Abstract
The multifaceted process of immune cell recruitment to sites of tissue injury is key to the development of an inflammatory response and involved in the pathogenesis of numerous cardiovascular disorders. We recently identified C-type natriuretic peptide (CNP) as an important endothelium-derived mediator that regulates vascular tone and protects against myocardial ischemia/reperfusion injury. Herein, we investigated whether CNP inhibits leukocyte recruitment and platelet aggregation and thereby exerts a potential antiinflammatory influence on the blood vessel wall. We assessed the effects of CNP on leukocyte-endothelial cell interactions in mouse mesenteric postcapillary venules in vivo in animals with high basal leukocyte activation (endothelial nitric oxide synthase knockout mice, eNOS-/-) or under acute inflammatory conditions (induced by interleukin-1β or histamine). CNP suppressed basal leukocyte rolling in eNOS-/- mice in a rapid, reversible, and concentration-dependent manner. These effects of CNP were mimicked by the selective natriuretic peptide receptor-C agonist cANF4-23. CNP also suppressed leukocyte rolling induced by IL-1β or histamine, inhibited platelet-leukocyte interactions, and prevented thrombin-induced platelet aggregation of human blood. Furthermore, analysis of human umbilical vein endothelial cells, leukocytes, and platelets revealed that CNP selectively attenuates expression of P-selectin. Thus, CNP is a modulator of acute inflammation in the blood vessel wall characterized by leukocyte and platelet activation. These antiinflammatory effects appear to be mediated, at least in part, via suppression of P-selectin expression. These observations suggest that endothelial CNP might maintain an anti-atherogenic influence on the blood vessel wall and represent a target for therapeutic intervention in inflammatory cardiovascular disorders.
Keywords: endothelium, natriuretic peptide receptor type C, atherosclerosis, thrombosis
Leukocyte recruitment and platelet activation at sites of tissue injury are important facets of an inflammatory response and are pivotal in the pathogenesis of a number of cardiovascular disorders including atherosclerosis and sepsis. These processes are triggered by specific inflammatory stimuli and are critically dependent on the expression of a series of adhesion molecules on the surface of leukocytes, platelets, and the vascular endothelium (1). However, this is a dynamic process; under basal (physiological) conditions, leukocyte and platelet activation is held in check by specific endothelium-derived mediators. Perhaps the most influential of these is nitric oxide (NO), which, in concert with analogous autacoids such as prostacyclin, maintains an active suppression of leukocyte and platelet activation under physiological conditions that is paramount for maintenance of blood vessel integrity and patency (2-4).
The importance of NO in the prevention of leukocyte recruitment has been established by studies demonstrating increased leukocyte-endothelial interactions in animals treated with NO-synthase (NOS) inhibitors and in endothelial NOS knockout (eNOS-/-) mice, both under basal conditions and after leukocyte activation (2, 3, 5). NO-induced inhibition of leukocyte recruitment is thought to be a consequence of suppression of adhesion molecules involved in the initial steps of leukocyte recruitment, principally P-selectin [but also the intercellular adhesion molecule (ICAM)-1 ligand, Cd11b] (2, 6-9). In addition, NO is well established to inhibit platelet reactivity, at least in part, via suppression of P-selectin expression (10). Thus, regulatory mechanisms governing P-selectin expression have a significant impact on the development of an inflammatory response. Indeed, loss of this inhibitory effect of NO results in a change in the endothelial cell from its normal cytoprotective antiinflammatory, antithrombotic nature to a pathogenic pro-inflammatory, prothrombotic phenotype; it is thought that such changes contribute to diseases such as sepsis and atherosclerosis, and restenosis after balloon angioplasty.
C-type natriuretic peptide (CNP) represents the paracrine element of the natriuretic peptide axis, complementing the endocrine actions of atrial natriuretic peptide and brain natriuretic peptide, to lower blood volume and pressure. We have recently identified CNP as an endothelium-derived hyperpolarizing factor (EDHF) in mesenteric resistance arteries (11) and defined a previously undescribed signaling pathway that is important in the regulation of vascular tone and local blood flow and involves activation of the natriuretic peptide receptor type C (NPR-C) and opening of a G protein-coupled inwardly rectifying K+ channel (GIRK) (11). Subsequently, we have demonstrated that this CNP/NPR-C signaling system is important in regulating coronary blood flow and represents a protective mechanism against myocardial ischemia-reperfusion (I/R) injury (12).
Previous reports allude to the possibility that CNP possesses antiinflammatory actions in the vasculature, akin to endothelium-derived NO. When administered locally by adenoviral gene transfer, CNP inhibits neointimal hyperplasia and thrombus formation in vein grafts (13, 14) and promotes vascular regeneration in models of limb ischaemia (15). Moreover, CNP expression is up-regulated in human atherosclerotic lesions (16). Therefore, endothelium-derived CNP may exert an important cytoprotective influence on the blood vessel wall and rationalize the beneficial actions of this peptide in the disorders described above. We have demonstrated that the (cardio)vascular effects of CNP, both in terms of its role as an EDHF and in protecting against myocardial I/R injury, are enhanced in the absence of NO. This phenomenon, in combination with the increased CNP expression in vasculopathies associated with NO deficiency (described above), intimates that this peptide may provide a salvage pathway to complement and compensate for endothelium-derived NO.
