table 4. Dunnett's t tests for differences in perikymata count means across populations, all tooth types combined.
| Comparison, Neandertal subtracted from | Difference of means | t value | P value |
|---|---|---|---|
| Inuit | 15.24 | 4.753 | 0.0000 |
| Newcastle | 7.94 | 2.706 | 0.0175 |
| Southern African | –19.50 | –6.945 | 0.0000 |
All one-way ANOVAs for difference in mean perikymata counts across populations for each tooth type were statistically significant, with P values <0.001 (results not shown). Table 5 shows Dunnett's simultaneous t tests by tooth type for mean differences in perikymata counts between Neandertals and the modern human samples. The Inuit have statistically significantly higher counts than Neandertals on UI1, UC, and LC, whereas the two populations do not show a statistically significant difference in UI2, LI1, and LI2. The Newcastle sample has statistically significantly higher counts than Neandertals on UI1 and LC but statistically significantly lower counts on LI2, and these two populations do not show statistically significantly different counts on UI2, UC, and LI1. Last, the southern African sample shows statistically significantly lower counts than Neandertals on all incisors, with statistically insignificant differences on both canines. In some cases, the comparisons at the level of tooth type are made with small sample sizes, potentially contributing to nonsignificant results. However, when the differences are significant, they confirm that Neandertal mean perikymata counts are lower than the mean perikymata counts of the Inuit, variable with respect to those of the sample from Newcastle, and higher than those of southern Africans.
Table 5. Dunnett's t tests for differences in perikymata count means across populations, separated by tooth type.
| Tooth type | Difference of means | t value | P value |
|---|---|---|---|
| UI1: Neandertal subtracted from | |||
| Inuit | 32.30 | 4.259 | 0.0002 |
| Newcastle | 27.18 | 4.103 | 0.0004 |
| Southern African | –20.60 | –3.137 | 0.0073 |
| UI2: Neandertal subtracted from | |||
| Inuit | 7.96 | 1.148 | 0.4936 |
| Newcastle | –9.69 | –1.542 | 0.2729 |
| Southern African | –28.63 | –4.765 | 0.0001 |
| UC: Neandertal subtracted from | |||
| Inuit | 25.738 | 3.0512 | 0.0082 |
| Newcastle | 10.584 | 1.7206 | 0.2007 |
| Southern African | –4.121 | –0.6296 | 0.8481 |
| LI1: Neandertal subtracted from | |||
| Inuit | –0.67 | –0.105 | 0.9986 |
| Newcastle | 2.10 | 0.344 | 0.9591 |
| Southern African | –25.22 | –4.286 | 0.0002 |
| LI2: Neandertal subtracted from | |||
| Inuit | –12.36 | –1.763 | 0.1743 |
| Newcastle | –22.60 | –3.185 | 0.0061 |
| Southern African | –42.23 | –6.461 | 0.0000 |
| LC: Neandertal subtracted from | |||
| Inuit | 38.500 | 4.2728 | 0.0002 |
| Newcastle | 40.085 | 4.7299 | 0.0001 |
| Southern African | 3.825 | 0.5044 | 0.9128 |
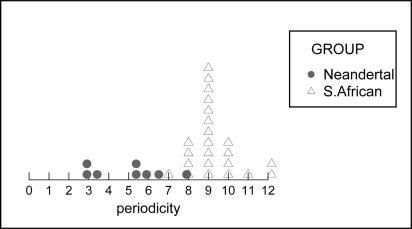
Hypothetical periodicities calculated for Neandertal teeth using regression equations relating periodicity to the total number of striae in the southern African sample reveal distributions for two tooth types (LI1 and LI2) that include periodicities <6, the lower limit of the known range of periodicities in African apes and humans. Neandertal LI1 and LI2 must therefore have grown more slowly than the LI1 and LI2 of southern Africans. It is possible that the UI1, UI2, UC, and LC of Neandertals grew in the same amount of time as in southern Africans, because the hypothetical periodicities fall in the range of 6–10 days. Under the assumption of growth periods abbreviated by 15% in Neandertal teeth relative to southern Africans, the hypothetical periodicities of all six anterior tooth types include periodicities <6. It is therefore not possible for Neandertal imbricational enamel formation times to have been 15% shorter than those of the southern Africans in our sample. To illustrate, Fig. 4 shows the hypothetical periodicities for the Neandertal lower I2 under the assumption of a growth period 15% shorter than that of the southern African lower I2. The actual periodicities of the southern African lower I2 sample are shown on the same histogram. Note that several teeth in the Neandertal sample would be required to have periodicities <6 for this assumption to be true.
