Abstract
Prostaglandin D2 (PGD2), a major cyclooxygenase product in a variety of tissues and cells, readily undergoes dehydration to yield the bioactive cyclopentenone-type PGs of the J2-series, such as 15-deoxy-Δ12,14-PGJ2 (15d-PGJ2). The observation that the level of 15d-PGJ2 increased in the tissue cells from patients with sporadic amyotrophic lateral sclerosis suggested that the formation of 15d-PGJ2 may be closely associated with neuronal cell death during chronic inflammatory processes. In vitro experiments using SH-SY5Y human neuroblastoma cells revealed that 15d-PGJ2 induced apoptotic cell death. An oligonucleotide microarray analysis demonstrated that, in addition to the heat shock-responsive and redox-responsive genes, the p53-responsive genes, such as gadd45, cyclin G1, and cathepsin D, were significantly up-regulated in the cells treated with 15d-PGJ2. Indeed, the 15d-PGJ2 induced accumulation and phosphorylation of p53, which was accompanied by a preferential redistribution of the p53 protein in the nuclei of the cells and by a time-dependent increase in p53 DNA binding activity, suggesting that p53 accumulated in response to the treatment with 15d-PGJ2 was functional. The 15d-PGJ2-induced accumulation of p53 resulted in the activation of a death-inducing caspase cascade mediated by Fas and the Fas ligand.
Prostaglandin (PG) D2 is a major cyclooxygenase-catalyzed reaction product in a variety of tissues and cells and has significant effects on a number of biological processes, including platelet aggregation, the relaxation of vascular and nonvascular smooth muscles, and nerve cell functions (1). It has been shown that PGD2 readily undergoes dehydration in vivo and in vitro to yield biologically active PGs of the J2 series, such as PGJ2, Δ12,14-PGJ2, and 15-deoxy-Δ12,14-PGJ2 (15d-PGJ2) (2–4). Members of the J2 series of the PGs, characterized by the presence of a reactive α,β-unsaturated ketone in the cyclopentenone ring (cyclopentenone PGs), have their own unique spectrum of biological effects, including antitumor activity, the inhibition of cell cycle progression, the suppression of viral replication, the induction of heat shock protein expression, and the stimulation of osteogenesis (5). In addition, they also show an anti-inflammatory effect, including inhibition of nuclear factor-κB activation by inhibiting IκB kinase (6, 7).
Many types of mammalian cells undergo apoptosis during normal development or in response to a variety of stimuli, including DNA damage, growth factor deprivation, and the abnormal expression of oncogenes or tumor suppressor genes (8–10). Apoptosis induced by these agents appears to be mediated by a common set of downstream elements that act as regulators and effectors of apoptotic cell death. In many cases, apoptosis requires the p53 tumor suppressor protein (11). The function of p53 in the destruction of aberrant cells has been well established in numerous organ systems. The loss of p53 activity undermines healthy cellular phenotypes, which can lead to cancer and neurodevelopmental diseases (12). The central involvement of p53 in initiating apoptosis after exposure to a pleiotropic array of stimuli results in the up-regulation of proapoptotic members of the bcl-2 family of proteins, such as Bcl-2 and Bax, or in cell cycle inhibitors p21 (13). Recent data suggest that p53-induced apoptosis is also through mechanisms other than Bax. Several laboratories have suggested the involvement of the Fas/Fas ligand (FasL) in p53-mediated apoptosis (14).
In the present study, we obtained in vivo evidence that the level of 15d-PGJ2 increased in the spinal cord motor neurons of sporadic amyotrophic lateral sclerosis (ALS) patients. Moreover, we investigated the molecular mechanisms involved in neuronal cell death induced by 15d-PGJ2.
Experimental Procedures
Material.
15d-PGJ2 was obtained from Cayman Chemicals (Ann Arbor, MI). Hoechst 33258 and propidium iodide (PI) were from Molecular Probes. The caspase inhibitors benzyloxycarbonyl-Val-Ala-Asp-fluoromethyl ketone (z-VAD-fmk) and acetyl-Ile-Glu-Thr-Asp-CHO (Ac-IETD-CHO) were obtained from Peptide Institute (Osaka). The antibodies against cyclooxygenase-2 (COX-2), p53, and phosphorylated p53 at Ser-15 were obtained from Santa Cruz Biotechnology. The anti-15d-PGJ2 monoclonal antibody (mAb11G2) was used for immunohistochemical detection of 15d-PGJ2 (15). The p53 sense (p53SE) and antisense (p53AS) oligonucleotides were obtained from Amersham Pharmacia Biosciences: p53SE, 5′-GGAGCCAGGGGGGAGCAGGG-3′; p53AS, 5′-CCCTGCTCCCCCCTGGCTCC-3′.
Cell Culture and Cell Viability.
SH-SY5Y cells were grown in Cosmedium-001 (Cosmo-Bio, Tokyo) containing 5% Nakashibetsu precolostrum new-born calf serum, 100 μg/ml penicillin, and 100 units/ml streptomycin. The cells were seeded in plates coated with polylysine and cultured at 37°C. Cell viability was quantified by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay as previously described (16).
Gene Chip Analysis.