Herein, we demonstrate that CNP inhibits endothelial-leukocyte interactions and platelet reactivity in an analogous fashion to NO. Moreover, the antileukocyte effects of CNP are the result, at least in part, of suppressed P-selectin expression. Thus, endothelial CNP might contribute to the maintenance of an antiatherogenic, antithrombotic phenotype on the blood vessel wall in combination with, and in lieu of, NO. These observations might also explain, at least in part, the previously reported beneficial effects of CNP in models of cardiovascular disease and therefore highlight a therapeutic target.
Methods
Intravital Microscopy. All experiments were conducted according to the Animals (Scientific Procedures) Act, 1986 (United Kingdom). Male WT and eNOS-/- mice (10-15 g; in-house colony) were anesthetized with diazepam (6 mg/kg; s.c.) and Hypnorm (0.7 mg/kg fentanyl citrate and 20 mg/kg fluanisone; i.m.). Cautery incisions were made along the abdominal region, and the mesenteric vascular bed was exteriorized for intravital recording, as we have described (6). Mesenteries were superfused with bicarbonate buffer at 37°C (132 mM NaCl/4.7 mM KCl/1.2 mM MgSO4/17.9 mM NaHCO3/2.0 mM CaCl2, pH 7.4, gassed with 5% CO2/95% N2) at a rate of 2 ml/min. Postcapillary venules (20- to 50-μm diameter; length ≥ 100 μm) were randomly chosen, and measurements were recorded for 1 min. Cell flux was assessed by counting the number of cells passing a fixed point per minute (Vwbc). Cell adhesion was quantified by counting, for each vessel, the number of adherent leukocytes (stationary for >30 s) in a 100-μm length. Red blood cell (RBC) velocity was measured in venules by using an optical doppler velocimeter (Microcirculation Research Institute, Texas A&M University, College Station), and venular blood flow was calculated from the product of mean RBC velocity (Vmean = center line velocity/1.6) and microvascular cross-sectional area, assuming a cylindrical geometry. Wall shear rates were calculated by the Newtonian definition: shear rate = 8,000 × (Vmean/diameter).
Assessment of the effect of CNP on basal leukocyte activation in eNOS-/-. To investigate whether CNP can modulate white cell recruitment, we used a model of high basal leukocyte rolling (i.e., eNOS-/- mice). CNP (0.1-1 μM) was superfused for 10 min, and leukocyte flux was recorded. To investigate the involvement of the NPR-C in responses to CNP, the ability of the selective NPR-C agonist (17), cANF4-23 (1 μM) to inhibit leukocyte rolling was also explored.
Assessment of the effect of CNP on acute inflammatory response to IL-1β or histamine. Murine recombinant IL-1β (5 ng i.p. in 0.1 ml of sterile saline, Preprotech) or histamine (100 μM) was used to elevate leukocyte rolling and adhesion in eNOS-/- and WT animals. Murine IL-1β was administered 90 min before exteriorization of the mesenteric vascular bed, as described (6, 18). Histamine (100 μM) was added to the superfusing buffer for 15 min before and during application of CNP (1 μM).
Flow Cytometry. Human umbilical vein endothelial cells (HUVECs). Confluent HUVECs were treated with histamine (100 μM, 15 min), TNF-α (20 ng/ml; 90 min), or IL-1β (200 ng/ml, 240 min) to induce P-selectin, E-selectin, and ICAM-1 expression, respectively. Samples were treated with vehicle (saline), CNP (0.1-1 μM), or cANF4-23 (1 μM) at the time of the inflammatory stimulus. Cells were then washed in Hanks' balanced salt solution (BioWhittaker) and incubated with appropriate mouse anti-human adhesion molecule monoclonal antibodies in the dark at 37°C for 30 min. HUVECs were dissociated with trypsin/EDTA at 37°C for 2 min. Trypsin activity was blocked by “trypsin neutralizing solution” (TNS; BioWhittaker), and FACS analysis was performed on isolated cells, as we have described (6).
Whole blood/platelets. Human blood was taken from individuals who had not taken nonsteroidal antiinflammatory drugs for the previous 14 days and collected into a citrated-vacutainer (ACD, Vacutainer, Becton Dickinson). Blood was diluted 1:5 with Tyrode's buffer containing 0.01% BSA (pH 7.4) and incubated with either thrombin (0.1 unit/ml for 10 min) to stimulate P-selectin expression on platelets, TNF-α (20 ng/ml, 30 min) to stimulate adhesion molecule expression on leukocytes, or buffer as control. In some samples, CNP (0.1-1 μM) was administered at the time of stimulation. FITC-labeled antibodies were added after stimulation for 30 min at 25°C, fixed with 0.5% paraformaldehyde (PFA) and FACS analysis conducted, as we have described (6). FITC-conjugated CD62L (clone FMC46), CD62E (clone CL2/6), CD62P (clone AK-6), CD11b (clone ICRF44), and CD54 (clone 15.2) were purchased from Serotec.
