Abstract
Acute pancreatitis is characterized by the pathologic activation of zymogens within pancreatic acinar cells. The process requires a rise in cytosolic Ca2+ from undefined intracellular stores. We hypothesized that zymogen activation is mediated by ryanodine receptor (RYR)-regulated Ca2+ release, because early zymogen activation takes place in a supranuclear compartment that overlaps in distribution with the RYR. Ca2+ signals in the basolateral, but not apical, region of acinar cells observed during supraphysiologic agonist stimulation were dependent on RYR Ca2+ release. Inhibition of RYR or depletion of RYR-sensitive Ca2+ pools each reduced pathologic zymogen activation in isolated acinar cells, but neither treatment affected amylase secretion. Inhibition of RYR also inhibited zymogen activation in vivo. We propose that Ca2+ release from the RYR mediates zymogen activation but not enzyme secretion. The findings imply a role for the RYR in acute pancreatitis.
Keywords: pancreatic acinar cell, dantrolene, calcium signaling
Acute pancreatitis is a life-threatening inflammatory disease. An early feature is the pathologic activation of zymogens, particularly of trypsinogen and other proteases, within the pancreatic acinar cell. Studies underscore the importance of zymogen activation in the pathogenesis of this disease. For example, pretreatment of rats with protease inhibitors blocks both trypsin activation and injury in the caerulein hyperstimulation model of pancreatitis (1, 2). Clinically, pretreatment with a protease inhibitor reduces procedure-related pancreatitis (3). Mice with a genetic deletion of the lysosomal hydrolase cathepsin B, an enzyme that can activate trypsinogen, have reduced trypsin activity and acinar tissue necrosis after caerulein hyperstimulation (4). Finally, hereditary pancreatitis results from mutations in the cationic trypsinogen gene that may increase trypsin activity (5). Together, such observations support a central role for pathologic intracellular zymogen activation in the pathogenesis of acute pancreatitis.
Enzyme secretion and the intracellular activation of zymogens both require an increase in acinar cell cytosolic Ca2+ [(Ca2+)i], but the intracellular source of this Ca2+ remains unclear (1, 6, 7). In pancreatic acinar cells, two spatially distinct Ca2+ channels mediate the initial release of Ca2+ from stores in the endoplasmic reticulum. The inositol 1,4,5-trisphosphate receptor (IP3R) channel is concentrated in a restricted area close to the apical membrane (8-10). The distribution of the IP3R overlaps with the sites of zymogen granule exocytosis required for enzyme secretion, although the IP3R is more apical than most of the granules (9). The ryanodine receptor (RYR) channel is diffusely distributed in the basolateral region of the acinar cell (11, 12). Early zymogen activation takes place in a supranuclear vesicular compartment that does not overlap with zymogen granules (2, 13). These results suggest that the distribution of pathologically activated zymogens would overlap with RYR, but not IP3R, channels. Based on these relationships, we hypothesized that zymogen activation, but not digestive enzyme secretion, is primarily mediated by release of Ca2+ from a storage pool that is regulated by the RYR. In this study, we used the carbachol- and caerulein-stimulation models of pancreatitis to examine the role of RYR in zymogen activation and amylase secretion.
Materials and Methods
Purification of Trypsinogen Activation Peptide (TAP) Antibody, Preparation of Pancreatic Sections, and Immunofluorescence. Antibodies to the marker of zymogen activation, TAP, were prepared as described in ref. 2. All reagents were purchased from Sigma unless otherwise noted. For detection of IP3R and RYR by immunofluorescence labeling, pancreatic sections were fixed in cold acetone for 5 min. Sections were incubated with either monoclonal anti-RYR antibody 34C (diluted 1:25) or monoclonal anti-IP3R-III antibody (diluted 1:100; Transduction Laboratories, Lexington, KY). Primary antibodies were detected with Alexa 488-conjugated goat anti-mouse secondary antibodies (diluted 1:00; Molecular Probes). Tissue sections for TAP labeling were prepared by in vivo perfusion as described in ref. 2. After labeling with anti-TAP IgG (diluted 1:200), primary antibodies were detected with FITC-labeled goat anti-rabbit IgG F′ab fragments (diluted 1:500). Nuclei were stained with TOPRO-3 (diluted 1:200; Molecular Probes). Specimens were imaged on a Zeiss META LSM 510 laser scanning confocal microscope.
Preparation of Pancreatic Acini. Groups of pancreatic acinar cells, known as acini, were isolated as described in ref. 14. Briefly, fasted male Sprague-Dawley rats weighing 50-100 g (Charles River Laboratories) were killed by using a protocol approved by the Animal Care and Use Committee. The pancreas was minced in buffer containing 40 mM Tris (pH 7.4), 95 mM NaCl, 4.7 mM KCl, 0.6 mM MgCl2, 1.3 mM CaCl2, 1 mM NaH2PO4, 10 mM glucose, and 2 mM glutamine, plus 0.1% BSA, 1× MEM nonessential amino acids (GIBCO/BRL), and 50 units/ml type-4 collagenase (Worthington) and then incubated for 60 min at 37°C.
Stimulation of Acini. After acini were incubated at 37°C for 60 min under constant O2 with shaking (80 rpm), they were pretreated with dantrolene (10-75 μM) or ryanodine (100 μM) to inhibit the RYR. Some preparations were pretreated with either caffeine (10 mM) for 10 min or EGTA (2 mM) for 2 min. Acini then were stimulated with either the muscarinic agonist carbachol (1 μM or 1 mM) or the cholecystokinin (CCK) ortholog caerulein (100 nM) for 60 min in the presence or absence of vasointestinal polypeptide (VIP) (100 nM), secretin (100 nM), or 8-bromo-cAMP (100 μM).