Fig. 4.
Distribution of hypothetical periodicities (represented by solid circles) for Neandertal lower I2 sample under the assumption of 15% shorter growth periods than the southern African lower I2 sample. Actual periodicities for the southern African lower I2 sample shown on same histogram, represented by triangles.
Discussion
This study demonstrates that Neandertal anterior tooth imbricational enamel formation times are within the range of variation that three modern human populations exhibit. Our results challenge the central conclusion of Ramirez-Rozzi and Bermúdez de Castro (15) that Neandertals had significantly abbreviated anterior tooth growth periods relative to modern humans. Based solely on a modern human sample from the Upper Paleolithic–Mesolithic, Ramirez-Rozzi and Bermúdez de Castro (15) suggested that Neandertals had anterior tooth imbricational enamel formation spans 15% shorter than modern humans. Yet if we assume, as these authors do (15), a common average periodicity across all human samples, then the Neandertals in our study would have been forming their teeth over longer time periods than a sample of modern southern Africans, in about the same amount of time as the sample from Newcastle, and in shorter periods than a sample of Inuit. When the relationship between periodicity and total perikymata counts is used to analyze the unknown periodicities in our Neandertal sample, our analysis indicates that the lower incisors of Neandertals are growing more slowly than those of southern Africans, whereas for canines and upper incisors, Neandertal and southern African imbricational enamel growth may be comparable. Again, using the relationship between periodicity and total perikymata counts, we show that Neandertal teeth could not have been growing in 15% less time than those of southern Africans.
Do our results therefore imply that Neandertals shared the prolonged childhoods of modern humans? This question could be answered with a definitive “yes” if enamel formation in anterior teeth were directly linked to the length of the childhood growth period. Unfortunately, it is currently not known whether this is true. What is known is that across primate species, gestation length, age of weaning, age of female sexual maturity, and life span are highly correlated with the age at which the permanent first molar erupts (11, 12). In addition, brain weight correlates with first molar eruption with an r of 0.98 across 21 species of primates (11, 12). Dean et al. (13) have noted that, in modern humans and Paranthropus, the lower canine completes enamel formation at about the same time that the first molar emerges. If this were also the case for Neandertals, whose calcification patterns are similar to those of modern humans (27), then our data indicate that the time of Neandertal first molar emergence was not different from that of modern southern Africans.
It has previously been suggested that the third molar erupted at ≈15 years of age in Neandertals (28), possibly because of a systemically accelerated dental development schedule (15). Yet, the possibility that Neandertals erupted their third molars early does not necessarily imply rapid overall dental development. Advanced third molar eruption in Neandertals may have been the result of their large jaws and retromolar spaces that made early eruption of the third molar possible (29).
It has also been suggested that if Neandertals suffered high adult mortality rates, then they might be expected to have had abbreviated periods of childhood growth (10, 15). Adult mortality rates directly select for the timing of maturation across mammals; a larger risk of dying selects for rapid maturation (9, 30, 31). However, Smith (32) notes that if Neandertals had accelerated life histories, then this would leave them with a “peculiar” relationship between brain size and maturation, “two variables that are rarely of step.” Because large brains require extended periods of childhood growth (1–7, 33), the presence of large brains in Neandertals suggests that their adult mortality risks were not high enough to have prevented them from evolving prolonged growth periods.
