Abstract
Hutchinson-Gilford progeria syndrome (HGPS) is a devastating premature aging disease resulting from a mutation in the LMNA gene, which encodes nuclear lamins A and C. Lamin A is synthesized as a precursor (prelamin A) with a C-terminal CaaX motif that undergoes farnesylation, endoproteolytic cleavage, and carboxylmethylation. Prelamin A is subsequently internally cleaved by the zinc metalloprotease Ste24 (Zmpste24) protease, which removes the 15 C-terminal amino acids, including the CaaX modifications, to yield mature lamin A. HGPS results from a dominant mutant form of prelamin A (progerin) that has an internal deletion of 50 aa near the C terminus that includes the Zmpste24 cleavage site and blocks removal of the CaaX-modified C terminus. Fibroblasts from HGPS patients have aberrant nuclei with irregular shapes, which we hypothesize result from the abnormal persistence of the farnesyl and/or carboxylmethyl CaaX modifications on progerin. If this hypothesis is correct, inhibition of CaaX modification by mutation or pharmacological treatment should alleviate the nuclear morphology defect. Consistent with our hypothesis, we find that expression in HeLa cells of GFP-progerin or an uncleavable form of prelamin A with a Zmpste24 cleavage site mutation induces the formation of abnormal nuclei similar to those in HGPS fibroblasts. Strikingly, inhibition of farnesylation pharmacologically with the farnesyl transferase inhibitor rac-R115777 or mutationally by alteration of the CaaX motif dramatically reverses the abnormal nuclear morphology. These results suggest that farnesyl transferase inhibitors represent a possible therapeutic option for individuals with HGPS and/or other laminopathies due to Zmpste24 processing defects.
Keywords: aging, posttranslational processing, laminopathy, Ste24p, Zarnestra
Hutchinson-Gilford progeria syndrome (HGPS) is a rare genetic disorder resulting in accelerated aging (1). Children with this condition generally appear normal at birth, but within one to two years they begin to exhibit profound phenotypes characteristic of premature aging, including bone defects and alopecia. The predominant cause of death is artherosclerosis at around age 13. The mutation responsible for HGPS was recently mapped to the LMNA gene, which encodes lamin A and the splice variant lamin C (2, 3). These proteins, together with lamin B, form the nuclear lamina, a protein meshwork underlying the inner nuclear envelope that helps to maintain nuclear architecture and that interacts with chromatin and regulators of transcription (4, 5).
Lamin A is generated from a precursor, prelamin A, that terminates with a C-terminal CaaX motif (C, cysteine; a, aliphatic amino acids; X, a variety of amino acids). Prelamin A undergoes a characteristic series of posttranslational modifications typical of CaaX proteins (6). First, the cysteine side chain is farnesylated; second, the three terminal amino acids are removed by an endoproteolytic cleavage (“aaXing”); and third, the farnesyl cysteine is carboxylmethylated (Fig. 1A, steps 1-3) (7). A distinctive feature of lamin A biogenesis is that, upon completion of CaaX processing, a second proteolytic processing event between Y646 and L647 mediated by zinc metalloprotease Ste24 (Zmpste24) removes the C-terminal 15 aa, including the farnesyl and carboxylmethyl CaaX modifications (Fig. 1A, step 4) (8-10). Zmpste24 is a protease in the endoplasmic reticulum membrane and was first identified in budding yeast, where it catalyzes aaXing (in which it is functionally redundant with Rce1) and an internal cleavage in the a-factor mating pheromone, as also occurs in prelamin A (11-13). Lamin B, like lamin A, undergoes CaaX modifications; however, notably, lamin B is not internally cleaved. Lamin C does not contain a CaaX motif and is neither CaaX-modified nor cleaved.
Fig. 1.
Model for the posttranslational C-terminal CaaX processing of prelamin A in normal and disease states. (A) WT prelamin A processing. WT prelamin A undergoes posttranslational CaaX modifications [farnesylation (step 1), proteolysis of the SIM residues (step 2), and carboxylmethylation (step 3)] followed by Zmpste24-mediated cleavage that removes the C-terminal 15 aa including the CaaX modifications (gray box) to generate mature lamin A (white box) (step 4). (B) Progerin processing in HGPS. The HGPS mutation results in a 50-aa deletion within prelamin A (Δ607-656) to generate progerin, which is expected to undergo C-terminal CaaX processing (steps 1-3) like prelamin A. However, because the Zmpste24 cleavage site is deleted in progerin, the modified C terminus persists (i.e., step 4 is blocked). (C) Prelamin A processing in Zmpste24 mutant. Zmpste24 activity is absent in the Zmpste24 knockout mouse and in mandibuloacryl dysplasia or restrictive dermopathy patients with mutations in Zmpste24. Prelamin A is expected to undergo normal C-terminal CaaX processing but aberrantly retain its CaaX modifications because of the absence of Zmpste24 activity. In this study, GFP-lamin A-UC (mutated in the Zmpste24 cleavage site) is used to mimic the uncleaved prelamin A resulting from Zmpste24 mutations. *, Zmpste24 cleavage site; CSIM, CaaX motif of prelamin A.