Enzyme Activity Assays. Protease activity assays were performed by using fluorogenic substrates as described in ref. 14. Briefly, enzyme substrate (chymotrypsin or trypsin; Calbiochem) was added to each homogenized sample and read by using 380-nm excitation and 440-nm emission wavelengths. Total amylase values were used for normalization because the enzymatic activity is stable in pancreatic homogenates and the assay is reproducible (Phadebas kit; Amersham Pharmacia Diagnostic, Rochester, NY). Amylase secretion was expressed as the percent of total released into medium.
Detection of Cellular Ca2+ Signals. Ca2+ signals were detected in acinar cells as described in ref. 15. Acini were incubated for 30 min with the medium-affinity Ca2+-sensing dye fluo-5F/AM and in limited experiments mag-fluo-4 (both at 6 μM and from Molecular Probes). These dyes were used because (Ca2+)i signals in acinar cells can exceed 1 μM and saturate high-affinity dyes such as fluo-4 (16). A Bio-Rad MRC-1024 confocal microscope was used with a 63×, 1.4 numerical aperture objective. An argon laser was used to excite the dye at 488-nm wavelength, and emission signals of >515 nm were collected. Signals were recorded at a rate of 2-10 frames/sec with full-screen scanning. Apical and basolateral regions were identified within individual acinar cells, and the fluorescence intensity of the Ca2+ signals in those regions was determined.
In Vivo Caerulein Pancreatitis. Based on pharmacokinetic studies, dantrolene (5 mg/kg) was given as a single tail vein injection to fasted rats (17). After 60 min, an i.p. dose of caerulein (40 μg/kg) was given to induce pancreatic zymogen activation. Control animals received an equivalent volume of normal saline. After another 60 min, rats were euthanized, and pancreatic homogenates were assayed for chymotrypsin and trypsin activities. In other experiments rats were given three additional hourly injections of caerulein, and the parameters of pancreatitis were evaluated 4 h after the first injection.
Statistical Analysis. Data represent means ± SEM of at least three individual experiments unless otherwise noted, with each experiment performed in duplicate. Statistical significance (P < 0.05) was determined by Student's t test analysis. Overlap between TAP, RYR, and IP3R was quantified by examining pixel intensity line scans in immunofluorescence sections. This value was calculated by using the Pearson overlap coefficient r (18).
Results
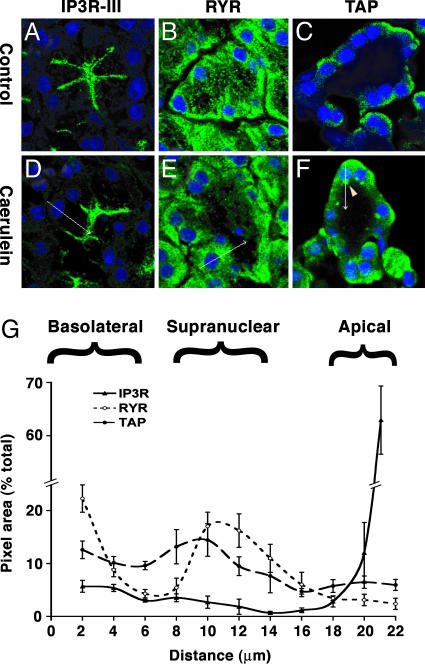
Zymogen Activation Overlaps with RYR but Not with IP3R. The subcellular distribution of the RYR and IP3R was compared with TAP, a marker of zymogen activation that appears early in disease, in pancreatic tissue after saline or 30 min of caerulein treatment in vivo. Because the labeling patterns of all three IP3Rs are similar in the pancreatic acinar cell, labeling for only the IP3R-III was performed (8-10). We have shown that preservation of TAP immunoreactivity requires gluteraldehyde fixation (2). This fixation, however, eliminated signals for RYR or IP3R labeling (data not shown). Thus, to compare TAP with the Ca2+-release channels, we performed single-label immunofluorescence for each molecule and then determined their overlaps within common cellular regions (Fig. 1). To control for the focal plane, we only analyzed images from confocal sections in which the nuclei were maximally sized. TAP labeling appeared as intense supranuclear vesicles after caerulein hyperstimulation (Fig. 1F, arrowhead). The faint, diffuse labeling of TAP in the basolateral regions of some cells was likely nonspecific. The distribution of labeled structures was quantified relative to the distance from the basolateral membrane by using the nucleus (TOPRO-3 stained) as a reference point. Overlap was measured along five parallel line scans in each section oriented basal-to-apical, which evenly dissected the nucleus (Fig. 1 D-F, arrows). IP3R labeling was localized to a discrete apical compartment, whereas RYR was diffusely distributed in the basolateral two-thirds of the cell but was concentrated in the supranuclear region (Fig. 1 A, B, D, and E). In contrast to IP3R, RYR was excluded from the apical region. The findings are consistent with previous localization studies in acinar cells (11, 12, 19). Significant coincidence in the distribution of TAP and RYR was demonstrated by a Pearson's overlap coefficient of 0.78 (± 0.02, SEM) (18). In contrast, the overlap between TAP and IP3R was minimal (coefficient 0.31 ± 0.02). The difference between the two overlap coefficients was statistically significant (P < 0.001). The data show that the cellular distribution of TAP overlaps with the distribution of RYR but not IP3R. Thus, the RYR Ca2+ release pool is in close proximity to the compartment in which zymogens are initially activated. The overlap between IP3R and RYR had a coefficient of 0.21 ± 0.03 (P < 0.0001). The results also demonstrate that the subcellular distribution of Ca2+-release channels is not changed in the early phase (30 min) of caerulein-hyperstimulation pancreatitis.