Platelet-leukocyte interactions. Blood was collected as described above and pretreated with CNP (1 μM), the NO-donor sodium nitroprusside (10 μM; positive control; Sigma), or Hepes-Tyrodes buffer for 5 min. Platelet activation was induced by TRAP-6 (1 μM; 10 min; Bachem). A mouse anti-CD14 monoclonal antibody conjugated with phycoerythrin (Beckman Coulter) and anti-CD 42a antibody conjugated to FITC (Immunotech) were added to the samples and incubated for 15 min at 22°C in the dark. Negative mouse isotype-matched controls (Beckman Coulter) of the relevant antibodies were used to enable the platelet-leukocyte gate to be established. The samples were then resuspended in Hepes-Tyrodes and analyzed by FACS (as we have described in ref. 6).
Platelet Aggregometry. Human blood was collected as above, and prostacyclin-washed platelet suspensions (WP) were prepared as described (19). Platelet aggregation was measured in a Whole Blood Ionised Calcium Lumi-Aggregometer (Chronolog, Havertown, PA). CNP (0.1-1 μM) was incubated with WP (2.5 × 108 platelets per ml; in the presence of 300 μM l-NAME to prevent endogenous NO synthesis) for 1 min before addition of thrombin (1 unit/ml), and effects on platelet aggregation were monitored for 10 min.
Statistical Analysis. All data are plotted graphically as mean values with vertical bars representing SEM. Tests of significance (prism, GraphPad, San Diego) were conducted by using two-way ANOVA for multiple comparisons followed by Bonferonni's posttest analysis. A probability (P) value of <0.05 was taken as an appropriate level of significance.
Results
CNP Inhibits Basal Leukocyte Rolling in eNOS-/- Mice. Basal leukocyte rolling in postcapillary mesenteric venules of eNOS-/- mice was 22 ± 1.7 cells per min (n = 52), which is significantly elevated compared to WT animals as we (6) and others (2, 3) have demonstrated. Superfusion of CNP (0.1-1 μM) produced a concentration-dependent decrease in basal leukocyte flux in mesenteric venules (Fig. 1). This inhibitory effect of CNP on leukocyte activation was rapid in onset and completely reversed within 10 min after washout (Fig. 1). Superfusion of the selective NPR-C agonist, cANF4-23 (1 μM, 10 min) (17), also significantly attenuated leukocyte flux (Fig. 2), intimating that NPR-C activation results in inhibition of leukocyte rolling.
Fig. 1.
CNP elicits a rapid, reversible, concentration-dependent inhibition of leukocyte activation in eNOS-/- mesenteric venules in vivo. Concentration-dependent inhibition of leukocyte rolling by CNP (0.1-1 μM; Upper) and time course of these effects (Lower) are shown. n ≥ 6; *, P < 0.05.
Fig. 2.
NPR-C activation underlies the antileukocyte activity of CNP in eNOS-/- mesenteric venules in vivo. Effect of the selective NPR-C agonist cANF4-23 (1 μM) on leukocyte rolling is shown. n ≥ 6; *, P < 0.05.
Although basal leukocyte flux was elevated in venules of eNOS-/- mice, there were few adherent leukocytes evident in these vessels, and application of CNP had no significant effect on leukocyte adherence (Table 1).
Table 1.
Leukocyte adherence in mesenteric postcapillary venules from WT and eNOS−/− mice
| Leukocyte adherence, cells per 100 μm (n)
|
||
|---|---|---|
| Condition | WT | eNOS−/− |
| Control | 0 ± 0 (6) | 0.9 ± 0.26 (9) |
| + CNP (1 μM) | ND | 0.7 ± 0.24 (9) |
| + cANF4-23 (1 μM) | ND | 0.7 ± 0.17 (9) |
| + IL-1β (5 ng; i.p.) | 2.0 ± 0.7 (6) | 2.7 ± 0.3 (6) |
| + IL-1β (5 ng; i.p.) + CNP (1 μM) | 1.3 ± 0.4 (6) | 1.8 ± 0.4 (6) |
| + Histamine (100 μM) | 1.5 ± 0.3 (8) | 2.7 ± 0.39 (5) |
| + Histamine (100 μM) + CNP (1 μM) | 1.3 ± 0.4 (8) | 2.4 ± 0.16 (5) |
| ND, not determined. | ||
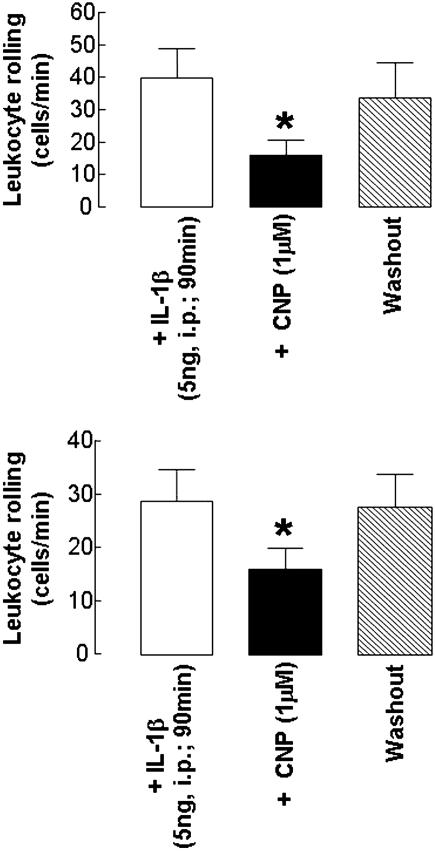
The observed reduction in leukocyte flux was not due to a change in blood flow because neither CNP nor cANF4-23 (or any of the other interventions used) caused a significant change in venule diameter or wall shear rates (data not shown). CNP Inhibits IL-1β- and Histamine-Induced Leukocyte Rolling. Treatment of eNOS-/- mice in vivo with IL-1β (5 ng, i.p.; 90 min) significantly elevated basal leukocyte rolling (control: 23 ± 4 cells per min; in the presence of IL-1β:41 ± 8 cells per min; n = 6; P < 0.05), a response that was inhibited (≈65%) by CNP (1 μM; Fig. 3). Interleukuin-1β also caused an increase in leukocyte rolling in WT animals (where basal leukocyte rolling is low; control: 10 ± 3 cells per min; in the presence of IL-1β:29 ± 6 cells per min; n = 6; P < 0.05) that was similarly attenuated in the presence of CNP (1 μM; Fig. 3). At this time point (90 min), IL-1β elicited a minor increase in leukocyte adherence in eNOS-/- and WT animals; however, this was not significantly modulated by CNP (Table 1). In a similar fashion, histamine (100 μM) elicited significant increases in leukocyte rolling in venules from WT and eNOS-/- mice that were attenuated in the presence of CNP (1 μM; Fig. 4).
