Abstract
Contraction in striated and cardiac muscles is regulated by the motions of a Ca2+-sensitive tropomyosin/troponin switch. In contrast, troponin is absent in other muscle types and in nonmuscle cells, and actomyosin regulation is myosin-linked. Here we report an unusual crystal structure at 2.7 Å of the C-terminal 31 residues of rat striated-muscle α-tropomyosin (preceded by a fragment of the GCN4 leucine zipper). The C-terminal 22 residues (263–284) of the structure do not form a two-stranded α-helical coiled coil as does the rest of the molecule, but here the α-helices splay apart and are stabilized by the formation of a tail-to-tail dimer with a symmetry-related molecule. The site of splaying involves a small group of destabilizing core residues that is present only in striated muscle tropomyosin isoforms. These results reveal a specific recognition site for troponin T and clarify the physical basis for the unique regulatory mechanism of striated muscles.
The tropomyosin (Tm) molecule exemplifies both the general features of the α-helical coiled-coil structure and the special features of a molecule that plays a key regulatory role in muscle contraction. Tm is a parallel two-stranded α-helical coiled coil whose sequence (284 amino acid residues long in most isoforms) displays two types of repeats. There is a short-range repeat of (up to) 40 continuous seven-residue segments in the form a-b-c-d-e-f-g where the a and d positions are usually apolar. The interlocking of these apolar residues in a knobs-into-holes fashion by the winding of α-helices around one another produces a coiled-coil structure (1). There is also a long-range repeat of charged and apolar surface side chains that was considered to correspond to seven pairs of consecutive actin-binding sites (2, 3); it appears, however, that the Tm molecule uses only the more regular set of sites to bind weakly to seven actin monomers (4). It has recently been shown that an additional long-range semiregular repeat of core alanine residues can generate about seven bends in the molecule's axis (5). Together, these periodic features promote the winding of a Tm filament (composed of head-to-tail noncovalently bonded molecules) around the actin helix.
In addition to these periodicities, there are regions of the molecule with unique sequences that have specialized roles in the regulatory mechanism. The highly conserved N-terminal region, and the more variable C terminus, are notable here in forming specific overlapping and short (≈9 residues) head-to-tail linkages between molecules. In striated muscles, a longer segment of the C terminus (≈27 residues) appears to constitute the primary anchoring site for troponin T (TnT) (6). This subunit is the Tm-binding component of troponin, the Ca2+-dependent regulatory complex. The TnT–Tm interaction, which is specific to striated-muscle Tm isoforms, is required for troponin binding to the thin filament with high affinity and proper stoichiometry (1 to 1 with Tm) (7–9).
In all muscles, Tm functions as a filament, produced by the head-to-tail assembly. The cooperativity of myosin binding to the thin filament is promoted by this linkage in native Tm isoforms (10–12). In striated muscles, TnT spans (13, 14) and apparently strengthens (15, 16) the head-to-tail joint. Current versions of the steric blocking mechanism for regulation in striated muscles clarify how the azimuthal position of the Tm filament on the actin helix—modulated by the troponin complex—controls the strong binding of myosin to actin (17–19).
At present, structure determinations of native Tm filaments (4, 20, 21) have been at too low a resolution to reveal details of the C-terminal region of the molecule, including the head-to-tail overlap. As a first step to understanding the design of this region at the atomic level, we have determined to 2.7-Å resolution the crystal structure of the chimeric peptide “GCN4-CTm,” which contains the C-terminal 31 residues of striated-muscle Tm from the α gene, preceded by a 24-residue fragment of the GCN4 leucine zipper. The structure consists of two distinct “domains”: the N-terminal three-fifths show a canonical two-stranded coiled coil, whereas the C-terminal region consists of α-helices (≈22 residues in length) that “splay out” from one another and are stabilized by crystal packing forces. In striated-muscle isoforms, the site of the splaying contains a small group of core residues that destabilize the coiled-coil conformation, whereas in vertebrate smooth-muscle Tm isoforms, the corresponding residues would promote coiled-coil formation. These results provide a physical basis for the strikingly different regulatory mechanisms in these two vertebrate muscle types. They also provide some insight into the design of the head-to-tail joint in the filament.