Fig. 1.
The distribution of TAP overlaps with RYR but not with IP3R. Confocal microscopy images of pancreas sections after 30 min of normal saline (A-C) or caerulein hyperstimulation (D-F) in vivo were labeled for IP3R-III (A and D), RYR (B and E), or TAP (C and F); nuclei were stained blue with TOPRO-3. Basal-to-apical line scans (see arrows in D-F) show that IP3R labeling is apical; RYR is distributed in the basolateral region, concentrated in the supranuclear region but excluded from the apical region. TAP appears as discrete supranuclear structures (arrowhead). (G) Overlap between TAP and RYR, but not IP3R, in the supranuclear region is quantified in five line scans from each section, relative to the distance from the basolateral membrane with the nucleus as a reference point.
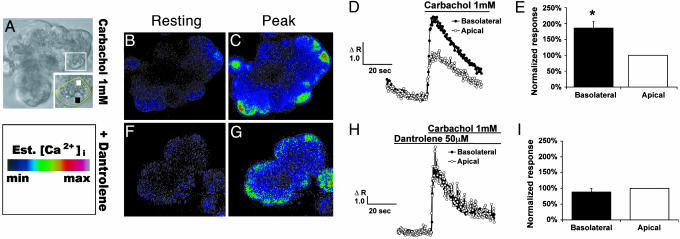
RYR Inhibition Selectively Reduces Basolateral Ca2+ Signals. Because RYR, but not IP3R, is localized to the region of the acinar cell in which trypsinogen becomes activated, we investigated whether the RYR is responsible for the Ca2+ signal required for zymogen activation. Supraphysiologic concentrations of both caerulein and carbachol induce prominent zymogen activation in pancreatic acinar cells (20). Carbachol was used as a representative agonist because it may be most relevant to human disease. Specifically, although rodent and human acinar cells have muscarinic receptors, only rodent acinar cells may have the CCK1 receptor that is linked to pancreatitis (21, 22). Further, muscarinic hyperstimulation is implicated in certain forms of human pancreatitis. Similar to what is described for CCK, both supraphysiologic (1 mM) and physiologic (1 μM) carbachol concentrations evoke a global (Ca2+)i wave that travels along an apical-to-basal axis (20). In addition to this pattern, by using the intermediate sensitivity Ca2+ dye fluo-5F/AM (KCa ∼ 2.3 μM; Fig. 2 B-E) and a lower-affinity Ca2+ dye mag-fluo-4 (KCa ∼ 22 μM), we also observed an enhanced basolateral Ca2+ signal with supraphysiologic carbachol. However, the enhanced signal was not observed using the high-affinity Ca2+ dye fluo-4 (KCa ∼ 0.35 μM), probably because of dye saturation. To inhibit Ca2+ flux through the RYR, acini were preincubated for 1-2 h with the cell-permeant RYR antagonist dantrolene (23). The preincubation time resulted in the maximum inhibitory effect. The advantage of using dantrolene to inhibit the RYR over caffeine-ryanodine pretreatment is that unlike the latter, it does not deplete endoplasmic reticulum Ca2+ stores (24). The concentrations used (10-75 μM) inhibit Ca2+ release from all three RYR isoforms, although there is greater affinity for RYR1 (23, 25). Dantrolene (50 μM) selectively reduced the carbachol (1 mM)-induced Ca2+ signal in the basolateral, but not the apical, region of the acinar cell (Fig. 2). When peak Ca2+ signals were compared in the two regions, dantrolene reduced the basolateral signal relative to the apical signal by 52% (P < 0.001). Dantrolene pretreatment did not change the baseline Ca2+ fluorescence in the apical or basolateral region (Fig. 2 B and F). Thus, dantrolene selectively reduced the Ca2+ signal in the same region of the acinar cell in which trypsinogen is activated. To examine whether extracellular Ca2+ contributes to the basolateral Ca2+ signal, cells were perfused with EGTA (2 mM) before supraphysiologic stimulation. There was no change in the comparison of apical and basolateral peak Ca2+ signals (data not shown). Thus, extracellular Ca2+ does not appear to be responsible for the basolateral Ca2+ signal. The finding is consistent with the observation that the initial agonist-evoked Ca2+ rise in acinar cells comes from intracellular stores and that the subsequent Ca2+ influx is needed to generate a sustained plateau (6).
Fig. 2.
RYR inhibition selectively reduces the Ca2+ peak in the basolateral region induced by carbachol (1 mM). (A) Representative phase image. Inset highlights regions of interest: black box, basolateral; white box, apical. (B and F) Resting Ca2+ fluorescence after cells were loaded with fluo-5F/AM. The images are pseudocolored using the Ca2+ scale. (D) Carbachol (1 mM) induced a higher basolateral than did apical Ca2+ signal. (E) Quantitation of studies from D (n = 17). (H) Carbachol-induced basolateral Ca2+ signal is selectively reduced by dantrolene (50 M). (I) Quantitation of studies from H (n = 6). *, P < 0.001 for basolateral region peak Ca2+ response, compared with the apical region in carbachol-alone condition.