Our study demonstrates that Neandertal anterior tooth formation times are encompassed within the wide range of variation that we have shown exists within modern humans. Based on comparison to an Upper Paleolithic–Mesolithic modern human sample, Ramirez-Rozzi and Bermúdez de Castro (15) claimed that Neandertal tooth growth was abbreviated relative to modern humans, and from this inference, they concluded that Neandertals grew up more quickly. Our study arrives at a different conclusion for two reasons. First, we did not rely on the assumption of a constant periodicity; instead, we additionally considered the range of periodicities that are present within a sample. Second, unlike Ramirez-Rozzi and Bermúdez de Castro (15), we compared Neandertals to a broad modern human comparative sample.
Our data show that anterior tooth perikymata counts cannot be used to distinguish Neandertal growth rates from the range of variation in modern humans once the latter is adequately assessed. If anterior tooth crown formation periods reflect overall growth periods, then our study suggests that Neandertals did not reach adulthood any more quickly than do modern humans.
Acknowledgments
This study was supported by Leakey Foundation grants to D.G.-S. and C.S.L. and by a College of Social and Behavioral Sciences grant to D.G.-S. from Ohio State University. We are grateful to the following people and institutions for access to their collections: Kevin Kuykendall and Cynthia Reid (Medical School of the University of the Witwatersrand, Johannesburg, South Africa), Jakov Radovčić (Croatian Natural History Museum, Zagreb, Croatia), Christopher Stringer and Robert Kruszynski (Natural History Museum, London), Yoel Rak and Alon Barash (Tel Aviv University, Tel Aviv), Almutt Hoffman (Museum für Vor- und Frühgeschichte Archäeologie Europas, Berlin), Ivana Jarasova (Anthropos Institute, Sankt Augustin, Germany), Phillipe Mennecier (Musée de l'Homme, Paris), and Ian Tattersall and Ken Mowbray (American Museum of Natural History, New York). We acknowledge the skill and excellence of Pamela Walton, who prepared all of the slides used in this study. We also thank Dale Hutchinson for his advice, Dan Steinberg for extensive support, Cathy Cooke and James Patrick Bell for making epoxy replicas, and Cameron Begg and Hank Colijn (Center for Electron Optics, Ohio State University) for their SEM assistance. We thank Tanya Smith (Max Planck Institute for Evolutionary Anthropology, Leipzig, Germany) and Jay Kelley (University of Illinois, Chicago) for use of the images in Fig. 1. Christopher Dean, Gary Schwartz, Rebecca Ferrell, Bruce Floyd, and Tanya Smith provided helpful comments on the manuscript. We thank the anonymous reviewer for comments that helped improve the clarity of the paper. Finally, the following people gave advice on the collections, and their help is also greatly appreciated: Shara Bailey, Jeff Schwartz, and Trent Holliday.
Author contributions: D.G.-S. designed research; D.G.-S. and D.J.R. performed research; D.G.-S., D.J.R., and T.A.B. analyzed data; D.G.-S. wrote the paper; D.G.-S. made dental impressions; and C.S.L. made dental impressions and edited the paper.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Crews, D. E. & Gerber, L. M. (2003) Coll. Antropol. 27, 7-22. [PubMed] [Google Scholar]
- 2.Leigh, S. R. (2001) Evol. Anthropol. 20, 223-236. [Google Scholar]
- 3.Bogin, B. (1997) Yrbk. Phys. Anthropol. 40, 63-89. [Google Scholar]
- 4.Sacher, G. A. (1975) in Primate Functional Morphology and Evolution, ed. Tuttle, R. H. (Mouton, The Hague, The Netherlands), pp. 417-441.
- 5.Martin, R. D. (1983) Human Brain Evolution in an Ecological Context (American Museum of Natural History, New York).
- 6.Gould, S. J. (1977) Ontogeny and Phylogeny (Harvard Univ. Press, Cambridge, MA).
- 7.Mann, A. E. (1972) Man 7, 379-386. [Google Scholar]
- 8.Smith, B. H. & Tompkins, R. L. (1995) Annu. Rev. Anthropol. 24, 257-279. [Google Scholar]
- 9.Kelley, J. (2002) in Human Evolution Through Developmental Change, eds. McNamara, K. J. & Minugh-Purvis, N. (Johns Hopkins Univ. Press, Baltimore), pp. 223-248.
- 10.Trinkaus, E. & Tompkins, R. L. (1990) in Primate Life History and Evolution, ed. De Rousseau, C. J. (Wiley–Liss, New York), pp. 153-180.