Materials and Methods
Expression and Purification of GCN4-CTm.
The vector for the GCN4-CTm chimera was created with the ExSite Mutagenesis kit (Stratagene). Primers encoding residues 7–30 of the GCN4 leucine zipper were used to amplify the cDNA for residues 254–284 of rat striated muscle α-Tm. The construct was cloned initially into the vector pCALn, and then subcloned by PCR into the NcoI and BamHI sites of pET3d with the addition of an N-terminal methionine. DNA sequencing confirmed that no incidental changes had been introduced by PCR. The recombinant protein was expressed in DE3 cells and purified as described by Monteiro et al. (22). The translated amino acid sequence is: MDKVEELLSKNYHLENEVARLKKLVDDLEDELYAQKLKYKAISEELDHALNDMTSI.
Crystallization and Data Collection.
One microliter of precipitant solution [12% polyethylene glycol (PEG) 550 monomethyl ether (MME)/20% glycerol/300 mM NaCl/60 mM Mg(Ac)2/60 mM bicine, pH 9.0] was added to 5 μl of protein solution (5 mg/ml GCN4-CTm/20% glycerol/1 mM DTT/10 mM Tris, pH 7.5). The resultant mixture was equilibrated against a reservoir buffer (4% PEG 550 MME/100 mM NaCl/20 mM Mg(Ac)2/20 mM bicine, pH 8.3) by vapor diffusion at 22°C. Hexagonal crystals (P6422, a = b = 137.20 Å, c = 40.07 Å, 70.2% solvent) were harvested after 2–3 days and cryoprotected with 8% PEG 550 MME/25% glycerol/100 mM NaCl/20 mM Mg(Ac)2/20 mM bicine, pH 8.3. Data from a single crystal were collected at 100 K to 2.6-Å resolution with synchrotron radiation at the Cornell High Energy Synchrotron Source (beamline A1, λ = 0.909 Å). Data were reduced with the software package hkl (23). (See Table 1 for data collection statistics.)
Table 1.
Data collection and refinement statistics
| Data Collection (∞→2.6-Å resolution) | |
| Measured reflections | 86,920 |
| Unique reflections | 7,229 |
| Completeness, % | 99.9 (99.9)* |
| Rmerge† | 0.052 (0.217)* |
| Refinement (∞→2.7-Å resolution) | |
| No. of reflections | 6,498 |
| σ cutoff | None |
| Rcryst‡, Rfree§ | 0.251, 0.289 |
| No. of protein/water atoms | 894, 27 |
| rms bond lengths, Å, angles, ° | 0.007, 1.012 |
| Average B factors of backbone atoms, Å2 | |
| Residues 229–253, 254–262 | 67.2, 56.0 |
| Residues 263–270, 271–283 | 86.2, 157.2 |
Values for the highest-resolution bin (2.69–2.60 Å) are shown in parentheses.
Rmerge = Σhkl|I − 〈I〉|/ΣIhkl.
Rcryst = Σhkl∥Fobs| − |Fcalc∥/Σhkl|Fobs|, where Fcalc and Fobs are calculated and observed structure factor amplitudes, respectively.
Rfree is the same as Rcryst, except that the summation is over 8% of the reflections, which were randomly selected and excluded from the refinement.
Structure Determination.
A 56-residue-long α-helical coiled-coil search model for molecular replacement was constructed by extending and modifying the GCN4 leucine zipper (24) by using o (25). Details concerning the construction of this search model are published as supporting information on the PNAS web site (www.pnas.org). With this model, a molecular replacement solution was found, by using AMoRe (26) in the space group P6422, but not P6222. (correlation coefficient = 0.511, Rcryst = 0.506 with data from 10.0 to 3.5 Å). The model was initially refined to 3.1-Å resolution (Rcryst = 0.331) by using cns_solve (27). At this stage, the 2 Fobs − Fcalc electron density map superimposed well with the N-terminal 40 residues of the model but revealed that the two helices are widely splayed apart at the C-terminal end (269–284) of the molecule. This region was subsequently rebuilt in o. The model underwent several rounds of refinement (both simulated annealing and minimization) in cns_solve, with data up to 2.7-Å resolution (final Rcryst = 0.251; see Table 1 for refinement statistics). Together with the established conformation of the coiled coil, the known GCN4 structure (24) and the prominent tyrosine side-chain electron density in Tm (e.g., see Fig. 1D) helped confirm the register of the helices. The temperature factors of the refined structure steadily increase starting from residue 263 to the C terminus (Table 1), and the last two residues (283–284) of both chains are highly disordered and not included in the final model. Main-chain dihedral angles of all residues lie in the most-favored region of the Ramachandran plot.