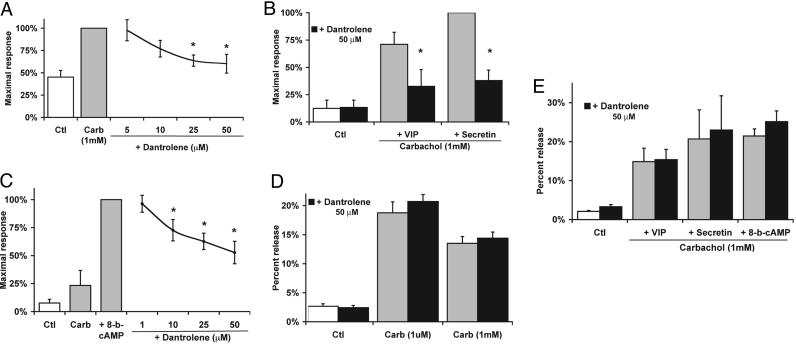
RYR Inhibition Reduces Intracellular Zymogen Activation. To examine the effects of RYR inhibition on zymogen activation, isolated acini were preincubated with dantrolene. Zymogen activation was induced by supraphysiologic carbachol (1 mM) (Fig. 3) or caerulein (100 nM) (Table 1). Assays of chymotrypsin rather than trypsin activity were measured in these cell studies because the generation of chymotrypsin more closely follows first-order kinetics over 60 min and is a more sensitive marker of zymogen activation than trypsin after 30 min of treatment (26). Dantrolene caused a concentration-dependent reduction in supraphysiologic carbachol-induced chymotrypsin activity by up to 40% (Fig. 3A). In preliminary studies, the trends for trypsin responses were similar to chymotrypsin. Because the pancreatic acinar cell has receptors that activate cAMP pathways, the effects of these ligands in combination with muscarinic stimulation were examined. We have shown that when carbachol-stimulated acini are cotreated with the cAMP agonists VIP or secretin, zymogen activation is enhanced (14, 27). We found that RYR inhibition by dantrolene (50 μM) reduced VIP- and secretin-enhanced chymotrypsin activity by 54% and 62%, respectively (Fig. 3B; P < 0.05). A similar effect was seen when cAMP levels were directly increased by 8-bromo-cAMP (Fig. 3C). Although the mechanism responsible for cAMP sensitization to zymogen activation is unclear, it also appears to be inhibited by dantrolene.
Fig. 3.
RYR inhibition reduces carbachol-induced zymogen activation but not enzyme secretion. (A) Dantrolene caused a concentration-dependent decrease in chymotrypsin activity induced by carb(achol) (1 mM). (B) Dantrolene (50 μM) reduced carbachol-induced chymotrypsin activity enhanced by VIP (100 nM) and secretin (100 nM). (C) Dantrolene caused a concentration-dependent reduction in chymotrypsin activity enhanced by 8-bromo-cAMP (100 μM). (D and E) Dantrolene did not affect carbachol-induced amylase secretion (D) with or without cAMP agonists (E) (n = 3-4). *, P < 0.05 with respect to the maximal response. Ctl, control.
Table 1. Caerulein responses in acinar cells.
| Zymogen activation: chymotrypin, %
|
Enzyme secretion: amylase, %
|
|||
|---|---|---|---|---|
| Treatment | − | + | − | + |
| Dantrolene (75 μM) | ||||
| Control | 14.4 ± 6.4 | 11.4 ± 4.9 | 3.4 ± 0.9 | 2.9 ± 0.6 |
| Caer (100 nM) | 71.5 ± 6.3 | 49.1 ± 4.3 | 10.4 ± 2.6 | 9.6 ± 2.5 |
| Caer + VIP | 100 | 74.0 ± 4.9 | 11.4 ± 2.1 | 11.4 ± 2.6 |
| Caer + 8-b-cAMP | 265.1 ± 20.6 | 79.2 ± 2.2 | 15.1 ± 1.5 | 15.0 ± 2.5 |
| Ryanodine (100 μM) | ||||
| Control | 2.6 ± 2.4 | 3.0 ± 3.0 | 2.1 ± 0.7 | 2.9 ± 1.8 |
| Caer (100 nM) | 100 | 62.2 ± 6.4 | 9.6 ± 0.5 | 9.5 ± 0.4 |
| Caffeine (10 mM) | ||||
| Control | 2.1 ± 0.7 | 4.0 ± 1.3 | 4.5 ± 0.9 | 5.3 ± 0.4 |
| Caer (100 nM) | 100 | 36.4 ± 12.1 | 12.5 ± 2.1 | 12.3 ± 2.2 |
Values are ±SD. Caer, caerulein; 8-b-cAMP, 8-bromo-cAMP. *, P < 0.01.
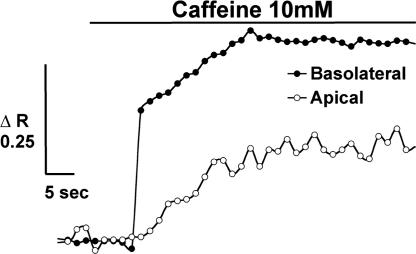
Because zymogen activation and secretion can be uncoupled in the pancreatic acinar cell and during pancreatitis, the effects of RYR inhibition on both responses were assayed. Dantrolene had no effect on either basal amylase secretion or that stimulated by physiologic (1 μM) or supraphysiologic (1 mM) carbachol (Fig. 3D). Dantrolene also lacked an effect when carbachol-induced amylase secretion was enhanced by VIP, secretin, or 8-bromo-cAMP (Fig. 3E). To ensure that RYR inhibition was not specific to carbachol, acini were treated with the CCK ortholog caerulein (Table 1). Dantrolene decreased caerulein-induced chymotrypsin activity without affecting amylase secretion (Table 1, dantrolene treatment). To confirm dantrolene's specificity, another RYR antagonist, ryanodine (100 μM), was used with caerulein (28). Ryanodine reduced chymotrypsin activity by 38% (P < 0.01) without affecting amylase secretion (Table 1, ryanodine treatment). To confirm that dantrolene and ryanodine are acting on RYR Ca2+ stores in the acinar cell, RYR-sensitive Ca2+ pools alternatively were depleted by using caffeine (10 mM) before caerulein stimulation. Previous studies have shown that this method of depletion is specific for RYR-sensitive Ca2+ pools (29). We found that perfusion of acinar cells with caffeine (10 mM) resulted in selective release of Ca2+ from the basolateral region (Fig. 4). A delayed apical rise also was seen and likely corresponds to diffusion of Ca2+ from the basolateral region. These findings are consistent with the basolateral distribution of RYR and reports of basolateral Ca2+ release induced by the RYR agonist cADP ribose (15). Caffeine pretreatment reduced caerulein-induced chymotrypsin activity by 64% (P < 0.01) without affecting amylase secretion (Table 1, caffeine treatment). Together these findings provide evidence that RYR-mediated Ca2+ release selectively modulates intracellular zymogen activation but not enzyme secretion.