An oligonucleotide microarray analysis was performed by using a gene chip, the HuGene human FL array (Affymetrix, Santa Clara, CA) as previously described (17).
Nuclear Staining Assay.
To determine the apoptotic nuclei, cells treated with 10 μM 15d-PGJ2 or vehicle for 24 and 48 h were fixed in 4% paraformaldehyde solution and stained with a fluorescent DNA-binding dye, Hoechst 33258, and observed by using a fluorescence microscope (Olympus, Tokyo).
Analysis of DNA Fragmentation.
For analysis of the DNA fragmentation by agarose gel electrophoresis, cellular DNA was extracted from whole cells treated with 10 μM 15d-PGJ2 for 24 and 48 h. After treatment with 15d-PGJ2, the cells were harvested, washed once with ice-cold PBS, and resuspended in 100 μl of lysis buffer (10 mM Tris⋅HCl, pH 7.4/10 mM EDTA, pH 8.0/0.5% Triton X-100), and the samples were then left on ice for 10 min. After centrifugation at 9,000 × g for 5 min, the supernatant was transferred to another Eppendorf tube, and sequential extractions were carried out. RNase was added to the samples at the final concentration of 0.5 mg/ml, and the mixture was incubated at 37°C for 1 h. Then, proteinase K was added to the mixture at the final concentration of 0.5 mg/ml, and the mixture was incubated at 50°C for 30 min. Electrophoresis was performed on a 2.0% agarose gel, and DNA was visualized by ethidium bromide staining.
Flow Cytometry Analysis of Cell Cycle.
The cells were washed and then collected with PBS. The samples were centrifuged and treated with PBS containing 0.1% Triton X-100 and resuspended in 0.5% RNase and 25 μg/ml PI. The stained cells were analyzed on a flow cytometer (Epics XL, Beckman Coulter).
Immunoblot Analysis.
Whole cell lysates from SH-SY5Y cells treated with 15d-PGJ2 were incubated with the SDS-sample buffer for 5 min at 100°C. The samples were then separated by 10% SDS/PAGE. One gel was used for staining with Coomassie Brilliant Blue; the other gel was transblotted onto a nitrocellulose membrane, incubated with Blockace for blocking, washed, and incubated with antibody. This procedure was followed by the addition of horseradish peroxidase conjugated to rabbit anti-mouse IgG and ECL reagents. The bands were visualized by Cool Saver AE-6955 (Atto, Tokyo).
Reverse Transcription (RT)-PCR.
The total RNA was isolated with isogen reagent (Nippon Gene, Tokyo) and spectrophotometrically quantified. The RT-PCR was performed as previously reported (18).
Localization of p53.
For the immunocytochemistry, cells were fixed overnight in PBS containing 2% paraformaldehyde and 0.2% picric acid at 4°C. After the membranes were permeabilized by exposing the fixed cells to PBS containing 0.3% Triton X-100, the cells were sequentially incubated in PBS solutions containing blocking serum (5% normal goat serum) and immunostained with the anti-p53 antibody. The cells were then incubated for 1 h in the presence of FITC-labeled goat anti-rabbit, rinsed with PBS containing 0.3% Triton X-100, and covered with anti-fade solution. Images of the cellular immunofluorescence were acquired by using a confocal laser microscope (Bio-Rad) with a ×40 objective (488-nm excitation and 518-nm emission).
Gel Shift Assay.
Nuclear proteins were prepared as previously reported (19). Binding reactions involved 22 μl volumes containing 5 μg of nuclear proteins, 3 μg of poly(dI-dC), and the [γ-32P]ATP-labeled oligonucleotide probe (2 × 104 cpm). After 30 min, the DNA–protein complexes were resolved in a 6% nondenaturing acrylamide gel and electrophoresed at room temperature. The gel was dried and exposed to Kodak film at −80°C overnight. The P53 consensus oligonucleotide probe used was as follow: 5′-TACAGAACATGTCTAAGCATGCTGGGGACT-3′ (Santa Cruz Biotechnology).
Immunohistochemical Detection of 15d-PGJ2 in Sporadic ALS Patients.
The presence of 15d-PGJ2 was examined on spinal cords obtained at autopsy from six sporadic ALS patients [sex: three males and three females; age: 31–73 (58.67 ± 15.17) years] and six age-matched, non-diabetic control individuals [sex: two males and four females; age: 50–78 (61.67 ± 11.34) years] without neurological disorders, after their family members granted informed consent in accordance with the Guideline for human materials in Tokyo Women's Medical University. Each spinal cord was processed with formlain (10%)-fixed, paraffin-embedded materials stored at room temperature for hematoxylin-eosin staining and COX-2 immunohistochemistry, and with OCT compound (Sakura Fine Technical, Tokyo)-embedded, frozen materials stored at −80°C for COX-2 and 15d-PGJ2 immunohistochemistry. An immunohistochemical analysis was performed as previously described (20).
Results
Immunochemical Detection of 15d-PGJ2 in Motor Neurons of Sporadic ALS Patients.