Figure 1.
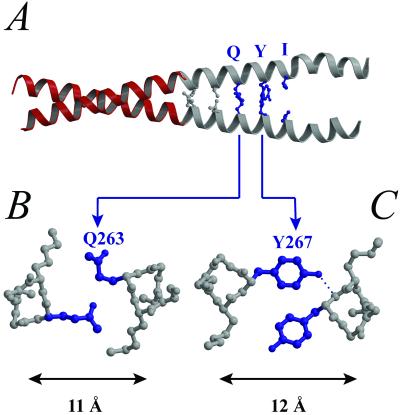
GCN4-CTm crystallizes as a “tail-to-tail” stabilized tetramer of α-helices. (A) The sequence of each chain consists of an N-terminal methionine, followed by residues 7–30 of the GCN4-leucine zipper (red) and then residues 254–284 of rat striated muscle α-Tm (gray). The 56 residues are numbered 229–284 in the current study. The N-terminal portion of GCN4-CTm forms a stable two-stranded α-helical coiled coil. However, the two helices are gradually separated from each other after residue 263. In the crystal, the splayed C-terminal end of each molecule has relatively high temperature factors (Table 1) but clearly forms a four-helix bundle with the corresponding region of a symmetry-related molecule. Four layers of apolar side chains constitute the core of the bundle. Each layer, in turn, contains two a residues from one molecule and two d residues from the other. The pairing is as follows: (B) Met-281 (a)-Ile-270 (d) for the first and fourth layers and (C) Leu-274 (a)-Ala-277 (d) for the second and third layers. Note that Met-281 and Leu-274 (both on a positions) also contact the side chains of Ser-271 and Leu-278 (both on e positions), respectively. (D) Stereo diagram of residues B255–266 and the corresponding omit electron density map contoured at 2σ using Fo − Fc coefficients. To remove bias, the phases for this map were calculated from a modified version of the final model that excluded the residues shown and that was then subjected to simulated annealing using CNS_SOLVE from a temperature of 1,000 K. The tyrosine shown is residue 261.
Prediction of Coiled-Coil Boundaries.
Initially, both the programs coils (28, 29) and paircoil (30) were used to predict the coiled-coil boundaries of different Tm isoforms. paircoil, which uses a 30-residue window for calculation, assigned very high probability scores (≈100%) for coiled-coil formation to the C-terminal region of striated muscle α-Tm. Similar results were obtained with coils, when a long (21- or 28-residue) window was chosen. In these cases, the overestimated scores for residues 263–284 probably arose from the inclusion of a large number of residues in a true coiled-coil conformation (i.e., those N-terminal to Gln-263). However, when the shortest (14-residue) window was selected in coils, the coiled-coil scores agreed well with a 22-residue splayed region observed in the current structure. We also compared predictions by coils with the actual structures of several DNA-binding proteins (31–35), which, like GCN4-CTm, contain both a coiled-coil region and a splayed α-helical extension. In all cases, the 14-residue window predicted the coiled-coil boundary most accurately and was considered most reliable for other Tm isoforms.
Results and Discussion
Description of the Structure.