Fig. 4.
Caffeine (10 mM) selectively releases Ca2+ from the basolateral region. Representative (Ca2+)i tracing from an acinar cell shows basolateral and apical regions upon caffeine stimulation (n = 9).
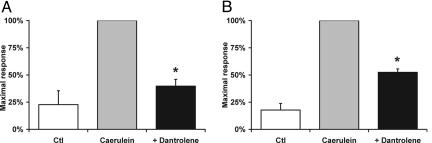
RYR Ca2+ Release Contributes to Early Zymogen Activation in Vivo. To examine the role of RYR Ca2+ release in vivo, rats were pretreated with dantrolene and then subjected to caerulein hyperstimulation for 1 h to induce zymogen activation. Dantrolene pretreatment reduced chymotrypsin and trypsin activities by 60% and 48%, respectively (Fig. 5; P < 0.05). Although this early time point is associated with intrapancreatic zymogen activation, it precedes the onset of extrapancreatic events such as neutrophil infiltration, which can affect zymogen activation in a later phase of pancreatitis (30, 31). These findings suggest that RYR Ca2+ release also regulates the early pathologic activation of pancreatic zymogens in vivo. However, 4 h after caerulein, dantrolene did not affect zymogen activation or other parameters of acute pancreatitis including edema, inflammation, cell vacuolization, or necrosis (data not shown).
Fig. 5.
RYR inhibition reduces zymogen activation induced by in vivo caerulein hyperstimulation. Chymotrypsin (A) and trypsin (B) activities are shown for rats injected with either normal saline control (Ctl), caerulein hyperstimulation alone, or with dantrolene pretreatment (n = 3). *, P < 0.05 with respect to the maximal response.
Discussion
Our data show that in pancreatic acinar cells, the normal RYR distribution in the basolateral and IP3R distribution in the apical region are unchanged during the early phase of pancreatitis. It is during this early phase that generation of TAP within discrete cytoplasmic organelles is initially detected. Based on the overlap between TAP and RYR compartments and the Ca2+-dependence of zymogen activation, we hypothesized that RYR-regulated Ca2+ release could modulate pathologic zymogen activation. We found that in both a cellular and animal model of acute pancreatitis, RYR inhibition reduced pathologic zymogen activation within acinar cells but did not affect enzyme secretion. Thus, Ca2+ release through the RYR appears to be selectively coupled to intracellular zymogen activation, but not secretion, in the acinar cell.
In this study, a distinct pattern of (Ca2+)i signaling was induced by supraphysiologic muscarinic receptor agonist stimulation. Studies in acinar cells stimulated with subphysiologic (nanomolar) carbachol concentrations have observed localized oscillations in a subcompartment of the apical region termed the trigger zone (32). These apical oscillations are sensitive to IP3R blockade (33). Confinement of these Ca2+ signals has been attributed to buffering of Ca2+ by apically distributed mitochondria (34). In contrast, physiologic (micromolar) carbachol concentrations initiate a Ca2+ wave that begins in the apical trigger zone and travels basolaterally (35-37). This global Ca2+ wave is induced by sequential activation of apical IP3R followed by basolateral RYR (15). We and others have observed that the peak level of this (Ca2+)i response is equivalent throughout the cell. By using a low-affinity dye to avoid saturation by high (Ca2+)i levels, it has been observed that maximum (Ca2+)i peaks induced by physiologic stimulation can reach 5-10 μM in the apical region (16). Because (Ca2+)i is much higher with a supraphysiologic than physiologic concentration of carbachol, we used a similar low-affinity Ca2+ dye to show that high (mM) carbachol concentrations cause an apical-to-basal Ca2+ wave. This treatment elicits a much higher Ca2+ peak in the basolateral region of the acinar cell than the apical region, thus underscoring the need to use Ca2+ dye sensors that do not become saturated. Notably, the difference in the peak Ca2+ levels between the two domains was eliminated by dantrolene (Fig. 1 E and I). Although RYR2 is the principal isoform in pancreatic acinar cells (12), these cells also may express RYR1 and RYR3 (11). Because the concentrations of dantrolene used in this study inhibit all three RYR isoforms, the target of this response is unclear. Together, these findings suggest that conditions that induce pancreatitis selectively increase (Ca2+)i in the basolateral, compared with the apical region of the acinar cell. RYR Ca2+ release is largely responsible for this distinct intracellular pattern of Ca2+ signaling.