- 11.Smith, B. H. (1989) Evolution (Lawrence, Kans.) 43, 683-688. [Google Scholar]
- 12.Smith, B. H. (1991) Am. J. Phys. Anthropol. 86, 157-174. [Google Scholar]
- 13.Dean, C., Leakey, M. G., Reid, D. J., Shrenk, F., Schwartz, G. T., Stringer, C. & Walker, A. (2001) Nature 414, 628-631. [DOI] [PubMed] [Google Scholar]
- 14.Trinkaus, E. (1995) J. Archaeol. Sci. 22, 121-142. [Google Scholar]
- 15.Ramirez-Rozzi, F. V. & Bermúdez de Castro, J. M. (2004) Nature 428, 936-939. [DOI] [PubMed] [Google Scholar]
- 16.Nancir, A., ed. (2003) Ten Cate's Oral Histology (Mosby, St. Louis), 6th Ed.
- 17.Hillson, S. (1996) Dental Anthropology (Cambridge Univ. Press, Cambridge, U.K.).
- 18.Fitzgerald, C. M. (1998) J. Hum. Evol. 35, 371-386. [DOI] [PubMed] [Google Scholar]
- 19.Bromage, T. G. (1991) Am. J. Phys. Anthropol. 86, 205-214. [Google Scholar]
- 20.Dean, M. C. & Reid, D. J. (2001) in Dental Morphology, ed. Brook, A. (Univ. of Sheffield, Sheffield, U.K.), pp. 135-143.
- 21.Smith, T. M., Reid, D. J., Dean, M. C., Olejniczak, A. J., Ferrell, R. J. &Martin, L. B. (2005) in Dental Perspectives in Human Evolution: State of the Art Research in Dental Anthropology, eds. Bailey, S. & Hublin, J. J. (Springer, Berlin), in press.
- 22.Reid, D. J. & Ferrell, R. J. (2005) J. Hum. Evol., in press. [DOI] [PubMed]
- 23.Dean, M. C. & Reid, D. J. (2001) Am. J. Phys. Anthropol. 116, 209-215. [DOI] [PubMed] [Google Scholar]
- 24.Reid, D. J. & Dean, M. C. (2005) J. Hum. Evol., in press.
- 25.Schwartz, J. H., Brauer, J. & Gordon-Larsen, P. (1995) Am. J. Phys. Anthropol. 97, 77-82. [DOI] [PubMed] [Google Scholar]
- 26.Dunnett, C. W. (1955) J. Am. Stat. Assoc. 50, 1096-1121. [Google Scholar]
- 27.Tompkins, R. L. (1996) Am. J. Phys. Anthropol. 99, 103-118. [DOI] [PubMed] [Google Scholar]
- 28.Wolpoff, M. H. (1979) Am. J. Phys. Anthropol. 50, 67-114. [DOI] [PubMed] [Google Scholar]
- 29.Stringer, C. B., Dean, M. C. & Martin, R. D. (1990) in Primate Life History and Evolution, ed. De Rousseau, C. J. (Wiley–Liss, New York), pp. 115-152.
- 30.Charnov, E. L. (1993) Life History Invariants: Some Explorations of Symmetry in Evolutionary Ecology (Oxford Univ. Press, Oxford).
- 31.Stearns, S. C. (1992) The Evolution of Life Histories (Oxford Univ. Press, Oxford).
- 32.Smith, B. H. (2004) Evol. Anthropol. 13, 239-241. [Google Scholar]
- 33.Leigh, S. R. & Park, P. B. (1998) Am. J. Phys. Anthropol. 107, 331-350. [DOI] [PubMed] [Google Scholar]
- 34.Radovčić, J., Smith, F. H., Trinkaus, E. & Wolpoff, M. H. (1988) The Krapina Hominids: An Illustrated Catalog of the Skeletal Collection (Mladost and Croatian Natural History Museum, Zagreb, Yugoslavia).
- 35.Kelley, J. & Smith, T. M. (2003) J. Hum. Evol. 44, 307-329. [DOI] [PubMed] [Google Scholar]