Many diseases are associated with mutations in the LMNA gene and are collectively known as laminopathies (14, 15). The most common LMNA mutation associated with HGPS is a de novo mutation that activates a cryptic splice site in exon 11, leading to an internal deletion of 50 codons that includes the Zmpste24 processing site but leaves the CaaX motif intact (2, 3). Because the internal processing site is absent, this mutant protein, referred to as progerin, is expected to retain its CaaX modifications (Fig. 1B). At the cellular level, the most striking phenotype associated with HGPS is irregularly shaped nuclei that display blebs, folds, and herniations in the nuclear envelope (16). Progerin is proposed to have a dominant-negative function, because individuals with HGPS still have a WT copy of the LMNA gene and overexpression of WT lamin A in HGPS fibroblasts does not reverse the phenotype (2, 17). In principle, the HGPS phenotypes could result from the internal deletion of 50 aa in progerin, from the retention of the CaaX modifications, or both. Interestingly, similar nuclear morphology defects are observed in mice deleted for Zmpste24, in which prelamin A retains its CaaX modifications because it is not internally cleaved (Fig. 1C) (8, 9). Here, we propose that the aberrant retention of the CaaX modifications (farnesyl and/or carboxylmethyl) on progerin is the principal cause of the dominant-negative phenotype in HGPS.
In this study, we sought to determine whether the effect of progerin on nuclear morphology is indeed due to the persistence of its CaaX modifications and whether blocking the CaaX modifications of progerin, either mutationally or pharmacologically, would prevent the appearance of HGPS phenotypes at the cellular level, namely the defects in nuclear morphology. We generated a HeLa cell model to study the effects on nuclear morphology of WT lamin A, progerin, and an uncleavable form of prelamin A (UC) mutated within the Zmpste24 cleavage site. Unlike the WT protein, progerin and UC are expected to retain their CaaX modifications because of the missing or mutated Zmpste24 cleavage site, respectively. Indeed, both proteins induced aberrant nuclear morphology similar to that observed in HGPS fibroblasts. Notably, when the CaaX motifs of progerin and UC were mutated to prevent modification (CaaX→SaaX), the nuclear morphology was normal, supporting the hypothesis that progerin retains its CaaX modifications and that this persistence is responsible for the dominant-negative phenotype. This striking result led us to examine whether farnesyl transferase inhibitors (FTIs) would likewise be effective in preventing the cellular phenotypes associated with expression of progerin. Excitingly, FTI treatment prevented the progerin phenotype, suggesting the possibility that these drugs could be an effective therapy in ameliorating the symptoms associated with HGPS.
Materials and Methods
DNA Manipulations. GFP-prelamin A (GFP-lamin A-WT) was constructed by fusing the GFP coding sequence (pQBI25, Quantum, Durham, NC) to the 5′ end of the prelamin A cDNA (gift from M. Sinensky, East Tennessee State University, Johnson City) by recombination in yeast, generating pSM1961. To generate GFP-progerin, 150 nt corresponding to the 50 codons absent in progerin (607-656 inclusive) were deleted in pSM1961 by recombinational cloning in yeast. GFP-uncleavable prelamin A (GFP-lamin A-UC), generated by recombinational cloning in pSM1961, introduces a point mutation in the Zmpste24 cleavage site of prelamin A (L647R). All constructs were subcloned into the mammalian expression vector pcDNA3.1 (Invitrogen) by using BamHI and XhoI. The C→S mutation (C661S) was introduced into all three constructs (WT, progerin, and UC) (QuickChange XLII mutagenesis kit, Stratagene) and verified by sequencing. Constructs used in this study contain a point mutation (L367P) to enhance the visual difference between forms of lamin A with and without the CaaX modifications.
Cell Culture, FTI Treatment, and Microscopy. HeLa cells were grown on coverslips in 6-well dishes in DMEM plus 10% FBS supplemented with penicillin and streptomycin. GFP-tagged constructs were transiently transfected with FuGene (Roche Molecular Biochemicals).
The racemic form of the FTI R115777, denoted rac-R115777, was synthesized as described in ref. 18. The compound was purified to homogeneity by HPLC on a reversed-phase column and the correct structure was confirmed by proton NMR and MS (data not shown). For FTI treatment of HeLa cells, rac-R115777 was added from a 20 mM stock solution in DMSO to cells 24 h after transfection.
Cells expressing the GFP-lamin A constructs were examined by fluorescence microscopy 48 h after transfection at ×40 magnification with a Zeiss Axioscope microscope. For quantitation of abnormal nuclear morphology and nuclear envelope localization, ≈150-300 HeLa cell nuclei were examined in two independent experiments, and results are expressed graphically as the average percentage of the total nuclei counted. For nuclear envelope localization, nuclei were scored as positive if there was enhanced staining at the nuclear envelope compared with the nucleoplasm. Lamina-associated polypeptide 2 (LAP2) was visualized by indirect immunofluorescence using anti-LAP2 antibody (gift of K. Wilson, Johns Hopkins University School of Medicine) and Cy3-conjugated secondary antibody.