In the crystal, two symmetry-related monomers of GCN4-CTm interact in an overlapping “tail-to-tail” fashion so that the entire structure is a tetramer of α-helices (Fig. 1A). The two α-helical chains of each monomer (“A” and “B”) are parallel and form distinctive structures along their axial length (Fig. 2). The N-terminal three-fifths of the chimeric monomer (corresponding to residues 7–30 of GCN4 followed by residues 254–262 of striated muscle α-Tm) is an uninterrupted canonical two-stranded coiled coil. The distance between the helical axes is about 10 Å, and this segment is stabilized by symmetrical “knobs-into-holes” packing between the two chains. Here, every core side chain from both helices, including the Tm residues Leu-256 (in a d position) and Leu-260 (in an a position), inserts into the hole formed by four side chains on the opposite helix. After residue 262, however, the two helices splay apart, and their axes are separated by 11 Å at Gln-263 (in a d position) and 12 Å at Tyr-267 (in an a position) (Fig. 2 B and C). These residues (in contrast to leucine) appear to be too bulky to fit within the core of a canonical two-stranded coiled coil, and instead they pack at the interface of the helices in an asymmetric manner. The side chains from the B helix fit into the (four-residue) holes on the A helix, but their counterparts on the A helix are oriented away from the core. [Similar asymmetric dispositions of bulky side chains in the cores of coiled coils with large diameters also occur in other structures, see the a-position Phe-358 of variable surface glycoprotein (36) and the d-position Arg-286 of cortexillin (37).] After residue 267 of the Tm fragment, the two helices from one monomer do not contact each other in the crystal but are stabilized instead (albeit with relatively high temperature factors) by interactions with the equivalent region of a symmetry-related molecule, forming an antiparallel four-helix bundle (Fig. 1A). [A similar arrangement of α-helices, consisting of a parallel coiled-coil dimer and a C-terminal antiparallel tetramer, also occurs in crystals of an antigen-presenting cell tumor repressor fragment (38)]. In the current structure, these tetrameric interactions include contacts made by d-position isoleucines (at position 270) (Fig. 1B), which are preferentially found in four- rather than two-stranded coiled coils (39). The conformation of the C-terminal region of striated-muscle Tm observed in the crystal suggests some of the structural features that may occur at the native head-to-tail joint in filaments of striated- and smooth-muscle Tm isoforms (see below).
Figure 2.
Structural basis for the splaying in the C-terminal region of striated muscle Tm. (A) In the C-terminal region of striated muscle α-Tm, there are three consecutive “core” residues (blue) that are generally disfavored in two-stranded coiled coils: Gln-263 (d position), Tyr-267 (a position), and Ile-270 (d position). The two equivalent Ile side chains do not contact each other, and the pairs of Gln and Tyr side chains do not display the usual symmetric “knobs-into-holes” packing pattern. In each of these two cases (B and C), only one of the two equivalent residues is inserted into the hole on the opposite helix. Moreover, the distance between the two helices increases to accommodate the insertion of such long or bulky side chains.
Structural Comparison of the C-terminal Regions of Various Vertebrate Tms.
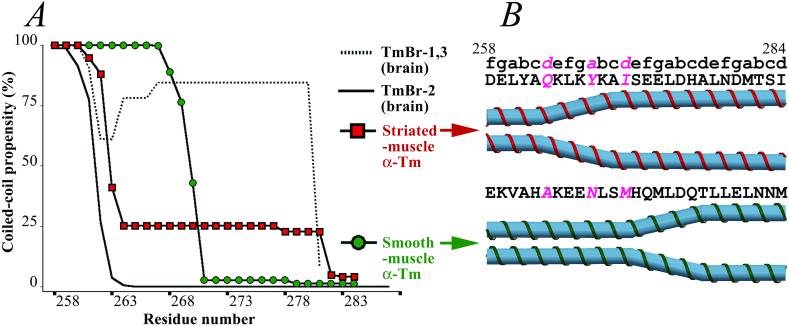
Although Tms are characterized by a common plan, these proteins display considerable diversity. They are encoded by multiple genes, and they display alternative exon expression, especially in their C-terminal regions (40). Here, for example, among the products of the α-Tm gene, the amino acid sequences of vertebrate smooth- and nonmuscle isoforms differ markedly from the striated-muscle isoform. (Similar results are found in products of other Tm genes.) Smooth-muscle α-Tm appears to adopt a canonical coiled-coil conformation that extends beyond residue 262 to residue 270. This conformation is predicted by the program coils (28–29) (see Fig. 3A and Materials and Methods), consistent with the observation that the residues at the interhelical interface in this region of smooth-muscle α-Tm (i.e., Ala-263, Asn-267, and Met-270) are generally common in the core of two-stranded coiled coils. The coiled-coil predictions produce variable results for the region beyond residue 270, but the parameters that most accurately predict the observed coiled-coil boundary in the α-striated-muscle Tm crystal structure (i.e., a 14-residue window size; see Materials and Methods) indicate that residues 271–284 of smooth-muscle α-Tm do not form a coiled-coil conformation (Fig. 3A). This region is nevertheless predicted to be completely α-helical according to various sequence analysis programs, including psipred (41), jpred (42), and phd (43), and does not contain any obvious helix-breaking residues (i.e., prolines). Taken together, these analyses suggest that the C-terminal region of vertebrate smooth-muscle Tm may also display a splayed conformational motif, but that the length of any splayed α-helix would be at least seven residues shorter than that observed in the striated-muscle isoform (Fig. 3B).