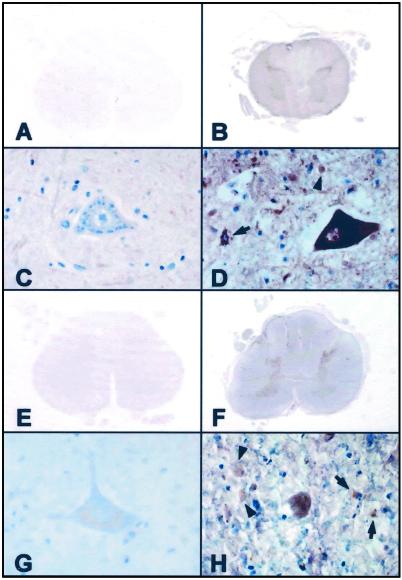
To determine the role for 15d-PGJ2 in neuronal degeneration, we investigated immunohistochemical localization of 15d-PGJ2 in the control and ALS spinal cords, by using anti-15d-PGJ2 monoclonal antibody that specifically recognizes free 15d-PGJ2 (15). No significant immunoreaction product deposits were seen in sections processed with the omission of the primary antibodies (data not shown). As shown in Fig. 1, the spinal cords in the control cases exhibited no significant immunoreactivities for COX-2 or 15d-PGJ2 (Fig. 1 A, C, E, and G). In the ALS cases, both the anti-COX-2 and 15d-PGJ2 antibodies reacted strongly with the spinal cord gray matter (Fig. 1 B and F), especially almost all of the anterior horn cells (Fig. 1 D and H). These immunoreactivities were more intense in the perikarya and proximal cell processes of the anterior horn cells than in the reactive astrocytes, microglia/macrophages, and surrounding neuropil (Fig. 1 D and H). The coexistence of COX-2 and 15d-PGJ2 in the neurons and glias was verified on consecutive sections. These data suggest that the intracellular production of 15d-PGJ2 may be closely associated with neuron death during inflammatory processes.
Figure 1.
Photomicrographs of spinal cord sections from control cases (A, C, E, and G) and ALS cases (B, D, F, and H) immunostained with antibodies specific for COX-2 (A–D) and 15d-PGJ2 (E–H). The control spinal cords were negatively stained with the antibodies to COX-2 (A and C) and 15d-PGJ2 (E and G). In the ALS spinal cords, both of these antibodies reacted with the gray matter but not with the white matter (B and F). These immunoreactivities were more intense in the perikarya and proximal cell processes of the anterior horn cells, compared with the reactive astrocytes (arrows), microglia/macrophages (arrowheads), and surrounding neuropil to a lesser extent. (A–D) Formalin-fixed, paraffin-embedded sections; (E-H) OCT compound-embedded, frozen sections; (A-H) avidin-biotin-immunoperoxidase complex method. (A, B, E, and F) ×6; (C, D, G, and H) ×40.
Induction of Apoptotic Cell Death of SH-SY5Y Neuroblastoma Cells by 15d-PGJ2.
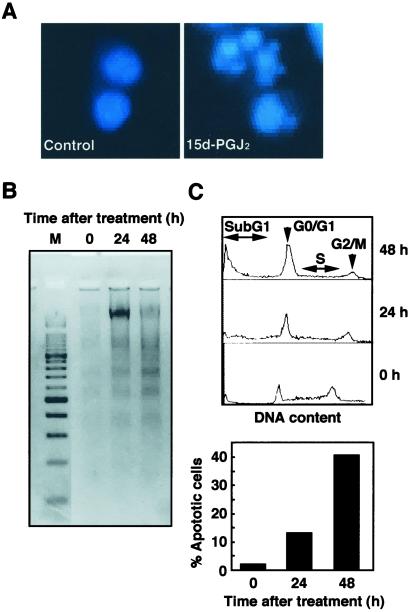
We have previously shown that the in vitro exposure of human nueroblastoma SH-SY5Y cells to 15d-PGJ2 led to a dose- and time-dependent decrease in the number of viable cells (16). Here, we characterized whether 15d-PGJ2-induced cell death included apoptosis. When human nueroblastoma SH-SY5Y cells were exposed to 10 μM 15-PGJ2, fragmented nuclei were found in cells exhibiting typical morphological features of apoptosis (Fig. 2A). The gel electrophoresis of DNA from SH-SY5Y cells exposed to 15-PGJ2 also displayed nucleosomal DNA fragmentation (Fig. 2B). Moreover, we measured the numbers of cells with a subG1 DNA content as a measure of apoptosis and observed a significant increase in the apoptotic cells (Fig. 2C). These results indicate that 15d-PGJ2 induces apoptotic cell death of SH-SY5Y cells.
Figure 2.
Induction of apoptosis in SH-SY5Y cells treated with 15d-PGJ2. (A) SH-SY5Y human neuroblastoma cells were fixed with paraformaldehyde, stained with Hoechst 33258, and examined by fluorescence microscopy. (Left) Untreated control cells. (Right) Cells treated with 10 μM 15d-PGJ2 for 24 h. (B) DNA fragmentation in SH-SY5Y cells treated with 10 μM 15d-PGJ2. Nucleosomal DNA fragmentation was visualized by agarose gel electrophoresis. M, DNA size markers. (C) SubG1 analysis of SH-SY5Y cells treated with 15d-PGJ2. SH-SY5Y cells treated with 10 μM 15d-PGJ2 were analyzed for DNA content by PI staining by using a flow cytometer (Upper). The subG1 DNA content was used as indicative of apoptotic cells (Lower). Cells with nuclei condensation, DNA fragmentation, and subG1 DNA contents as typical hallmarks of apoptosis revealed that 15d-PGJ2 induced apoptosis.