Fig. 3.
CNP inhibits IL-1β-stimulated leukocyte activation in eNOS-/- and WT mesenteric venules in vivo. Effect of CNP (1 μM) on leukocyte rolling in eNOS-/- (Upper) and WT (Lower) mice treated with IL-1β (5 ng, i.p., 90 min) is shown. n ≥ 6; *, P < 0.05.
Fig. 4.
CNP inhibits histamine-stimulated leukocyte activation in eNOS-/- and WT mesenteric venules in vivo. Effect of CNP (1 μM) on leukocyte rolling in eNOS-/- (Upper) and WT (Lower) mice treated with histamine (100 μM). n ≥ 6; *, P < 0.05 versus control; **, P < 0.05 versus histamine alone.
NPR-C Activation Inhibits P-Selectin Expression on Endothelial Cells. P-selectin expression was assessed in HUVECs after activation with histamine (100 μM), because the in vivo effects of IL-1β (used in the intravital studies) are mediated predominantly via mast cell degranulation and, consequently, histamine release (18, 20). P-selectin expression on HUVECs and platelets was below detection limits in the absence of inflammatory stimuli. Histamine (100 μM) increased P-selectin expression on HUVECs, a response that was suppressed by CNP (0.1-1 μM; Fig. 5) or cANF4-23 (1 μM; data not shown). TNF-α (20 ng/ml) and IL-1β (200 ng/ml) also caused a significant increase in expression of E-selectin and ICAM-1, respectively; however, unlike P-selectin, application of CNP (0.1-1 μM) did not alter expression of these adhesion molecules (data not shown). Moreover, CNP (0.1-1 μM) did not have any effect on whole-blood leukocyte expression of L-selectin or the ICAM-1 ligand CD11b in response to TNF-α (20 ng/ml; data not shown).
Fig. 5.
CNP inhibits P-selectin expression on HUVECs and platelets. Effect of CNP (0.1-1 μM) on P-selectin expression in HUVECs treated with histamine (100 μM; Upper) and platelets activated by thrombin (0.1 unit/ml; Lower). n ≥ 6; *, P < 0.05.
CNP Inhibits Platelet-Leukocyte Aggregate Formation and Platelet Aggregation. Platelet-leukocyte interactions, mediated by binding of platelet P-selectin with leukocyte P-selectin glycoprotein ligand-1 (PSGL-1), are a key process in thrombus formation and in the pathogenesis of a number of inflammatory cardiovascular disorders including atherosclerosis and myocardial infarction (21, 22). Because we observed a marked CNP-mediated suppression of P-selectin expression on endothelial cells, we investigated whether CNP also modulates P-selectin expression and reactivity in platelets.
The thrombin receptor activating peptide, TRAP-6 (Ser-Phe-Leu-Leu-Arg-Asn; 1 μM) caused a marked increase in platelet-leukocyte aggregates in whole blood (Fig. 6). In the presence of CNP (1 μM) or sodium nitroprusside (10 μM), formation of platelet-leukocyte aggregates in response to TRAP-6 was significantly reduced (Fig. 6).
Fig. 6.
CNP inhibits platelet-leukocyte interactions and thrombin-induced aggregation of human platelets. Effect of CNP (0.1-1 μM) on TRAP-6 (1 μM)-induced platelet-leukocyte aggregate formation (upper) and thrombin (1 unit/ml)-induced aggregation in prostacyclin-washed human platelets (Lower) is shown. Sodium nitroprusside (10 μM) was used as a positive control to inhibit platelet-leukocyte interactions (Upper). n = 5; *, P < 0.05.
Thrombin (1 unit/ml) elicited a significant and reproducible aggregation of prostacyclin-washed platelets. CNP (0.1-1 μM) produced a concentration-dependent inhibition of thrombin-induced platelet aggregation (Fig. 6). The potency of CNP was akin to that reported for NO released by isolated vascular endothelial cells (23, 24) and fits with the reported ability of CNP to block thrombus formation (25).