Figure 3.
Structural variations in the C-terminal region of different vertebrate Tm isoforms. (A) Coiled-coil predictions, using the program COILS (28–29) (with a 14-residue window size) for the C-terminal regions of the products of the α-Tm gene. In striated-muscle α-Tm, encoded by exon 9a (red squares), the coiled-coil propensity decreases sharply after Gln-263, consistent with a 22-residue splayed region observed in the current study (see Materials and Methods). Vertebrate smooth-muscle α-Tm and many nonmuscle isoforms, encoded by exon 9d (green circles), are also predicted to be α-helical but not in a two-stranded coiled-coil conformation at the C terminus. However, the coiled coil in these isoforms appears to extend beyond residue 262 to at least residue 270. In the brain isoforms, TmBr-1 and TmBr-3, encoded by exon 9c (dotted line), the C terminus is predicted to be an α-helical coiled coil to the very end. In another brain isoform, TmBr-2, encoded by exon 9b (solid line), the C-terminal region is mostly likely not α-helical because of three closely spaced prolines. (B) Schematic representation of the C-terminal ends of striated- and smooth-muscle α-Tms.
The conformational differences between the C-terminal regions of striated- and smooth-muscle Tm may be further enhanced by the location of the last d-position alanine. The coiled coil of Tm is unusual in having a large number of alanines in its core; seven semiregularly spaced clusters of generally two to three d-position alanines occur in the sequences of muscle Tms, and the most C-terminal “cluster” consists of only one alanine. The recent crystal structure of an N-terminal fragment of chicken striated-muscle α-Tm (5) showed that the small size (and unbranched nature) of this side chain produces locally narrow coiled-coil segments (≈8 Å in diameter in contrast to 9.5–10 Å for the leucine zipper); local narrowing (to about 9.0 Å) also occurs in the homodimeric coiled coil of cortexillin (37), where only one core alanine from each helix is present. In striated-muscle Tms, the C-terminal alanine is located at position 277, in the splayed region that participates in the tail-to-tail four-helix bundle. In the smooth-muscle Tm sequence, however, the last d-position alanine is located a couple of heptads earlier, at position 263, well within the coiled-coil region according to our predictions. The expected narrowing of the smooth-muscle Tm coiled coil in this region contrasts with the observed widening of the coiled coil in the striated-muscle isoform caused by Gln-263.
The amino acid sequences of all known nonmuscle Tms, like those of smooth-muscle Tm, also differ in their C-terminal regions from that of striated-muscle Tm. In fact, for both the α and β gene products, many of these nonmuscle Tms, including those in fibroblasts and certain cells of the liver and kidney (40), have the same C-terminal amino acid sequence as smooth-muscle Tm. The sequence identity occurs because in these different tissues the C terminus is encoded by the same alternatively spliced exon, “9d.” Three Tms expressed in the brain, however, have entirely different C-terminal amino acid sequences. Two of these—whose C-terminal region is encoded by exon 9c—are predicted to have an unsplayed fully α-helical coiled coil conformation; the other brain Tm isoform that uses exon 9b has three closely spaced prolines in its C-terminal sequence, which indicates a non-α-helical chain (Fig. 3A and legend). Exon 9a is expressed only in striated-muscle Tm, and the unique conformation of its C-terminal region appears to be critical for troponin-mediated regulation of muscle contraction.
C-Terminal Splaying and TnT Recognition.