15d-PGJ2-Mediated Gene Expression.
Clay et al. (21) have recently demonstrated that early de novo gene expression is required for 15-PGJ2-induced apoptosis in breast cancer cells. Hence, to investigate the 15-PGJ2-mediated expression of genes critical to the apoptosis of the SH-SY5Y cells, we performed an oligonucleotide microarrays analysis. As shown in Table 1, the expression of genes involved in the heat shock response, including heme oxygenase, HSP40, HSP70, and HSP28, and the genes involved in the redox regulation, including γ-glutamylcysteine synthetase, thioredoxin reductase, Nrf2, and glutathione peroxidase, was significantly up-regulated. More notably, we found that the p53-responsive genes, such as gadd45, cyclin G1, and cathepsin D, significantly rose by >3-fold in cells treated with 15d-PGJ2. The data suggest that p53, which is thought to monitor the integrity of the cellular genome and responds to DNA damage by inducing cell cycle arrest and/or apoptosis, is activated in cells treated with 15d-PGJ2.
Table 1.
Effect of 15d-PGJ2 on gene expression studied by gene chip
| GenBank accession no. | Name of gene | Fold increase |
|---|---|---|
| X06985 | Human mRNA for heme oxygenase | 75.5 |
| L35546 | Homo sapiens γ-glutamylcysteine synthetase light subunit mRNA; complete cds | 17.8 |
| M63138 | Cathepsin D (catD) gene | 14.9 |
| D85429 | H. sapiens gene for heat shock protein 40; complete cds | 12.2 |
| M60974 | Human growth arrest and DNA-damage-inducible protein (gadd45) mRNA; complete cds | 5.5 |
| L12723 | Human heat shock protein 70 (hsp70) mRNA; complete cds | 3.6 |
| X91247 | H. sapiens mRNA for thioredoxin reductase | 3.3 |
| S74017 | Nrf2 = NF-E2-like basic leucine zipper transcriptional activator (human; hemin-induced K562 cells; mRNA; 2304 nt) | 3.3 |
| X77794 | H. sapiens mRNA for cyclin G1 | 3.2 |
| Z23090 | H. sapiens mRNA for 28-kDa heat shock protein | 3.1 |
| D00632 | H. sapiens mRNA for glutathione peroxidase; complete cds | 2.6 |
Total RNA isolated from the SH-SY5Y cells treated with 10 μM 15d-PGJ2 for 24 h was subjected to oligonucleotide microarray analysis.
15d-PGJ2-Induced Apoptosis Is p53 Dependent.
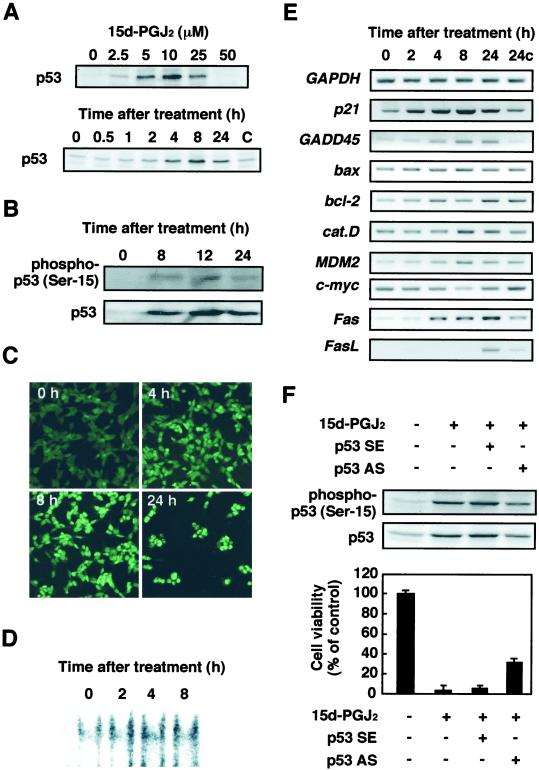
The tumor suppressor gene product p53 has been reported to mediate apoptosis in many experimental systems. It is believed that this activity is responsible for the tumor suppressive function of p53 (13, 22, 23). Therefore, we examined whether the p53 protein is involved in the 15d-PGJ2-induced apoptosis. As shown in Fig. 3A, 15d-PGJ2 induced p53 in time- and dose-dependent manners. To determine whether the p53 accumulated in response to the treatment with 15d-PGJ2 was functional, we assayed the phosphorylation and subcellular localization of p53 and expression of the p53 target genes in cells treated with 15d-PGJ2. As shown in Fig. 3B, 15d-PGJ2 significantly induced the phosphorylation of p53. In addition, the p53 phosphorylation was accompanied by a preferential redistribution of the p53 protein into the nuclei of the cells (Fig. 3C) and by significant increase in p53 DNA binding activity (Fig. 3D). Moreover, the RT-PCR analysis also demonstrated the up-regulation of a distinct set of p53-responsive genes, such as p21, Fas, FasL (Fas ligand), MDM2, and gadd45, in the cells treated with 15d-PGJ2 (Fig. 3E). 15d-PGJ2 scarcely induced the proapoptotic Bcl-2 family members, such as Bax and Bcl-2. Finally, the involvement of p53 in the 15d-PGJ2-induced cell death was examined by the effect of p53 antisense on the cytotoxic effect of 15d-PGJ2. As shown in Fig. 3F, the p53 antisense significantly suppressed the 15d-PGJ2-induced phosphorylation and expression of p53, which were well correlated with the inhibition of cell death. These data suggest that p53 is involved in the 15d-PGJ2-induced cell death.