Thrombin (0.1 unit/ml) also produced a significant up-regulation of platelet P-selectin expression (Fig. 5) that was significantly inhibited in the presence of CNP (0.1-1 μM; Fig. 5).
Discussion
Natriuretic peptides and their guanylate cyclase-linked receptors represent attractive therapeutic targets because of their pivotal role in the regulation of cardiovascular homeostasis. Although much interest has focused on the biological effects and therapeutic potential of atrial natriuretic peptide and brain natriuretic peptide, little is known about the (patho)physiological importance of CNP. Recently, we have characterized CNP as a local regulator of vascular tone and blood flow through activation of NPR-C and subsequent smooth muscle cell hyperpolarization; such a pathway is likely to underlie the action of EDHF in certain vascular beds (11). Moreover, we have shown that this signaling pathway also regulates coronary blood flow and protects against myocardial I/R injury (12). Herein, we demonstrate that CNP also modulates leukocyte-endothelial interactions in vivo by down-regulating P-selectin expression. In addition, CNP-mediated suppression of P-selectin expression on platelets is manifested as a marked inhibition of aggregation and platelet-leukocyte interactions, and therefore assigns an antithrombotic role to CNP. Together, these observations intimate that the CNP is likely to be an integral regulator of local inflammation and represents a mechanism to target for treatment of inflammatory cardiovascular disorders.
Chronic deficiency in endothelium-derived NO, as found in eNOS-/- mice, results in high basal leukocyte rolling (2, 3, 6) as a result of increased expression of P-selectin. We used this model to investigate the antiinflammatory effects of CNP in vivo. In mesenteric postcapillary venules, superfusion of CNP reduced leukocyte rolling in a concentration-dependent manner such that CNP (1 μM) reduced leukocyte flux by ≈65%. The concentrations of CNP that were effective in inhibiting leukocyte-endothelial cell interactions were commensurate with the local concentrations of CNP released from the vascular endothelium, as we have reported in the mesenteric and coronary vascular beds (11, 12). The inhibitory effect of CNP on leukocyte recruitment occurred immediately (within 1 min), was maximal within 10 min of exposure, and was lost rapidly after washout of the peptide. Such observations are consistent with a direct effect of CNP on leukocyte-endothelial interactions and suggest that CNP is invoking a fast biochemical change in adhesion molecule expression to exert its antileukocyte actions.
NO-dependent inhibition of leukocyte-endothelial cell interactions depends on the activation of soluble guanylate cyclase and elevations in cyclic guanosine-3′, 5′-monophosphate (cGMP) (6). Historically, the biological effects of CNP have been attributed almost without exception to NPR-B-dependent elevations in cGMP, because CNP is the only endogenous ligand for this NPR identified to date (CNP does not activate NPR-A, whereas all natriuretic peptides bind to the “clearance receptor” NPR-C) (26). Because we hypothesized that NO and CNP act as complementary vasoprotective molecules, it is not unreasonable to postulate that CNP mediates its antileukocyte effects through NPR-B activation. Importantly, however, we have recently demonstrated that CNP can also elicit responses in the vasculature via an NPR-C-dependent mechanism. Because of its short cytoplasmic domain and lack of GC activity, NPR-C was classically regarded as a clearance receptor that binds (all) natriuretic peptides and removes them from the circulation. However, our recent findings highlight an important positive signal transduction pathway initiated by NPR-C activation. Thus, we wished to obtain insight into which NPR was involved in mediating CNP-induced inhibition of leukocyte rolling. In this regard, we used the selective NPR-C agonist (17), cANF4-23 (because of the lack of availability of selective NPR-B antagonists). cANF4-23 mimicked the inhibitory actions of CNP on leukocyte recruitment; although this observation only provides circumstantial evidence that CNP exerts its antileukocyte effects via this NPR, it does demonstrate that NPR-C activation is functionally important in the regulation of leukocyte-endothelial cell interactions.
Having demonstrated that CNP activation can inhibit basal leukocyte rolling, we investigated whether CNP could also inhibit leukocyte activation induced by an inflammatory stimulus. Application of histamine or pretreatment in vivo with IL-1β elevated leukocyte flux in eNOS-/- mice. Subsequent administration of CNP inhibited leukocyte rolling to below basal level, indicating that CNP can also modulate leukocyte activation induced by inflammatory stimuli. The finding that CNP inhibited leukocyte rolling induced by both histamine and IL-1β demonstrates that the antiinflammatory effects of CNP are mediated by a common mechanism (i.e., inhibition of P-selectin expression) that is independent of the stimulus. These antileukocyte effects of CNP are not merely due to its ability to suppress basal leukocyte flux because CNP also inhibited leukocyte rolling in WT animals (where basal leukocyte flux is low) exposed to inflammatory stimuli (i.e., histamine or IL-1β). The potent antileukocyte effects of CNP exerted in eNOS-/- mice are likely to have important implications for inflammatory cardiovascular diseases such as sepsis and atherosclerosis, which are characterized by endothelial dysfunction and loss of NO. Under such conditions, the importance of CNP in preventing adherence of leukocytes (and perhaps platelets) is likely to be accentuated and might compensate for the NO deficiency.