The distinct spatial patterns of Ca2+ signaling are relevant to both pancreatic enzyme secretion and pathologic zymogen activation. Physiologic agonist stimulation is associated with maximal enzyme secretion and minimal zymogen activation; supraphysiologic stimulation reduces secretion and causes pathologic zymogen activation (38). Inhibiting RYR Ca2+ release during physiologic stimulation has been shown to diminish the basolateral component of the Ca2+ wave but does not affect its capacity to elicit maximal enzyme secretion (36). In our studies, inhibiting RYR Ca2+ release during supraphysiologic stimulation diminished the Ca2+ peak in the basolateral region but did not significantly change the apical Ca2+ peak (Fig. 2I). Furthermore, RYR inhibition reduced pathologic zymogen activation but did not affect secretion (Fig. 3 D and E and Table 1). Together, these data suggest that RYR Ca2+ release mediates zymogen activation but not secretion.
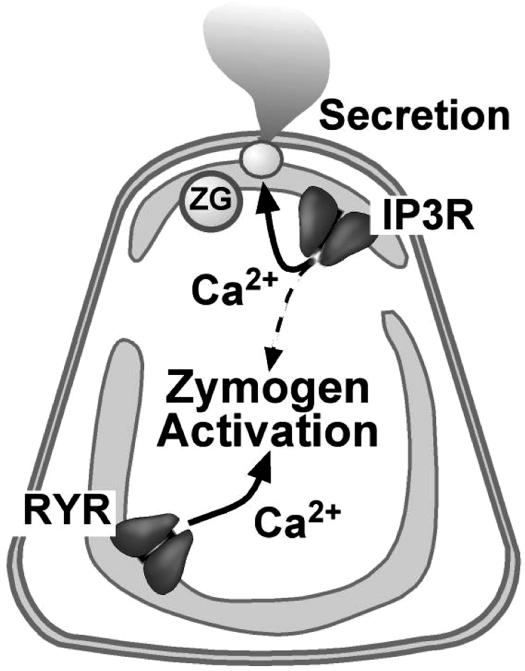
RYR inhibition by dantrolene, ryanodine, or depletion of RYR-sensitive Ca2+ stores by caffeine incompletely inhibited zymogen activation. Further, dantrolene reduced the peak Ca2+ signal in the basolateral region but did not eliminate it. There are several explanations for these results. The effects on RYR inhibition or RYR Ca2+ pool depletion may have been incomplete. Alternatively, RYR-independent pathways, such as those mediated by the IP3R, may contribute to the Ca2+ signal in the basolateral region that regulates zymogen activation (Fig. 6).
Fig. 6.
Model for the regulation of subcellular Ca2+ pools by RYR and IP3R in pancreatic acinar cells. Pathologic zymogen activation is primarily mediated by Ca2+ release from RYR-regulated pools. Pancreatic acinar cell secretion is mediated by Ca2+ release through IP3R, but not RYR. Note that some Ca2+ released through IP3R or other mechanisms may contribute to zymogen activation. ZG, zymogen granule.
The finding that RYR Ca2+ release modulates zymogen activation needs further study in other models of pancreatitis. It is intriguing, for example, that recent data suggest that pancreatic duct obstruction, a cause of pancreatitis, affects acinar cell Ca2+ signaling (39). We found that the premature zymogen activation was inhibited by dantrolene pretreatment for 1 h, but not 4 h, after caerulein hyperstimulation in vivo. In addition, edema and histological pancreatitis severity were not affected. The lack of a dantrolene effect on longer durations of pancreatitis might be due to pharmacologic factors that reduce RYR inhibition over time. Furthermore, IP3R-regulated Ca2+ release may independently contribute by permitting a threshold amount of zymogen activation required to cause pancreatitis.
The findings of this study raise issues relating to both the physiologic and pathologic importance of RYR in acinar cells. In this context, we have observed that even physiologic CCK concentrations cause a modest increase in zymogen activation (40). However, unlike supraphysiologic treatment, during physiologic treatment the active enzymes are secreted from the cells (41). One possible role for secreting active enzymes under physiologic conditions may be to coordinate enzyme secretion with fluid secretion in a paracrine fashion, possibly by activating protease-activated receptors such as PAR-2 on duct and acinar cells (42). Thus, Ca2+ release by RYR may regulate zymogen activation under both physiologic and pathologic conditions.
Although aberrant Ca2+ signaling is a central feature of acute pancreatitis (43), this work reports a previously undescribed link between RYR and pancreatitis. RYR dysfunction, often caused by RYR mutations, is associated with disordered channel function and disease. Mutations in RYR1, the major isoform expressed in skeletal muscle, lead to abnormal (Ca2+)i release and cause malignant hyperthermia and central core disease (44). Notably, dantrolene is used as first-line therapy for malignant hyperthermia (23). Mutations in RYR2, the major isoform expressed in heart, increase channel activity during exercise and are associated with cardiac arrhythmia (45). Polymorphisms in RYR that affect its function could potentially contribute to the pathogenesis of acute pancreatitis. Furthermore, some forms of pancreatitis, such as that caused by ethanol, seem to be initiated by factors that sensitize the pancreas to harmful stimuli (26, 46). Factors that enhance the functioning of RYR might contribute to such sensitization. For example, two that sensitize acinar cells to zymogen activation, increased cellular cAMP (14, 27) or free fatty acids (47), also increase RYR channel opening (48, 49). Further understanding of the factors causing RYR-mediated zymogen activation may help elucidate the mechanisms causing pancreatitis.