Figure 3.
Induction of p53 in SH-SY5Y cells treated with 15d-PGJ2. SH-SY5Y cells were treated for 8 h with 10 μM of the indicated PGs, and p53 induction was examined by immunoblot analysis. (A Upper) Dose-dependent induction of p53 in SH-SY5Y cells treated with 0–50 μM 15d-PGJ2 for 8 h. (Lower) Time-dependent induction of p53 in SH-SY5Y cells treated with 10 μM 15d-PGJ2. (B) Analysis of activated p53 in SH-SY5Y cells treated with 10 μM 15d-PGJ2. (C) Induction of nuclear translocation of p53 in SH-SY5Y cells exposed to 10 μM 15d-PGJ2. The RL34 cells were fixed in 2% paraformaldehyde and 0.2% picric acid and then immunostained with the anti-p53 antibody. Images of the cellular immunofluorescence were acquired by using a confocal laser scanning microscope. (D) 15d-PGJ2 induces nuclear protein-p53 binding activity. SH-SY5Y cells were treated with 10 μM 15d-PGJ2 for different time intervals as indicated in the figures. (E) RT-PCR analysis of p53-responsive gene expression in SH-SY5Y cells treated with 10 μM 15d-PGJ2 for different time intervals. (F) Inhibition of p53 phosphorylation, p53 protein expression, and cell death by p53AS in SH-SY5Y cells treated with 15d-PGJ2. Indicated antisense oligonucleotides (2 μg) were transfected for 12 h in SH-SY5Y cells, and then the cells were treated for 8 h with 10 μM 15d-PGJ2. The induction of p53 was examined by immunoblot analysis, and cell viability was measured by the MTT assay. In the MTT assay, data are expressed as the percentage of control culture conditions.
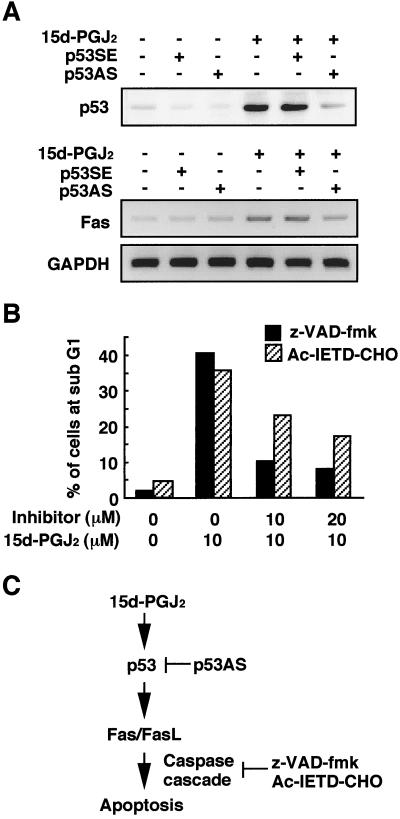
Activation of p53-Mediated Fas/FasL Signaling Pathway by 15d-PGJ2.
It was found that the p53-mediated apoptosis required transcriptional activation, because actinomycin D and cycloheximide, which block RNA and protein synthesis, significantly inhibited the 15d-PGJ2 cytotoxicity (data not shown). These results were consistent with the previous observation that early de novo gene expression is required for the 15-PGJ2-induced apoptosis in breast cancer cells (21). Therefore, it is reasonable to hypothesize that the p53-responsive gene products may be involved in the signaling cascade of apoptosis. In this context, based on the finding (Fig. 3E) that 15d-PGJ2 significantly induced the gene expressions of Fas and FasL that elicit the activation of a death-inducing caspase cascade, we examined the involvement of the Fas/FasL signaling pathway in the p53-mediated apoptosis induced by 15d-PGJ2. As shown in Fig. 4A, the p53 antisense suppressed the expression of Fas, suggesting that the p53 accumulation might be associated with the up-regulations of Fas and its ligand. To examine the involvement of the caspase cascade, broad spectrum of the caspase inhibitor, z-VAD-fmk, and a specific caspase-8 inhibitor, Ac-IETD-CHO, were used to treat the cells after exposure to 15d-PGJ2. As shown in Fig. 4B, both inhibitors significantly prevented the 15d-PGJ2-induced neuronal apoptosis monitored by the accumulation of the subG1 fraction. Thus, it appears that 15d-PGJ2 is a potential activator of the p53-mediated Fas/FasL signaling pathway (Fig. 4C).