Early leukocyte rolling in vivo is mediated by interaction of adhesion molecules on activated leukocytes and endothelial cells. P-selectin is stored in endothelial cells and platelets and is rapidly mobilized to the cell surface upon stimulation. In this way, regulation of P-selectin expression rapidly alters leukocyte-endothelial interactions (6, 27, 28). Leukocyte rolling is also mediated by constitutively expressed L-selectin on leukocytes and transcription-regulated E-selectin expression on endothelial cells (29). The rapid antileukocyte effects of CNP (both in terms of onset and resolution) are consistent with modulation of selectin expression. To determine which adhesion molecules might be involved in CNP-mediated regulation of leukocyte rolling, we used FACS analysis to investigate adhesion molecule expression on activated endothelial cells and leukocytes in the absence and presence of CNP. Although the profile of proinflammatory stimuli required to elicit specific adhesion molecule expression does differ between murine and human cells (30), these studies were conducted in HUVECS and whole blood samples isolated from healthy volunteers to provide evidence that CNP can modulate adhesion molecule expression (and by inference leukocyte recruitment) in humans. Because the in vivo effects of IL-1β (used in the intravital studies) are mediated predominantly via mast cell degranulation (18, 20), we used histamine as a stimulus to elicit P-selectin expression on HUVECs. Consistent with the temporal effects of CNP on leukocyte activation, expression of P-selectin, but not E-selectin (which is not stored but regulated by transcription), was significantly suppressed by CNP. Although the maximum inhibitory effect of CNP on P-selectin expression was ≈45%, this was manifested as a much greater (≈65%) attenuation of leukocyte rolling. However, we have demonstrated that P-selectin expression is obligatory for leukocyte rolling in murine mesenteric postcapillary venules (a specific neutralizing antibody virtually abolishes leukocyte rolling) and that the rapid inhibition of leukocyte rolling by NO (or soluble guanylate cyclase activators) is mediated by down-regulation of endothelial P-selectin expression (6). Thus, a moderate inhibition of P-selectin expression is likely to produce a marked attenuation of leukocyte rolling because there appears to be no compensatory pathway involving E- or L-selectin in these animals (and the fact that leukocytes require contact with multiple P-selectin molecules to initiate rolling and arrest).
Firm adhesion of leukocytes to the endothelium requires integrin CD11b expression on leukocytes and ICAM-1 on the endothelium. CNP did not affect expression of endothelial ICAM-1 or leukocyte CD11b, suggesting that CNP is unlikely to modulate adherence of leukocytes to the vascular endothelium directly. In our studies, observations were made at early time points that coincide with the first step in leukocyte recruitment (i.e., rolling), meaning that leukocyte adhesion was minimal. However, the marked effect of CNP on leukocyte rolling should result in a knock-on, downstream decrease in leukocyte adhesion due to the sequential processes involved in leukocyte recruitment; this is supported by the fact that a similar degree of inhibition of platelet P-selectin expression resulted in a significant functional inhibition of aggregation and platelet-leukocyte interactions.
The observation that the antileukocyte effects of CNP were the result of down-regulation of P-selectin expression suggested that this peptide might also play a role in modulating platelet reactivity; P-selectin is a well characterized marker of platelet activation that is important in platelet-leukocyte interactions (31), and haemostasis is defective in P-selectin-deficient mice (32). In the present study, CNP inhibited platelet-leukocyte interactions/aggregate formation and produced a concentration-dependent inhibition of thrombin-induced platelet aggregation. These inhibitory effects were accompanied by a reduced expression of P-selectin on the platelet surface. Because NO also inhibits platelet aggregation and P-selectin expression (10), this provides further biochemical and functional data indicating that modulation of P-selectin is likely to a physiological role fulfilled by both CNP and NO to maintain an antiatherogenic influence on the blood vessel wall.
The mechanism(s) underlying CNP-mediated inhibition of P-selectin expression are unclear and merit further study. Interestingly, epoxyeicosatrienoic acids (EETs), proposed to represent EDHF in some tissues (33), have been reported to inhibit platelet aggregation via hyperpolarization, intimating that platelet reactivity is sensitive to changes in membrane potential. Because we have established CNP to act as an EDHF in the mesenteric and coronary circulation and shown that NPR-C activation leads to smooth muscle cell hyperpolarization (6, 12), such a mechanism may also account for the antiplatelet action of CNP. Yet, if G-protein coupling is responsible for transducing the signals conveyed by NPR-C activation (as appears to be the case in the mesenteric and coronary vasculature; refs. 11 and 12), one might expect CNP to promote platelet aggregation because activation of Gi signaling pathways is now well established to be a prerequisite for maximal platelet aggregation (34-36), including a role for GIRKs (37). However, previous reports suggest that only NPR-C (not NPR-A or NPR-B) are expressed on human platelets (38), intimating that an NPR-B-mediated increase in platelet cGMP (the second messenger invoked by NO to inhibit platelet reactivity) does not underlie the antiaggregatory effects of CNP. Thus, a potential NPR-C/Gi-coupled effector pathway that inhibits platelet aggregation might represent a target for antithrombotic therapy.