Acknowledgments
We thank T. Gniadek, D. Gomes, A. Shah, D. Jain, and C. Shugrue for technical advice and B. Ehrlich and E. Thrower for critical reading of the manuscript. This work was supported by National Institutes of Health Grants T32 DK07017, KO8 DK68116, and K12 HD001401 (to S.Z.H.), DK54021 (to F.S.G.), and DK45710, TW01451, and DK34989 (to M.H.N.); an American Gastroenterological Association AstraZeneca Award (to S.Z.H.); a Veterans Administration Merit Grant (to F.S.G.); and a Grant-in-Aid from the American Heart Association (to M.H.N.).
Author contributions: S.Z.H., M.H.N., and F.S.G. designed research; S.Z.H., P.P., W.M.G., and T.R.K. performed research; S.Z.H., P.P., W.M.G., T.R.K., M.H.N., and F.S.G. analyzed data; and S.Z.H., W.M.G., M.H.N., and F.S.G. wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: (Ca2+)i, cytosolic Ca2+; RYR, ryanodine receptor; IP3R, inositol 1,4,5-trisphosphate receptor; CCK, cholecystokinin; TAP, trypsinogen activation peptide; VIP, vasointestinal polypeptide.
References
- 1.Saluja, A. K., Bhagat, L., Lee, H. S., Bhatia, M., Frossard, J. L. & Steer, M. L. (1999) Am. J. Physiol. 276, G835-G842. [DOI] [PubMed] [Google Scholar]
- 2.Otani, T., Chepilko, S. M., Grendell, J. H. & Gorelick, F. S. (1998) Am. J. Physiol. 275, G999-G1009. [DOI] [PubMed] [Google Scholar]
- 3.Cavallini, G., Tittobello, A., Frulloni, L., Masci, E., Mariana, A. & Di Francesco, V. (1996) N. Engl. J. Med. 335, 919-923. [DOI] [PubMed] [Google Scholar]
- 4.Halangk, W., Lerch, M. M., Brandt-Nedelev, B., Roth, W., Ruthenbuerger, M., Reinheckel, T., Domschke, W., Lippert, H., Peters, C. & Deussing, J. (2000) J. Clin. Invest. 106, 773-781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Whitcomb, D. C., Gorry, M. C., Preston, R. A., Furey, W., Sossenheimer, M. J., Ulrich, C. D., Martin, S. P., Gates, L. K., Jr., Amann, S. T., Toskes, P. P., et al. (1996) Nat. Genet 14, 141-145. [DOI] [PubMed] [Google Scholar]
- 6.Raraty, M., Ward, J., Erdemli, G., Vaillant, C., Neoptolemos, J. P., Sutton, R. & Petersen, O. H. (2000) Proc. Natl. Acad. Sci. USA 97, 13126-13131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kruger, B., Albrecht, E. & Lerch, M. M. (2000) Am. J. Pathol. 157, 43-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee, M. G., Xu, X., Zeng, W., Diaz, J., Wojcikiewicz, R. J., Kuo, T. H., Wuytack, F., Racymaekers, L. & Muallem, S. (1997) J. Biol. Chem. 272, 15765-15770. [DOI] [PubMed] [Google Scholar]
- 9.Nathanson, M. H., Fallon, M. B., Padfield, P. J. & Maranto, A. R. (1994) J. Biol. Chem. 269, 4693-4696. [PubMed] [Google Scholar]
- 10.Yule, D. I., Ernst, S. A., Ohnishi, H. & Wojcikiewicz, R. J. (1997) J. Biol. Chem. 272, 9093-9098. [DOI] [PubMed] [Google Scholar]
- 11.Fitzsimmons, T. J., Gukovsky, I., McRoberts, J. A., Rodriguez, E., Lai, F. A. & Pandol, S. J. (2000) Biochem. J. 351, 265-271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leite, M. F., Dranoff, J. A., Gao, L. & Nathanson, M. H. (1999) Biochem. J. 337, 305-309. [PMC free article] [PubMed] [Google Scholar]
- 13.Hofbauer, B., Saluja, A. K., Lerch, M. M., Bhagat, L., Bhatia, M., Lee, H. S., Frossard, J. L., Adler, G. & Steer, M. L. (1998) Am. J. Physiol. 275, G352-G362. [DOI] [PubMed] [Google Scholar]
- 14.Lu, Z., Kolodecik, T. R., Karne, S., Nyce, M. & Gorelick, F. (2003) Am. J. Physiol. 285, G822-G828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leite, M. F., Burgstahler, A. D. & Nathanson, M. H. (2002) Gastroenterology 122, 415-427. [DOI] [PubMed] [Google Scholar]
- 16.Ito, K., Miyashita, Y. & Kasai, H. (1997) EMBO J. 16, 242-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Flewellen, E. H., Nelson, T. E., Jones, W. P., Arens, J. F. & Wagner, D. L. (1983) Anesthesiology 59, 275-280. [DOI] [PubMed] [Google Scholar]
- 18.Manders, E. M. M., Verbeek, F. J. & Aten, J. A. (1993) J. Microsc. (Oxford) 169, 375-382. [DOI] [PubMed] [Google Scholar]
- 19.Wojcikiewicz, R. J., Ernst, S. A. & Yule, D. I. (1999) Gastroenterology 116, 1194-1201. [DOI] [PubMed] [Google Scholar]
- 20.Williams, J. A. (2002) Curr. Opin. Gastroenterol. 18, 529-535. [DOI] [PubMed] [Google Scholar]
- 21.Ji, B., Bi, Y., Simeone, D., Mortensen, R. M. & Logsdon, C. D. (2001) Gastroenterology 121, 1380-1390. [DOI] [PubMed] [Google Scholar]