Figure 4.
p53-dependent activation of Fas/FasL pathway by 15d-PGJ2. (A) Inhibition of p53 protein expression (Upper) and Fas expression (Lower) by p53AS in SH-SY5Y cells treated with 15d-PGJ2. The p53 sense (p53SE) or antisense (p53AS) oligonucleotides were transfected with Lipofectin (GIBCO) reagents for 12 h in SH-SY5Y cells, and then the cells were treated with 10 μM 15d-PGJ2 for 8 h. Fas induction was examined by RT-PCR analysis. Treatment with Lipofectin alone did not affect the expression of p53 and Fas (data not shown). (B) Effect of caspase inhibitors on 15d-PGJ2-induced increase in number of cells with a subG1 DNA content. The inhibitors used were z-VAD-fmk (hatched bar) and Ac-IETD-CHO (filled bar). The SH-SY5Y cells were treated with 10 μM 15d-PGJ2 in the presence or absence of inhibitor for 8 h. The subG1 DNA contents were analyzed by PI staining by using a flow cytometer. (C) Model for mechanisms by which 15d-PGJ2 exerts p53-mediated neuronal apoptosis.
Discussion
In the present study, we demonstrated that 15d-PGJ2 was accumulated in the spinal cord of sporadic ALS patients, mainly occurring in the motor neurons of the anterior horn (Fig. 1). Many of the COX-2- and 15d-PGJ2-positive neurons were devoid of pathological changes, but some were clearly distorted and smaller, probably corresponding to the more advanced injury of the neurons. To a lesser extent, the positive cells in the gray matter were non-neuronal and primarily corresponded to the reactive astrocytes and occasionally to the activated microglial cells. These data raise the possibility that COX-2 up-regulation, through its pivotal role in inflammation, followed by the enhanced intracellular production of 15d-PGJ2 is involved in the ALS neurodegenerative process.
The in vitro studies using SH-SY5Y neuroblastoma cells showed that 15d-PGJ2-induced cell death included apoptosis (Fig. 2). Moreover, the gene chip and RT-PCR analyses identified p53 as a key molecule in the 15d-PGJ2-induced apoptosis. The p53 protein is a tumor suppressor protein that transmits signals arising from various forms of cellular stress, including DNA damage and hypoxia, to genes and factors that induce cell cycle arrest and apoptosis (24). Not only is p53 induced by a variety of apoptotic stimuli, but the overexpression of p53 has been demonstrated to induce apoptosis in a variety of cell types (11, 25). Based on the enhanced gene expression of p53-responsive genes in the 15d-PGJ2-treated cells, we hypothesized that p53 might be accumulated and activated in the cells. Consistent with this hypothesis, we found that the treatment with 15d-PGJ2 resulted in the rapid accumulation of p53 in the cells (Fig. 3 A–C) and that, although there was a possibility that the PG could inactivate the wild-type p53 (26), the accumulated p53 was shown to be biologically active, based on the increased p53 DNA binding activity (Fig. 3D) and transactivation of the p53 target genes (Fig. 3E). Moreover, the 15d-PGJ2-induced apoptosis was blocked by the expression of the p53 antisense oligonucleotide (Fig. 3F). Although other molecules may also be involved in cell death, these data suggest that p53 is a key molecule in the apoptosis induced by 15d-PGJ2.
The precise mechanism by which p53 modulates cell viability has not yet been determined. However, the prime candidates for its regulation may be Fas and its ligand (FasL), which are known to be one of the downstream mediators in the p53-dependent apoptotic pathway (14). Indeed, the p53- and Fas-associated apoptosis has been suggested to be a common mechanism of cell loss in several neurodegenerative diseases (27). The Fas (CD95/APO-1) receptor is a member of the death receptor subfamily of the tumor necrosis factor/nerve growth factor superfamily. The receptor has an extracellular domain for ligand binding and an intracellular death domain. After binding to its specific ligand FasL, the trimerization of Fas recruits the Fas-associated death domain (FADD) via interactions between the death domains of Fas and FADD. This is followed by FADD-like IL-1β-converting enzyme (FLICE)/caspase-8 binding via interactions between the death-effector domains of FADD and caspase-8, and by activation of caspase-8. Activation of caspase-8, in turn, activates the caspase cascade leading to apoptosis (14). The present study showed that 15d-PGJ2 induced the gene expression of Fas and FasL (Fig. 3E) and that the 15d-PGJ2-induced Fas expression was significantly blocked by the expression of the p53 antisense oligonucleotide (Fig. 4A). These and the observations that the 15d-PGJ2-induced apoptosis monitored by the accumulation of the subG1 fraction was completely inhibited by the addition of a broad spectrum of caspase inhibitor and a specific caspase-8 inhibitor (Fig. 4B) suggest that 15d-PGJ2 is a potential activator of the Fas/FasL signaling pathway in SH-SY5Y neuroblastoma cells.