In sum, we have extended our previous work characterizing an important role for CNP in the vasculature by identifying a significant antileukocyte, antiplatelet effect of this peptide. This observation emphasises the importance of this novel signal transduction system in the vasculature and suggests that endothelial CNP likely possesses antiatherogenic properties akin to those exerted by endothelial NO. In concert, these mediators appear to play a key role in coordinating local blood flow and maintaining the luminal patency and integrity of the vascular endothelium to prevent pathogenesis. Moreover, in cardiovascular disorders characterized by NO deficiency, CNP is likely to act in a compensatory fashion, not only restoring the vasodilator capacity of the endothelium but also its antiadhesive and antiaggregatory influence. Previous studies have alluded to a potential therapeutic role for CNP in reducing neointimal hyperplasia and thrombogenicity and promoting reendothelialization after vascular damage, which has important implications for balloon-angioplasty and vein grafting (14, 39); this is supported by the enhanced expression of CNP in human atherosclerotic lesions (16). CNP is also released from the myocardium during heart failure and acts in a cytoprotective fashion (12, 40). Thus, the findings of the present study, in combination with the recent identification of a key role for CNP/NPR-C in regulating vascular tone and vascular smooth muscle proliferation, provide the rationale for the effectiveness of this peptide in the treatment of inflammatory cardiovascular conditions such as sepsis, atherosclerosis, coronary artery disease, and I/R injury.
Acknowledgments
This work was funded by a project grant from The Wellcome Trust. A.J.H. is the recipient of a Wellcome Trust Senior Research Fellowship.
Author contributions: R.S.S., P.F., A.M., A.A., and A.J.H. designed research; R.S.S., M.C., P.F., M.L., and A.J.H. performed research; R.S.S., M.C., P.F., M.L., A.M., A.A., and A.J.H. analyzed data; and R.S.S., A.M., A.A., and A.J.H. wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: eNOS, endothelial NOS; ICAM, intercellular adhesion molecule; CNP, C-type natriuretic protein; EDHF, endothelium-derived hyperpolarizing factor; GIRK; G protein-coupled inwardly rectifying K+ channel; NPR, natriuretic peptide receptor; I/R, ischemia-reperfusion; HUVEC, human vascular endothelial cell.
References
- 1.Granger, D. N. & Kubes, P. (1994) J. Leukocyte Biol. 55, 662-675. [PubMed] [Google Scholar]
- 2.Lefer, D. J., Jones, S. P., Girod, W. G., Baines, A., Grisham, M. B., Cockrell, A. S., Huang, P. L. & Scalia, R. (1999) Am. J. Physiol. 276, H1943-H1950. [DOI] [PubMed] [Google Scholar]
- 3.Sanz, M. J., Hickey, M. J., Johnston, B., McCafferty, D. M., Raharjo, E., Huang, P. L. & Kubes, P. (2001) Br. J. Pharmacol. 134, 305-312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Radomski, M. W., Palmer, R. M. & Moncada, S. (1987) Br. J. Pharmacol. 92, 639-646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kubes, P., Suzuki, M. & Granger, D. N. (1991) Proc. Natl. Acad. Sci. USA 88, 4651-4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ahluwalia, A., Foster, P., Scotland, R. S., McLean, P. G., Mathur, A., Perretti, M., Moncada, S. & Hobbs, A. J. (2004) Proc. Natl. Acad. Sci. USA 101, 1386-1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Armstead, V. E., Minchenko, A. G., Schuhl, R. A., Hayward, R., Nossuli, T. O. & Lefer, A. M. (1997) Am. J. Physiol. 273, H740-H746. [DOI] [PubMed] [Google Scholar]
- 8.Liu, P., Xu, B., Hock, C. E., Nagele, R., Sun, F. F. & Wong, P. Y. (1998) Am. J. Physiol. 275, H2191-H2198. [DOI] [PubMed] [Google Scholar]
- 9.Kubes, P., Kurose, I. & Granger, D. N. (1994) Am. J. Physiol. 267, H931-H937. [DOI] [PubMed] [Google Scholar]
- 10.Langford, E. J., Brown, A. S., Wainwright, R. J., de Belder, A. J., Thomas, M. R., Smith, R. E., Radomski, M. W., Martin, J. F. & Moncada, S. (1994) Lancet 344, 1458-1460. [DOI] [PubMed] [Google Scholar]
- 11.Chauhan, S. D., Nilsson, H., Ahluwalia, A. & Hobbs, A. J. (2003) Proc. Natl. Acad. Sci. USA 100, 1426-1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hobbs, A., Foster, P., Prescott, C., Scotland, R. & Ahluwalia, A. (2004) Circulation 110, 1231-1235. [DOI] [PubMed] [Google Scholar]