Western Blotting. HeLa cell extracts were prepared by harvesting cells in SDS sample buffer. Proteins were separated on 4-15% gradient SDS/PAGE gels and transferred onto nitrocellulose membranes. Antibodies used were mouse anti-lamin A/C (1:50) (Chemicon), goat anti-prelamin A (1:400) (Santa Cruz Biotechnology), mouse anti-HDJ-2 (1:400) (NeoMarkers, Fremont, CA), and mouse anti-β-tubulin (1:4,000) (Upstate Biotechnology, Lake Placid, NY). Proteins were detected with horseradish peroxidase-conjugated secondary antibodies and BM chemiluminescence blotting substrate (Roche Diagnostics).
Results
GFP-Progerin Causes a Distinctive Nuclear Morphology Defect in HeLa Cells and Shows a Localization Pattern Different from GFP-Lamin A-WT. Normally, prelamin A undergoes CaaX processing at its C terminus, followed by an internal cleavage by Zmpste24 to remove these modifications (Fig. 1A). In the case of HGPS, the LMNA mutation leads to deletion of the Zmpste24 cleavage site, and the resulting mutant protein, progerin, is expected to retain its CaaX modifications (Fig. 1B) (2). CaaX-modified prelamin A also accumulates in the Zmpste24 knockout mouse (Fig. 1C). Interestingly, the Zmpste24 knockout mouse shares a number of phenotypes with HGPS patients, including the striking nuclear morphology defects at the cellular level and progeroid appearance at the organismal level (8, 9). It has recently been reported that an individual with progeroid-like phenotypes (diagnosed as mandibuloacryl dysplasia) and several cases of restrictive dermopathy also have loss-of-function mutations in Zmpste24 (19, 20). In each of these instances, a form of prelamin A with its CaaX modifications intact is predicted to accumulate, leading us to hypothesize that it is specifically the persistence of these modifications that is at least in part responsible for the disease phenotypes.
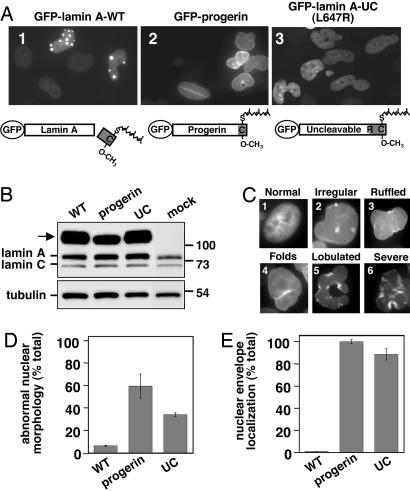
To test our hypothesis, we developed a HeLa cell culture system to examine the effect of misprocessed forms of prelamin A on nuclear morphology. GFP-tagged WT prelamin A (GFP-lamin A-WT) and GFP-progerin mimic the normal and HGPS states, respectively. We also mutated the Zmpste24 cleavage site in prelamin A (L647R) to generate an uncleavable molecule (GFP-lamin A-UC), which represents the Zmpste24 mutant state (Fig. 2A) (21). Transient transfection of GFP-lamin A-WT had no effect on nuclear morphology, given that the nuclei maintained their normal shape (Fig. 2A, image 1). In contrast, GFP-progerin resulted in strikingly abnormal nuclei (Fig. 2A, image 2) with a wide range of nuclear morphology defects (Fig. 2C). The number of cells with abnormal nuclei was almost 10-fold higher in cells expressing GFP-progerin as compared with GFP-lamin A-WT (Fig. 2D). The localization of the GFP-tagged proteins also varied: GFP-lamin A-WT localized to prominent nuclear foci and to the nucleoplasm (see also below), whereas GFP-progerin was primarily at the nuclear envelope with some nucleoplasmic staining, but importantly no nuclear foci were apparent (Fig. 2A, compare images 1 and 2; see also Fig. 2E). Similar results to GFP-progerin were observed with GFP-lamin A-UC (Fig. 2A, image 3; see also Fig. 2 D and E), consistent with the progerin phenotypes being specifically due to the retention of the CaaX modifications. The observed effects were not due to perturbations in the levels of endogenous lamin A and C (Fig. 2B). Overall, there was a clear correlation between the presence of abnormal nuclear morphology, the localization of GFP-progerin and GFP-lamin A-UC strictly to the nuclear envelope, and the expected persistence of CaaX modifications.
Fig. 2.