This crystallographic and sequence analysis suggests a structural basis for the known differences in regulation between vertebrate striated- and smooth-muscle Tms. In smooth muscles, contraction is not controlled by the Tm–troponin switch on the thin filament but instead by the phosphorylation of myosin itself. Troponin is an elongated (≈265-Å) complex of three subunits, where troponin I (TnI), troponin C (TnC), and the C-terminal region of TnT form a globular head, and the remainder of TnT forms a rod-like (≈160-Å-long) tail (13, 44). TnT appears to bind Tm in an overall antiparallel manner: its C-terminal region (corresponding to chymotryptic fragment T2) is positioned near residue 190 of Tm, and the N-terminal tail of TnT (corresponding to chymotryptic fragment T1) extends toward Tm's C terminus, overlapping the head-to-tail joint of Tm as well as ≈10–30 residues of the N terminus of the next molecule along the filament (13, 14, 45). In contrast to the variable and Ca2+-sensitive linkages between the C-terminal region of TnT and the central region of Tm in the vicinity of residue 190 (46, 47), the N-terminal region of TnT binds to the Tm C terminus relatively strongly and in a conserved manner regardless of the level of Ca2+ (8, 46, 48). On the basis of a number of additional truncation and missense mutation studies of TnT (9, 16, 49–51), it appears that a segment of TnT spanning at least residues 64–92 (in rabbit skeletal numbering) may contain important interaction sites for the C-terminal region of Tm.
According to our study, the segment of Tm predicted to adopt different conformations in vertebrate striated- and smooth-muscle isoforms (residues 263–270) (Fig. 3) is located within a region that appears to be the C-terminal binding site for TnT. The C-terminal 27 residues (258–284) encoded by exon 9a are clearly critical for binding troponin. Although C-terminal truncation experiments of Tm indicate that residues 274–284 contribute to the binding affinity (7, 45, 46), chimeric 9a/9d exon results suggest that the first 18 residues of this exon (258–275) are especially important in determining the striated-specific interaction with troponin (6). Note that residues Gln-263, Tyr-267, and Ile-270, which account for the “9a-like” splaying observed in the current structure, are conserved in vertebrate striated-muscle Tm sequences. Moreover, residues 263 and 267 in the core positions of striated-muscle α-Tm (as well as Tyr-261 on the outer surface) have also been shown to interact with TnT by tryptophan fluorescence and tyrosine iodination experiments (45, 52).‖ The presence of these relatively bulky side chains at the splayed interhelical interface provides a locally larger surface area than would be found in a canonical coiled coil predicted for the corresponding region in smooth-muscle Tm (where the comparatively small side chains of Ala-263 and Asn-267 would be for the most part buried). This widened groove may be a significant conformational feature in striated-muscle Tm that enchances the specific binding of the T1 region of TnT. The structure of the Tm–TnT complex, however, is not known in detail and has been visualized only at low resolution (13).
The regulation of invertebrate muscles presents a more complex picture than in vertebrates: there is a great variety of invertebrate muscle types but relatively little biochemical information. Nevertheless, some generalizations may be made. In contrast to vertebrate muscles, which use either myosin-linked regulation or thin-filament regulation with troponin, most invertebrate muscles have dual regulation and correspondingly show the presence of troponin. [Note that myosin-linked regulation in invertebrates can involve light-chain phosphorylation as in Limulus muscles (53) or the direct binding of Ca2+ as in molluscan muscles (54).] In the C-terminal region of both vertebrate and invertebrate Tms, residues 258–275 (just before the head-to-tail overlap) are for the most part conserved (i.e., “9a-like”) in those isoforms that are expressed in troponin-containing muscles (Fig. 4). This result suggests that this 18-residue segment is a critical TnT recognition site in diverse phyla. On this basis, Tms from these muscle types are predicted to have similarly splayed C-terminal ends, especially because the core residues are either homologous (Glu instead of Gln at core position 263) or identical [Tyr-267 and Ile-270 (except Val in a few cases)]. The sequences of invertebrate and vertebrate TnTs also display a highly conserved segment (residues 83–106 in rabbit fast skeletal numbering) that overlaps the region shown to bind to the C terminus of Tm (unpublished data). Thus, both invertebrates and vertebrates appear to have similar interactions between Tm and troponin in this region. The few Tms found in invertebrate tissues that lack troponin [e.g., the pharyngeal and intestinal isoforms (TmIII and TmIV) from Caenorhabditis elegans] do not have 9a-like sequences and are predicted (by coils) not to adopt the splayed conformation but instead to form a stable coiled coil in the C-terminal end. In the case of nonmuscle tissues from both vertebrates and invertebrates, troponin appears to be absent, and the corresponding Tm sequences [e.g., the “cytoskeletal isoform” from Drosophila] are not predicted to have the splayed recognition site for TnT.