- 13.Schachner, T., Zou, Y., Oberhuber, A., Mairinger, T., Tzankov, A., Laufer, G., Ott, H. & Bonatti, J. (2004) Eur. J. Cardiothorac. Surg. 25, 585-590. [DOI] [PubMed] [Google Scholar]
- 14.Ohno, N., Itoh, H., Ikeda, T., Ueyama, K., Yamahara, K., Doi, K., Yamashita, J., Inoue, M., Masatsugu, K., Sawada, N., et al. (2002) Circulation 105, 1623-1626. [DOI] [PubMed] [Google Scholar]
- 15.Yamahara, K., Itoh, H., Chun, T. H., Ogawa, Y., Yamashita, J., Sawada, N., Fukunaga, Y., Sone, M., Yurugi-Kobayashi, T., Miyashita, K. et al. (2003) Proc. Natl. Acad. Sci. USA 100, 3404-3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Naruko, T., Ueda, M., van der Wal, A. C., van der Loos, C. M., Itoh, H., Nakao, K. & Becker, A. E. (1996) Circulation 94, 3103-3108. [DOI] [PubMed] [Google Scholar]
- 17.Maack, T., Suzuki, M., Almeida, F. A., Nussenzveig, D., Scarborough, R. M., McEnroe, G. A. & Lewicki, J. A. (1987) Science 238, 675-678. [DOI] [PubMed] [Google Scholar]
- 18.McLean, P. G., Ahluwalia, A. & Perretti, M. (2000) J. Exp. Med. 192, 367-380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Radomski, M. & Moncada, S. (1983) Thromb. Res. 30, 383-389. [DOI] [PubMed] [Google Scholar]
- 20.Subramanian, N. & Bray, M. A. (1987) J. Immunol. 138, 271-275. [PubMed] [Google Scholar]
- 21.Huo, Y., Schober, A., Forlow, S. B., Smith, D. F., Hyman, M. C., Jung, S., Littman, D. R., Weber, C. & Ley, K. (2003) Nat. Med. 9, 61-67. [DOI] [PubMed] [Google Scholar]
- 22.Ley, K. (2003) Trends Mol. Med. 9, 263-268. [DOI] [PubMed] [Google Scholar]
- 23.Radomski, M. W., Palmer, R. M. & Moncada, S. (1987) Br. J. Pharmacol. 92, 181-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Salvemini, D., Korbut, R., Anggard, E. & Vane, J. (1990) Proc. Natl. Acad. Sci. USA 87, 2593-2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qian, J. Y., Haruno, A., Asada, Y., Nishida, T., Saito, Y., Matsuda, T. & Ueno, H. (2002) Circ. Res. 91, 1063-1069. [DOI] [PubMed] [Google Scholar]
- 26.Chen, H. H. & Burnett, J. C., Jr. (1998) J. Cardiovasc. Pharmacol. 32, Suppl. 3, S22-S28. [PubMed] [Google Scholar]
- 27.Hicks, A. E., Nolan, S. L., Ridger, V. C., Hellewell, P. G. & Norman, K. E. (2003) Blood 101, 3249-3256. [DOI] [PubMed] [Google Scholar]
- 28.Hicks, A. E., Leppanen, A., Cummings, R. D., McEver, R. P., Hellewell, P. G. & Norman, K. E. (2002) FASEB J. 16, 1461-1462. [DOI] [PubMed] [Google Scholar]
- 29.Norman, K. E., Katopodis, A. G., Thoma, G., Kolbinger, F., Hicks, A. E., Cotter, M. J., Pockley, A. G. & Hellewell, P. G. (2000) Blood 96, 3585-3591. [PubMed] [Google Scholar]
- 30.Yao, L., Setiadi, H., Xia, L., Laszik, Z., Taylor, F. B. & McEver, R. P. (1999) Blood 94, 3820-3828. [PubMed] [Google Scholar]
- 31.McEver, R. P. (1991) J. Cell Biochem. 45, 156-161. [DOI] [PubMed] [Google Scholar]
- 32.Subramaniam, M., Frenette, P. S., Saffaripour, S., Johnson, R. C., Hynes, R. O. & Wagner, D. D. (1996) Blood 87, 1238-1242. [PubMed] [Google Scholar]
- 33.Fisslthaler, B., Popp, R., Kiss, L., Potente, M., Harder, D. R., Fleming, I. & Busse, R. (1999) Nature 401, 493-497. [DOI] [PubMed] [Google Scholar]
- 34.Roger, S., Pawlowski, M., Habib, A., Jandrot-Perrus, M., Rosa, J. P. & Bryckaert, M. (2004) FEBS Lett. 556, 227-235. [DOI] [PubMed] [Google Scholar]
- 35.Gratacap, M. P., Herault, J. P., Viala, C., Ragab, A., Savi, P., Herbert, J. M., Chap, H., Plantavid, M. & Payrastre, B. (2000) Blood 96, 3439-3446. [PubMed] [Google Scholar]
- 36.Paul, B. Z., Jin, J. & Kunapuli, S. P. (1999) J. Biol. Chem. 274, 29108-29114. [DOI] [PubMed] [Google Scholar]
- 37.Shankar, H., Murugappan, S., Kim, S., Jin, J., Ding, Z., Wickman, K. & Kunapuli, S. P. (2004) Blood 104, 1335-1343. [DOI] [PubMed] [Google Scholar]
- 38.Blaise, V., Wolf, J. P., Regnard, J. & Berthelay, S. (1994) Cell Mol. Biol. 40, 309-317. [PubMed] [Google Scholar]
- 39.Doi, K., Ikeda, T., Itoh, H., Ueyama, K., Hosoda, K., Ogawa, Y., Yamashita, J., Chun, T. H., Inoue, M., Masatsugu, K., et al. (2001) Arterioscler. Thromb. Vasc. Biol. 21, 930-936. [DOI] [PubMed] [Google Scholar]
- 40.Kalra, P. R., Clague, J. R., Bolger, A. P., Anker, S. D., Poole-Wilson, P. A., Struthers, A. D. & Coats, A. J. (2003) Circulation 107, 571-573. [DOI] [PubMed] [Google Scholar]