On the other hand, mitochondrial dysfunction is also a well-documented event in apoptosis (28, 29). A process known as permeability transition appears to be responsible for the loss of the mitochondrial membrane potential, leading to the opening of the permeability transition pore and release of solutes from the mitochondria (30–32). Among the proteins released are the apoptosis-inducing factor and cytochrome c. The release of these proteins leads to activation of caspases, a family of serine threonine proteases, and subsequently apoptosis (33). The exposure of cells to 15d-PGJ2 indeed resulted in a significant decrease in the mitochondrial membrane potential after exposure to 15d-PGJ2 (16). Moreover, we have observed cytochrome c release after 48 h of 15d-PGJ2 treatment (M. Kondo. & K.U., unpublished observation), which correlated with the time that apoptosis was observed (Fig. 2C), suggesting that the Fas/FasL-independent and mitochondria-dependent apoptosis pathway may also be involved.
It was also anticipated that reactive oxygen species (ROS) might be involved in the p53-dependent apoptotic pathway (34, 35). Indeed, the following observations have suggested the involvement of ROS in the 15d-PGJ2-induced cell death (16): (i) reaction of 15d-PGJ2 with cells induces mitochondrial damage and generates ROS; (ii) 15d-PGJ2-induced cell death can be partially inhibited by antioxidants, including α-tocopherol and N-acetylcysteine; and (iii) The cytotoxic potential of the cyclopentenone PGs is associated with the ability to generate ROS. However, our preliminary experiment has shown that the 15d-PGJ2-induced p53 accumulation in the cells was scarcely affected by pretreatment with N-acetylcysteine (M. Kondo & K.U., unpublished observation). Thus, further studies are required for elucidation of the role of ROS in the 15d-PGJ2-induced cell death.
Acknowledgments
We thank Dr. P. Boon Chock (National Institutes of Health) for critical reading of this manuscript. This work was supported in part by the Program for Promotion of Basic Research Activities for Innovative Biosciences (PROBRAIN).
Abbreviations
- PGs
prostaglandins
- 15d-PGJ2
15-deoxy-Δ12,14-PGJ2
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- ALS
amyotrophic lateral sclerosis
- FasL
Fas ligand
- COX-2
cyclooxygenase-2
- z-VAD-fmk
benzyloxycarbonyl-Val-Ala-Asp-fluoromethyl ketone
- Ac-IETD-CHO
acetyl-Ile-Glu-Thr-Asp-CHO
- PI
propidium iodide
- RT
reverse transcription
References
- 1.Giles H, Leff P. Prostaglandins. 1988;35:277–300. doi: 10.1016/0090-6980(88)90093-7. [DOI] [PubMed] [Google Scholar]
- 2.Fitzpatrick F A, Wynalda M A. J Biol Chem. 1983;258:11713–11718. [PubMed] [Google Scholar]
- 3.Kikawa Y, Narumiya S, Fukushima M, Wakatsuka H, Hayaishi O. Proc Natl Acad Sci USA. 1984;81:1317–1321. doi: 10.1073/pnas.81.5.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hirata Y, Hayashi H, Ito S, Kikawa Y, Ishibashi M, Sudo M, Miyazaki H, Fukushima M, Narumiya S, Hayaishi O. J Biol Chem. 1988;263:16619–16625. [PubMed] [Google Scholar]
- 5.Fukushima M. Prostaglandins Leukot Essent Fatty Acids. 1992;47:1–12. doi: 10.1016/0952-3278(92)90178-l. [DOI] [PubMed] [Google Scholar]
- 6.Rossi A, Kapahi P, Natoli G, Takahashi T, Chen Y, Karin M, Santoro M G. Nature (London) 2000;403:103–108. doi: 10.1038/47520. [DOI] [PubMed] [Google Scholar]
- 7.Straus D, Pascual G, Li M, Welch J S, Ricote M, Hsiang C-H, Sengchanthalangsy L L, Ghosh G, Glass C K. Proc Natl Acad Sci USA. 2000;97:4844–4849. doi: 10.1073/pnas.97.9.4844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raff M C, Barres B A, Burne J F, Coles H S, Ishizaki Y, Jacobson M D. Science. 1993;262:695–700. doi: 10.1126/science.8235590. [DOI] [PubMed] [Google Scholar]
- 9.Thompson C B. Science. 1995;267:1456–1462. doi: 10.1126/science.7878464. [DOI] [PubMed] [Google Scholar]
- 10.Vaux D L, Strasser A. Proc Natl Acad Sci USA. 1996;93:2239–2244. doi: 10.1073/pnas.93.6.2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fisher D E. Cell. 1994;78:539–542. doi: 10.1016/0092-8674(94)90518-5. [DOI] [PubMed] [Google Scholar]