Expression of GFP-progerin and GFP-lamin A-UC in HeLa cells results in abnormal nuclear morphology and a localization pattern distinct from GFP-lamin A-WT. (A) HeLa cells were transiently transfected with the indicated constructs (containing the L367P mutation) as described in Materials and Methods; representative fields of cells are shown. Schematics of the expected processing status of the GFP-tagged proteins are shown. (B) Expression of the GFP-tagged proteins (arrow) and endogenous lamin A and C was assessed by Western blotting with anti-lamin A/C antibodies (Upper), with tubulin as a loading control (Lower). (C) The different types of normal (image 1) or aberrant (images 2-6) nuclei observed in cells expressing GFP-progerin, ranging from mild to severe, are indicated. (D and E) Nuclei from HeLa cells transfected with the GFP-tagged constructs were scored for abnormal nuclear morphology (D) and nuclear envelope localization (E) of the GFP-tagged proteins as described in Materials and Methods. Graphs represent the average of two experiments with the range indicated by the bar.
Endogenous lamin A has been previously shown to localize variably to the nuclear envelope, the nucleoplasm, nuclear speckles, and/or foci (22). In our studies, we made use of a point mutation in lamin A (L367P) that had no effect on the localization of GFP-progerin or GFP-lamin A-UC (Fig. 6A, which is published as supporting information on the PNAS web site; compare images 3 and 4 with images 7 and 8) but which altered GFP-lamin A-WT localization. Without the mutation, GFP-lamin A-WT was observed primarily at the nuclear envelope and in the nucleoplasm, although a small percentage of cells contained large intranuclear foci (8%) (Fig. 6A, images 1 and 2). With the L367P mutation, GFP-lamin A-WT was excluded from the nuclear envelope and instead found in the nucleoplasm and in foci (45%) (Figs. 2 A, image 1, and 6A, images 5 and 6). These foci may represent a nonphysiological aggregation of GFP-lamin A-WT due to its overexpression relative to endogenous lamin A (Fig. 2B), because foci were apparent only in the most highly expressing cells and were much larger that the speckles described for endogenous lamin A. However, although the foci are likely a nonphysiological localization pattern for lamin A, they provide a distinctive “signature” for a lamin A molecule lacking CaaX modifications, as shown below. Incorporating the L367P mutation into our GFP-lamin A constructs promoted foci formation, resulting in a visual enhancement of the localization difference between lamin A molecules with or without CaaX modifications (nuclear envelope vs. nucleoplasm/foci, respectively), which facilitated our analyses.
Mutational Alteration of the GFP-Progerin CaaX Motif Prevents Aberrant Nuclear Morphology Phenotype and Nuclear Envelope Localization. Farnesylation involves the attachment of the farnesyl group from farnesyl pyrophosphate to the sulfhydryl group of the CaaX motif cysteine by means of a thioether linkage. If farnesylation is prevented, all subsequent CaaX processing steps, as well as the internal cleavage of prelamin A, are blocked (23). Mutation of this critical cysteine to serine prevents prenylation of prelamin A and all subsequent CaaX modifications (24). If the aberrant retention of the CaaX modifications on progerin (and UC) is indeed the cause of the abnormal nuclear morphology, then blocking farnesylation of the molecules (i.e., by introducing the C→S mutation) should prevent the abnormal nuclear morphology phenotype (Fig. 3B).
Fig. 3.
Block in the CaaX processing of progerin by mutation of the CaaX motif (C→S) and pharmacological (FTI) treatment. (A) Normally, progerin is expected to undergo C-terminal CaaX processing (steps 1-3) but no Zmpste24-mediated internal processing (step 4), resulting in persistent CaaX modifications. (B) A mutational block at step 1 in CaaX processing by introduction of a C→S mutation prevents the farnesylation and subsequent processing of progerin. (C) Pharmacological treatment with an FTI will likewise prevent farnesylation of progerin at step 1 and, thus, its subsequent processing.
To examine this hypothesis, we created CaaX mutant (C→S) forms of GFP-lamin A-WT, GFP-progerin, and GFP-lamin A-UC and examined the effect of expressing these proteins in HeLa cells. Strikingly, the C→S mutation in GFP-progerin and GFP-lamin A-UC prevented the formation of abnormal nuclei (Fig. 4A, compare images 5 and 6 with images 2 and 3), with the percentage of abnormal nuclei observed reduced to WT levels (Fig. 4B). In addition, the GFP-tagged proteins showed substantially reduced nuclear envelope localization (Fig. 4C) and instead were found in the nucleoplasm and intranuclear foci, like GFP-lamin A-WT. Consistent with the findings of Sinensky and coworkers (7), the C→S mutation had no effect on GFP-lamin A-WT. In this case, the nuclei remained normal and the localization of the GFP-tagged proteins was unchanged (Fig. 4A, compare image 4 with image 1; see also Fig. 4 B and C). The phenotypic changes resulting from the C→S mutations in GFP-progerin and GFP-lamin A-UC were not due to alteration in their expression level or in the levels of endogenous lamin A and C (Fig. 7A, which is published as supporting information on the PNAS web site). Taken together, there is a clear correlation between retention of CaaX modifications, induction of abnormal nuclei, and nuclear envelope localization. The only proteins expected to retain their CaaX modifications, namely GFP-progerin and GFP-lamin A-UC, are the only ones that cause an abnormal nuclear morphology and that localize to the nuclear envelope.