- 22.Reubi, J. C., Waser, B., Gugger, M., Friess, H., Kleeff, J., Kayed, H., Buchler, M. W. & Laissue, J. A. (2003) Gastroenterology 125, 98-106. [DOI] [PubMed] [Google Scholar]
- 23.Krause, T., Gerbershagen, M. U., Fiege, M., Weisshorn, R. & Wappler, F. (2004) Anaesthesia 59, 364-373. [DOI] [PubMed] [Google Scholar]
- 24.Johenning, F. W., Zochowski, M., Conway, S. J., Holmes, A. B., Koulen, P. & Ehrlich, B. E. (2002) J. Neurosci. 22, 5344-5353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu, G., Zucchi, R., Ronca-Testoni, S. & Ronca, G. (2000) Basic Res. Cardiol. 95, 137-143. [DOI] [PubMed] [Google Scholar]
- 26.Lu, Z., Karne, S., Kolodecik, T. & Gorelick, F. S. (2002) Am. J. Physiol. 282, G501-G507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chaudhuri, A., Kolodecik, T. R. & Gorelick, F. S. (2005) Am. J. Physiol. 288, G235-G243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ashby, M. C., Petersen, O. H. & Tepikin, A. V. (2003) Biochem. J. 369, 441-445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sasajima, H., Wang, X. & van Breemen, C. (1997) Biochem. Biophys. Res. Commun. 241, 471-475. [DOI] [PubMed] [Google Scholar]
- 30.Grady, T., Saluja, A., Kaiser, A. & Steer, M. (1996) Am. J. Physiol. 271, G20-G26. [DOI] [PubMed] [Google Scholar]
- 31.Gukovskaya, A. S., Vaquero, E., Zaninovic, V., Gorelick, F. S., Lusis, A. J., Brennan, M. L., Holland, S. & Pandol, S. J. (2002) Gastroenterology 122, 974-984. [DOI] [PubMed] [Google Scholar]
- 32.Kasai, H., Li, Y. X. & Miyashita, Y. (1993) Cell 74, 669-677. [DOI] [PubMed] [Google Scholar]
- 33.Wakui, M., Osipchuk, Y. V. & Petersen, O. H. (1990) Cell 63, 1025-1032. [DOI] [PubMed] [Google Scholar]
- 34.Straub, S. V., Giovannucci, D. R. & Yule, D. I. (2000) J. Gen. Physiol. 116, 547-560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kasai, H. & Augustine, G. J. (1990) Nature 348, 735-738. [DOI] [PubMed] [Google Scholar]
- 36.Nathanson, M. H., Padfield, P. J., O'Sullivan, A. J., Burgstahler, A. D. & Jamieson, J. D. (1992) J. Biol. Chem. 267, 18118-18121. [PubMed] [Google Scholar]
- 37.Toescu, E. C., Lawrie, A. M., Petersen, O. H. & Gallacher, D. V. (1992) EMBO J. 11, 1623-1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schmid, S. W., Modlin, I. M., Tang, L. H., Stoch, A., Rhee, S., Nathanson, M. H., Scheele, G. A. & Gorelick, F. S. (1998) Am. J. Physiol. 274, G734-G41. [DOI] [PubMed] [Google Scholar]
- 39.Mooren, F., Hlouschek, V., Finkes, T., Turi, S., Weber, I. A., Singh, J., Domschke, W., Schnekenburger, J., Kruger, B. & Lerch, M. M. (2003) J. Biol. Chem. 278, 9361-9369. [DOI] [PubMed] [Google Scholar]
- 40.Leach, S. D., Modlin, I. M., Scheele, G. A. & Gorelick, F. S. (1991) J. Clin. Invest. 87, 362-366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grady, T., Mah'Moud, M., Otani, T., Rhee, S., Lerch, M. M. & Gorelick, F. S. (1998) Am. J. Physiol. 275, G1010-G1017. [DOI] [PubMed] [Google Scholar]
- 42.Nguyen, T. D., Moody, M. W., Steinhoff, M., Okolo, C., Koh, D. S. & Bunnett, N. W. (1999) J. Clin. Invest. 103, 261-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sutton, R., Criddle, D., Raraty, M. G., Tepikin, A., Neoptolemos, J. P. & Petersen, O. H. (2003) Pancreatology 3, 497-505. [DOI] [PubMed] [Google Scholar]
- 44.Melzer, W. & Dietze, B. (2001) Acta. Physiol. Scand. 171, 367-378. [DOI] [PubMed] [Google Scholar]
- 45.Wehrens, X. H., Lehnart, S. E., Huang, F., Vest, J. A., Reiken, S. R., Mohler, P. J., Sun, J., Guatimosim, S., Song, L. S., Rosemblit, N., et al. (2003) Cell 113, 829-840. [DOI] [PubMed] [Google Scholar]
- 46.Pandol, S. J., Periskic, S., Gukovsky, I., Zaninovic, V., Jung, Y., Zong, Y., Solomon, T. E., Gukovskaya, A. S. & Tsukamoto, H. (1999) Gastroenterology 117, 706-716. [DOI] [PubMed] [Google Scholar]
- 47.Werner, J., Saghir, M., Warshaw, A. L., Lewandrowski, K. B., Laposata, M., Iozzo, R. V., Carter, E. A., Schatz, R. J. & Fernandez-Del Castillo, C. (2002) Am. J. Physiol. 283, G65-G73. [DOI] [PubMed] [Google Scholar]
- 48.Fill, M. & Copello, J. A. (2002) Physiol. Rev. 82, 893-922. [DOI] [PubMed] [Google Scholar]
- 49.Fitzsimmons, T. J., McRoberts, J. A., Tachiki, K. H. & Pandol, S. J. (1997) J. Biol. Chem. 272, 31435-31440. [DOI] [PubMed] [Google Scholar]