- 12.Fulci G, Van Meir E G. Mol Neurobiol. 1999;19:61–77. doi: 10.1007/BF02741378. [DOI] [PubMed] [Google Scholar]
- 13.Brown J M, Wouters B G. Cancer Res. 1999;59:1391–1399. [PubMed] [Google Scholar]
- 14.Sharma K, Wang R X, Zhang L Y, Yin D L, Luo X Y, Solomon J C, Jiang R F, Markos K, Davidson W, Scott D W, Shi Y F. Phamacol Ther. 2000;88:333–347. doi: 10.1016/s0163-7258(00)00096-6. [DOI] [PubMed] [Google Scholar]
- 15.Shibata T, Kondo M, Osawa T, Shibata N, Kobayashi M, Uchida K. J Biol Chem. 2002;277:10459–10466. doi: 10.1074/jbc.M110314200. [DOI] [PubMed] [Google Scholar]
- 16.Kondo M, Oya-Ito T, Kumagai T, Osawa T, Uchida K. J Biol Chem. 2001;276:12076–12083. doi: 10.1074/jbc.M009630200. [DOI] [PubMed] [Google Scholar]
- 17.Takabe W, Mataki C, Wada Y, Ishii M, Izumi A, Aburatani H, Hamakubo T, Niki E, Kodama T. J Atheroscl Thromb. 2001;7:223–230. doi: 10.5551/jat1994.7.223. [DOI] [PubMed] [Google Scholar]
- 18.Kumagai T, Kawamoto Y, Osawa T, Nakamura Y, Hatayama I, Satoh K, Uchida K. Biochem Biophys Res Commun. 2000;273:437–441. doi: 10.1006/bbrc.2000.2967. [DOI] [PubMed] [Google Scholar]
- 19.Schreiber E, Matthias P, Müller M M, Schaffner W. Nucleic Acids Res. 1989;17:6419. doi: 10.1093/nar/17.15.6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yamada S, Kumazawa S, Ishii T, Nakayama T, Itakura K, Shibata N, Kobayashi M, Sakai K, Osawa T, Uchida K. J Lipid Res. 2001;42:1187–1196. [PubMed] [Google Scholar]
- 21.Clay C E, Atsumi G, High K P, Chilton F H. J Biol Chem. 2001;276:47131–47135. doi: 10.1074/jbc.C100339200. [DOI] [PubMed] [Google Scholar]
- 22.Bunz F, Dutriaux A, Lengauer C, Waldman T, Zhou S, Brown J P, Sedivy J M, Cagen L M, Fales H M, Pisano J J. J Biol Chem. 1976;251:6550–6554. [PubMed] [Google Scholar]
- 23.Lin D L, Chang C. J Biol Chem. 1996;271:14649–14652. doi: 10.1074/jbc.271.25.14649. [DOI] [PubMed] [Google Scholar]
- 24.Prives C. Cell. 1998;95:5–8. doi: 10.1016/s0092-8674(00)81774-2. [DOI] [PubMed] [Google Scholar]
- 25.Yonish-Rouach E, Resnitzky D, Lotem J, Sachs L, Kimchi A, Oren M. Nature (London) 1991;352:345–347. doi: 10.1038/352345a0. [DOI] [PubMed] [Google Scholar]
- 26.Moos P J, Edes K, Fitzpatrick F A. Proc Natl Acad Sci USA. 2000;97:9215–9220. doi: 10.1073/pnas.160241897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de la Monte S M, Sohn Y K, Ganju N, Wands J R. Lab Invest. 1998;78:401–411. [PubMed] [Google Scholar]
- 28.Cossarizza A, Kalashnikova G, Grassilli E, Chiappelli F, Salvioli S, Capri M, Barbieri D, Troiano L, Monti D, Franceschi C. Exp Cell Res. 1994;214:323–330. doi: 10.1006/excr.1994.1264. [DOI] [PubMed] [Google Scholar]
- 29.Zamzami N, Marchetti P, Castedo M, Decaudin D, Macho A, Hirsch T, Susin S A, Petit P X, Mignotte B, Kroemer G. J Exp Med. 1995;182:367–377. doi: 10.1084/jem.182.2.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Susin S A, Lorenzo H K, Zamzami N, Marzo I, Snow B E, Brothers G M, Mangion J, Jacotot E, Costantini P, Loeffler, et al. Nature (London) 1999;397:441–446. doi: 10.1038/17135. [DOI] [PubMed] [Google Scholar]
- 31.Larochette N, Decaudin D, Jacotot E, Brenner C, Marzo I, Susin S A, Zamzami N, Xie Z, Reed J, Kroemer G. Exp Cell Res. 1999;249:413–421. doi: 10.1006/excr.1999.4519. [DOI] [PubMed] [Google Scholar]
- 32.Zamzami N, Marzo I, Susin S A, Brenner C, Larochette N, Marchetti P, Reed J, Kofler R, Kroemer G. Oncogene. 1998;16:1055–1063. doi: 10.1038/sj.onc.1201864. [DOI] [PubMed] [Google Scholar]
- 33.Perkins C L, Fang G, Kim C N, Bhalla K N. Cancer Res. 2000;60:1645–1653. [PubMed] [Google Scholar]
- 34.Polyak K, Xia Y, Zweier J L, Kinzler K W, Vogelstein B. Nature (London) 1997;389:300–305. doi: 10.1038/38525. [DOI] [PubMed] [Google Scholar]
- 35.Johnson T M, Yu Z X, Ferrans V J, Lowenstein R A, Finkel T. Proc Natl Acad Sci USA. 1996;93:11848–11852. doi: 10.1073/pnas.93.21.11848. [DOI] [PMC free article] [PubMed] [Google Scholar]