Fig. 4.
Mutation (C→S) of the CaaX motif to prevent farnesylation of GFP-progerin and GFP-lamin A-UC reverses the nuclear morphology defects. (A) HeLa cells were transiently transfected with the indicated constructs and examined by fluorescence microscopy as in Fig. 2. Expected structures of the GFP-tagged proteins are shown. (B and C) Nuclei from HeLa cells transfected with the GFP-tagged constructs were scored for abnormal nuclear morphology (B) and nuclear envelope localization (C) of the GFP-tagged proteins as in Fig. 2. Graphs represent the average of two experiments with the range indicated by the bar.
Pharmacological Inhibition of Farnesylation Prevents the Aberrant Nuclear Morphology Phenotype Associated with GFP-Progerin. FTIs were originally developed to block the farnesylation of Ras, a CaaX protein that is mutated and hyperactive in a number of cancers. Although FTIs block farnesylation (and subsequent processing steps) of all CaaX proteins, they have been shown to be well tolerated in patients undergoing anticancer clinical trials, indicating that blocking farnesylation of at least a subset of farnesylated proteins does not produce severe side effects. R115777 (Tipifarnib; also known as Zarnestra) is an FTI in phase III clinical trials (25). We synthesized the racemic form of this compound, rac-R115777 (18), to test whether it would be effective in preventing the nuclear morphology defects caused by GFP-progerin by inhibiting its farnesylation (Fig. 3C). rac-R115777 is active in HeLa cells, as shown by the appearance of (unfarnesylated) endogenous prelamin A and unfarnesylated pre-HDJ-2 (Fig. 8, which is published as supporting information on the PNAS web site), consistent with other studies (25). The concentrations tested are commonly used with HeLa cells and were not toxic (data not shown).
Quite strikingly, FTI treatment reversed the abnormal nuclear morphology associated with GFP-progerin and GFP-lamin A-UC (Fig. 5A, compare images 5 and 6 with images 2 and 3). Although some abnormal nuclei were still observed, FTI treatment reduced the proportion of abnormal nuclei by 2- to 3-fold, close to WT levels (Fig. 5B). A similar effect was observed with GFP-progerin without the L367P mutation (Fig. 6B). The nuclear envelope localization of the GFP-tagged proteins was also reduced by ≈2-fold (Fig. 5C). The residual nuclear envelope localization was presumably due to preexisting GFP-progerin and GFP-lamin A-UC produced by the cells after transfection but before FTI addition. In addition to abnormal nuclear morphology, progerin expression has also been reported to reduce the cellular level of LAP2, a lamin-associated protein (17). Indeed, we observed a very low level of LAP2 in cells expressing GFP-progerin, and FTI treatment restored LAP2 to the WT level (Fig. 5D).
Fig. 5.
The FTI rac-R115777 blocks the formation of abnormal nuclei by GFP-progerin and GFP-lamin A-UC. (A) HeLa cells were transiently transfected with the indicated constructs, treated with DMSO (images 1-3) or 1 μM rac-R115777 in DMSO (images 4-6), and examined by fluorescence microscopy, as in Fig. 2. Expected structures of the GFP-tagged proteins are shown. (B and C) Nuclei from HeLa cells transfected with the GFP-tagged constructs with or without FTI treatment were scored for abnormal nuclear morphology (B) and for nuclear envelope localization (C) of the GFP-tagged proteins as in Fig. 2. Graphs represent the average of two experiments with the range indicated by bars. (D) Nuclei from HeLa cells transfected with GFP-progerin with or without FTI treatment were examined by fluorescence microscopy for progerin expression (images 1 and 3) and by indirect immunofluorescence for LAP2 expression (images 2 and 4). Arrows indicate cells expressing GFP-progerin and the corresponding cells for LAP2 immunofluorescence.
Similar effects were observed at all concentrations of rac-R115777 tested (1-5 μM) and with another FTI, BMS-214662 (26) (data not shown). FTI treatment had no effect on GFP-lamin A-WT, consistent with the fact that it does not normally retain CaaX modifications (Fig. 5A, compare images 1 and 4). The FTI effect was not due to a change in protein levels by FTI treatment (Fig. 7B). Similar to what was observed with the C→S mutants (Fig. 4), there was a clear correlation between retention of CaaX modifications, induction of abnormal nuclei, and nuclear envelope localization. That is, GFP-progerin and GFP-lamin A-UC are both predicted to retain their CaaX modifications, and this abnormal retention is blocked by FTI treatment, which prevents CaaX processing from occurring and therefore prevents the formation of abnormal nuclei. This result is exciting because, if this cellular effect were to translate to a reversal of the organismal phenotype, then FTIs may be a viable therapeutic treatment for individuals suffering from HGPS.