Figure 4.
TnT-binding Tms from vertebrates and invertebrates (see sequences 1–7 for example) all share a well-conserved C-terminal segment (residues 258–275) before the head-to-tail overlap. Moreover, in all these Tms, the core positions 263 (d), 267 (a), and 270 (d) (shown in pink) are occupied by residues that destabilize two-stranded coiled coils, suggesting a splayed C-terminal region similar to the one observed in the current structure. By contrast, Tms from troponin-deficient tissues (see sequences 8–13 for example) lack such a TnT-recognition site. Shown are selected C-terminal sequences from various Tm isoforms, including (1) striated muscle α-Tm from Rattus norvegicus (all identities to this sequence are shaded), (2) striated muscle β-Tm from R. norvegicus, (3) Tm from the notochord of Branchiostoma belcheri, (4) the “thoracic isoform” of Tm I from the flight and leg muscles of Drosophila melanogaster, (5) Tm from the striated adductor muscle of Chlamys nipponensis, (6) Tm from anterior byssus retractor muscle of Mytilus galloprovincialis, (7) TmI from the body wall, anus, and tail muscles of C. elegans, (8) brain isoforms TmBr-1 and TmBr-3 (9c-encoded), (9) brain isoform TmBr-2 (9b-encoded) and (10) smooth muscle α-Tm (9d-encoded) from R. norvegicus, (11) the “cytoskeletal isoform” of Tm II from D. melanogaster, and (12 and 13) two pharyngeal and intestinal isoforms (TmIII and TmIV) from C. elegans.
Implications of C-Terminal Splaying for the Native Tm Head-to-Tail Junction.
Both vertebrate striated- and smooth-muscle Tms, when bound to actin, form head-to-tail noncovalently bonded filaments with similar but distinctive properties. In both isoforms, the N- and C-terminal ends of adjacent molecules appear to overlap by ≈9 residues. This extent of overlap allows the Tm filament to make regular interactions with the actin helix (55). The low-resolution crystal structures of native Tm filaments are consistent with this picture (4, 20, 21) but cannot reveal the atomic details of this linkage. In this connection, the high-resolution crystal structures of the N-terminal region (5) and the C-terminal fragment described here are also not helpful. In the first study, the N terminus was not acetylated (but see ref. 56), and in the current structure, segments of 15 residues overlap in a nonnative antiparallel (tail-to-tail) fashion. Nevertheless, the C-terminal splaying, observed here in striated-muscle Tm and suggested to occur as well (albeit for a shorter extent) in smooth muscles, indicates that formation of the head-to-tail junction in either isoform provides stabilization for this portion of the molecule. Note, however, that the intrinsic strength of the head-to-tail junction appears to be greater in smooth-muscle than in striated-muscle Tm (12, 57). This feature is caused by sequence differences in the C-terminal nine residues because the N-terminal sequences of these two isoforms are identical. A strengthening of the particularly weak striated-muscle Tm head-to-tail joint by the binding of troponin is consistent with the observation in low-resolution cocrystal studies that TnT spans this joint as well as the beginning of the splayed region (13). On the basis of the current data, a number of different models are possible for the arrangement of the head-to-tail junction, but in either isoform the structure would probably involve a parallel four-helix bundle. [Note, however, that the previous model by McLachlan and Stewart (55), in which both termini were assumed to be simple two-stranded coiled coils interacting in an overlapping manner, is inconsistent with our findings.]