Discussion
Inhibiting Farnesylation Reverses the Nuclear Morphology Defect Caused by Progerin. In this study, we developed a robust HeLa cell culture system to express GFP-tagged progerin (and other forms of prelamin A) that faithfully reproduced the nuclear morphology defects associated with HGPS. Our results strongly support the hypothesis that the aberrant retention of CaaX modifications on progerin is responsible for many of the disease phenotypes. Notably, the nuclear morphology defect resulting from expression of progerin or UC is completely blocked when farnesylation is prevented, either by mutation of the CaaX motif (C→S) (Fig. 4) or by FTI treatment (Fig. 5). Our results open up the possibility that FTIs could be used as a drug treatment for individuals suffering from HGPS. Importantly, even though FTIs will prevent farnesylation of other CaaX proteins, they have been shown to be well tolerated in clinical trials (25).
In addition to FTIs, drugs that target steps upstream in the farnesyl pyrophosphate/cholesterol biosynthesis pathway, such as statins and bisphosphonates, may also be effective in reversing the nuclear morphology defects associated with HGPS and would be worth testing in our cell culture system and in vivo. It is important to note that inhibition of farnesylation also blocks subsequent CaaX processing steps, namely aaXing and carboxylmethylation. Presently, we cannot rule out the possibility that it is specifically the retention of the carboxylmethyl modification rather than farnesylation that is responsible for the disease phenotypes. If this possibility were the case, then inhibition of isoprenylcysteine methyl-transferase to block carboxylmethylation may also be an effective treatment in addition to, or instead of, FTIs. Our cell culture system may also be ideal in screening for novel drugs that block the dominant-negative phenotype of progerin by taking advantage of the easily visualized dramatic change in GFP-progerin localization from the nuclear envelope to intranuclear foci that parallels the restoration of normal nuclear morphology.
Lack of Zmpste24 Processing of Prelamin A in Human Disease and Aging. The gene encoding Zmpste24 was discovered in our laboratory in a screen for sterile mutants in yeast (hence the designation STE24), and we established biochemically that it carries out critical proteolytic processing steps in the biogenesis of the mating pheromone a-factor (11-13). In mammals, the importance of Zmpste24 function is only now becoming appreciated through mouse knockout studies (8, 9) and the discovery of several human diseases that result from a lack of Zmpste24 cleavage of prelamin A. The lack of prelamin A cleavage in these diseases results from mutations in Zmspte24 itself (mandibuloacryl dysplasia and restrictive dermopathy) or mutations in LMNA that affect the Zmpste24 cleavage site (HGPS) (Fig. 1) (2, 19, 20). Regardless of how prelamin A internal cleavage is blocked, the result appears to be the same: aberrant nuclei at the cellular level and progeroid-like phenotypes at organismal level. Importantly, recent studies suggest that it is not the reduction or absence of mature lamin A that causes disease but rather the aberrant accumulation of an uncleaved CaaX-modified form of prelamin A (17, 27).
HGPS has been suggested to be a model system for studying normal human aging (14). It is tempting to speculate that in normal individuals, prelamin A processing might become less efficient with aging because of a decrease in Zmpste24 activity. Alternatively, the mRNA splicing machinery may become less precise with age, resulting in the occasional use of the cryptic splice site in LMNA to produce a low level of progerin even in the absence of the HGPS mutation. Although speculative, it may be of interest to examine cells from elderly individuals to determine whether uncleaved prelamin A or even some progerin is present in their nuclei, contributing to the phenotypes associated with normal aging.
Why Does the Persistence of Prelamin A CaaX Modifications Result in Aberrant Nuclear Morphology and Disease? What might be the mechanism whereby persistently CaaX-modified progerin or prelamin A could result in the disruption of nuclear morphology? Maintaining the proper balance of CaaX-modified proteins at the nuclear envelope seems to be critical, because not all lamin A mutations have an effect on nuclear morphology, but the mutations that are predicted to lead to retention of the CaaX modifications always affect morphology (i.e., HGPS and loss of Zmpste24 activity) (2, 8, 9, 19, 20). An excess of persistently CaaX-modified progerin or uncleaved prelamin A could displace normal CaaX-modified proteins (i.e., lamin B and prelamin A before Zmpste24 cleavage) from their binding partners, leading to nuclear aberrations. One potential binding partner is the lamin B receptor (LBR), which interacts with lamin B (28). LBR has been directly implicated in maintaining neutrophil nuclear architecture because LBR mutations result in the Pelger-Huet anomaly, which is characterized by abnormal neutrophil nuclei (29). Another possible binding partner is Narf (nuclear prelamin A recognition factor), whose cellular function remains elusive (30). Clearly a better understanding of the determinants of nuclear architecture and of lamin A, B, and C interactions with each other and their various binding partners will be important in formulating clearer mechanistic models to test how it is that progerin and uncleaved prelamin A can influence nuclear architecture.
How is the striking cellular defect of aberrant nuclear morphology associated with progeroid phenotypes at the organismal level? One model proposes that the aberrant nuclei are more susceptible than normal nuclei to mechanical stress, ultimately leading to cell death (31). Alternatively, gene expression may be affected when nuclei are aberrant, because the nuclear lamina has been shown to interact with an ever-growing number of transcription factors (e.g., Rb and SREBP), other lamin-associated proteins, chromatin, and chromatin-binding proteins (e.g., HP1-α) (4). Indeed, transcriptional profiling of HGPS fibroblasts has revealed expression changes in >300 genes (32). It has also recently been reported that, in HGPS fibroblasts, several nuclear proteins (including lamin B and members of the LAP2 family) are specifically mislocalized and/or underexpressed (i.e., HP1-α levels are decreased) and that histone modifications are altered (17). Consistent with these findings, we observed that the level of LAP2 was decreased in cells expressing GFP-progerin and restored upon FTI treatment (Fig. 5D). An important and daunting challenge is to understand which cellular phenotypes actually contribute to progeroid disorders and which are simply a secondary consequence of the disease. At present, all of the cellular effects, including aberrant nuclear morphology, can only be characterized as associative phenotypes in a complex disease, and, although they may or may not be causes of the disease, they are highly useful as markers for the disease state.
FTIs as a Treatment for HGPS? Our studies raise the interesting possibility that FTIs may be useful in treating individuals with HGPS. Although FTIs will reduce the farnesylation of many CaaX proteins in addition to progerin, it is important to note that these drugs are well tolerated in humans; indeed, R115777 is in phase III clinical trials (25). In fact, the efficacy of FTIs in preventing the progerin-induced nuclear morphology defects could be due to decreased farnesylation of targets in addition to progerin, such as lamin B. Blocking farnesylation and subsequent carboxylmethylation has also been reported to affect protein stability and can sometimes lead to cross-prenylation by geranylgeranyl transferase, either of which may also contribute to the effects we observe with FTI treatment (25). It has recently been demonstrated that a modified oligonucleotide can be used to correct the splicing defect in HGPS fibroblasts and prevent the production of progerin, resulting in a reversal of the abnormal nuclear morphology and other cellular phenotypes (17). This exciting result provides proof of principle that drugs that target progerin, like FTIs, may be effective in treating individuals suffering from HGPS. The fact that FTIs are readily available and well tolerated in humans, coupled with the devastating effects of not treating HGPS, suggests that it will be worthwhile to examine their efficacy as a therapy for this tragic disease.
Supplementary Material
Acknowledgments
We thank Dr. M. Sinensky for the prelamin A cDNA, M. Zastrow for helpful discussions, and Drs. D. Raben and P. Espenshade for critical reading of the manuscript. This work was supported by National Institutes of Health Grants GM41223 (to S.M.) and AI54384 (to M.H.G.) and by grants from the Progeria Research Foundation (to M.P.M. and S.M.) and the Medicines for Malaria Venture of Geneva (to M.H.G.).
Author contributions: M.P.M., G.H., and S.M. designed research; M.P.M. performed research; P.B. and M.H.G. contributed new reagents/analytic tools; M.P.M., G.H., M.H.G., and S.M. analyzed data; and G.H. and S.M. wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: HGPS, Hutchinson-Gilford progeria syndrome; FTI, farnesyl transferase inhibitor; UC, uncleavable form of prelamin A; Zmpste24, zinc metalloprotease Ste24; LAP2, lamina-associated polypeptide 2.
References
- 1.Pollex, R. L. & Hegele, R. A. (2004) Clin. Genet. 66, 375-381. [DOI] [PubMed] [Google Scholar]
- 2.Eriksson, M., Brown, W. T., Gordon, L. B., Glynn, M. W., Singer, J., Scott, L., Erdos, M. R., Robbins, C. M., Moses, T. Y., Berglund, P., et al. (2003) Nature 423, 293-298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Sandre-Giovannoli, A., Bernard, R., Cau, P., Navarro, C., Amiel, J., Boccaccio, I., Lyonnet, S., Stewart, C. L., Munnich, A., Le Merrer, M., et al. (2003) Science 300, 2055. [DOI] [PubMed] [Google Scholar]
- 4.Hutchison, C. J. & Worman, H. J. (2004) Nat. Cell Biol. 6, 1062-1067. [DOI] [PubMed] [Google Scholar]
- 5.Burke, B. & Stewart, C. L. (2002) Nat. Rev. Mol. Cell. Biol. 3, 575-585. [DOI] [PubMed] [Google Scholar]
- 6.Zhang, F. L. & Casey, P. J. (1996) Annu. Rev. Biochem. 65, 241-269. [DOI] [PubMed] [Google Scholar]
- 7.Lutz, R. J., Trujillo, M. A., Denham, K. S., Wenger, L. & Sinensky, M. (1992) Proc. Natl. Acad. Sci. USA 89, 3000-3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bergo, M. O., Gavino, B., Ross, J., Schmidt, W. K., Hong, C., Kendall, L. V., Mohr, A., Meta, M., Genant, H., Jiang, Y., et al. (2002) Proc. Natl. Acad. Sci. USA 99, 13049-13054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pendas, A. M., Zhou, Z., Cadinanos, J., Freije, J. M., Wang, J., Hultenby, K., Astudillo, A., Wernerson, A., Rodriguez, F., Tryggvason, K., et al. (2002) Nat. Genet. 31, 94-99. [DOI] [PubMed] [Google Scholar]
- 10.Corrigan, D. P., Kuszczak, D., Rusinol, A. E., Thewke, D. P., Hrycyna, C. A., Michaelis, S. & Sinensky, M. S. (2005) Biochem. J. 387, 129-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fujimura-Kamada, K., Nouvet, F. J. & Michaelis, S. (1997) J. Cell Biol. 136, 271-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tam, A., Nouvet, F. J., Fujimura-Kamada, K., Slunt, H., Sisodia, S. S. & Michaelis, S. (1998) J. Cell Biol. 142, 635-649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tam, A., Schmidt, W. K. & Michaelis, S. (2001) J. Biol. Chem. 276, 46798-46806. [DOI] [PubMed] [Google Scholar]
- 14.Mounkes, L. C. & Stewart, C. L. (2004) Curr. Opin. Cell Biol. 16, 322-327. [DOI] [PubMed] [Google Scholar]
- 15.Muchir, A. & Worman, H. J. (2004) Physiology (Bethesda) 19, 309-314. [DOI] [PubMed] [Google Scholar]
- 16.Goldman, R. D., Shumaker, D. K., Erdos, M. R., Eriksson, M., Goldman, A. E., Gordon, L. B., Gruenbaum, Y., Khuon, S., Mendez, M., Varga, R., et al. (2004) Proc. Natl. Acad. Sci. USA 101, 8963-8968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scaffidi, P. & Misteli, T. (2005) Nat. Med. 11, 440-445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Venet, M. G., Angibaud, P. R., Muller, P. & Sanz, G. C. (2000) U.S. Patent 6,037,350.
- 19.Agarwal, A. K., Fryns, J. P., Auchus, R. J. & Garg, A. (2003) Hum. Mol. Genet. 12, 1995-2001. [DOI] [PubMed] [Google Scholar]
- 20.Navarro, C. L., Cadinanos, J., De Sandre-Giovannoli, A., Bernard, R., Courrier, S., Boccaccio, I., Boyer, A., Kleijer, W. J., Wagner, A., Giuliano, F., et al. (2005) Hum. Mol. Genet. 14, 1503-1513. [DOI] [PubMed] [Google Scholar]
- 21.Hennekes, H. & Nigg, E. A. (1994) J. Cell Sci. 107, 1019-1029. [DOI] [PubMed] [Google Scholar]
- 22.Goldman, R. D., Goldman, A. E. & Shumaker, D. K. (2005) Novartis Found. Symp. 264, 3-21. [PubMed] [Google Scholar]
- 23.Beck, L. A., Hosick, T. J. & Sinensky, M. (1990) J. Cell Biol. 110, 1489-1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Holtz, D., Tanaka, R. A., Hartwig, J. & McKeon, F. (1989) Cell 59, 969-977. [DOI] [PubMed] [Google Scholar]
- 25.Bishop, W. R., Kirschmeier, P. & Baum, C. (2003) Cancer Biol. Ther. 2, S96-104. [PubMed] [Google Scholar]
- 26.Hunt, J. T., Ding, C. Z., Batorsky, R., Bednarz, M., Bhide, R., Cho, Y., Chong, S., Chao, S., Gullo-Brown, J., Guo, P., et al. (2000) J. Med. Chem. 43, 3587-3595. [DOI] [PubMed] [Google Scholar]
- 27.Fong, L. G., Ng, J. K., Meta, M., Cote, N., Yang, S. H., Stewart, C. L., Sullivan, T., Burghardt, A., Majumdar, S., Reue, K., et al. (2004) Proc. Natl. Acad. Sci. USA 101, 18111-18116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ye, Q. & Worman, H. J. (1994) J. Biol. Chem. 269, 11306-11311. [PubMed] [Google Scholar]
- 29.Hoffmann, K., Dreger, C. K., Olins, A. L., Olins, D. E., Shultz, L. D., Lucke, B., Karl, H., Kaps, R., Muller, D., Vaya, A., et al. (2002) Nat. Genet. 31, 410-414. [DOI] [PubMed] [Google Scholar]
- 30.Barton, R. M. & Worman, H. J. (1999) J. Biol. Chem. 274, 30008-30018. [DOI] [PubMed] [Google Scholar]
- 31.Lammerding, J., Schulze, P. C., Takahashi, T., Kozlov, S., Sullivan, T., Kamm, R. D., Stewart, C. L. & Lee, R. T. (2004) J. Clin. Invest. 113, 370-378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Csoka, A. B., English, S. B., Simkevich, C. P., Ginzinger, D. G., Butte, A. J., Schatten, G. P., Rothman, F. G. & Sedivy, J. M. (2004) Aging Cell 3, 235-243. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.