Perspective
A more complete understanding of the structural basis of thin-filament regulation will require additional atomic structures, including those of the head-to-tail joint as well as complexes with TnT and actin. The increased affinity of Tm for actin in the presence of troponin suggests that conformational changes may occur in Tm and/or actin on formation of the complex. Structures of the appropriate complexes should also reveal which residues of Tm contact TnT and actin. In this connection, it is not yet known whether the residues on Tm implicated in binding TnT from biochemical studies (described above) derive from one or both chains of the dimeric Tm molecule. Nevertheless, the splayed conformation of a key TnT-binding region discovered here appears to be a useful starting point for further studies.
Supplementary Material
Acknowledgments
We thank the staff of the Cornell High Energy Synchrotron Source for assistance with data collection. This work was supported by grants to C.C. from the National Institutes of Health (NIH) (AR17346) and the Muscular Dystrophy Association and to L.S.T. from the National Heart, Lung, and Blood Institute of the NIH (HL-38834).
Abbreviations
- Tm
tropomyosin
- TnT
troponin T
- TnC
troponin C
- TnI
troponin I
Footnotes
Data Deposition: The atomic coordinates have been deposited in the Protein Data Bank, www.rcsb.org (PDB ID code 1kql).
de Sousa, A. D. & Farah, C. S. (2001) Biophys. J. 80, 91a.
References
- 1.Crick F H C. Acta Crystallogr. 1957;6:689–697. [Google Scholar]
- 2.Parry D A D. J Mol Biol. 1975;98:519–535. doi: 10.1016/s0022-2836(75)80084-2. [DOI] [PubMed] [Google Scholar]
- 3.McLachlan A D, Stewart M. J Mol Biol. 1976;103:271–298. doi: 10.1016/0022-2836(76)90313-2. [DOI] [PubMed] [Google Scholar]
- 4.Phillips G N, Jr, Fillers J P, Cohen C. J Mol Biol. 1986;192:111–131. doi: 10.1016/0022-2836(86)90468-7. [DOI] [PubMed] [Google Scholar]
- 5.Brown J H, Kim K H, Jun G, Greenfield N J, Dominguez R, Volkmann N, Hitchcock-DeGregori S E, Cohen C. Proc Natl Acad Sci USA. 2001;98:8496–8501. doi: 10.1073/pnas.131219198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hammell R L, Hitchcock-DeGregori S E. J Biol Chem. 1996;271:4236–4242. doi: 10.1074/jbc.271.8.4236. [DOI] [PubMed] [Google Scholar]
- 7.Heeley D H, Golosinska K, Smillie L B. J Biol Chem. 1987;262:9971–9978. [PubMed] [Google Scholar]
- 8.Fisher D, Wang G, Tobacman L S. J Biol Chem. 1995;270:25455–25460. doi: 10.1074/jbc.270.43.25455. [DOI] [PubMed] [Google Scholar]
- 9.Hinkle A, Goranson A, Butters C A, Tobacman L S. J Biol Chem. 1999;274:7157–7164. doi: 10.1074/jbc.274.11.7157. [DOI] [PubMed] [Google Scholar]
- 10.Lehrer S S, Golitsina N L, Geeves M A. Biochemistry. 1997;36:13449–13454. doi: 10.1021/bi971568w. [DOI] [PubMed] [Google Scholar]
- 11.Pan B S, Gordon A M, Luo Z X. J Biol Chem. 1989;264:8495–8498. [PubMed] [Google Scholar]
- 12.Sano K, Maeda K, Oda T, Maeda Y. J Biochem. 2000;127:1095–1102. doi: 10.1093/oxfordjournals.jbchem.a022703. [DOI] [PubMed] [Google Scholar]
- 13.White S P, Cohen C, Phillips G N., Jr Nature (London) 1987;325:826–828. doi: 10.1038/325826a0. [DOI] [PubMed] [Google Scholar]
- 14.Cabral-Lilly D, Tobacman L S, Mehegan J P, Cohen C. Biophys J. 1997;73:1763–1770. doi: 10.1016/S0006-3495(97)78206-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maytum R, Lehrer S S, Geeves M A. Biochemistry. 1999;38:1102–1110. doi: 10.1021/bi981603e. [DOI] [PubMed] [Google Scholar]
- 16.Hill L E, Mehegan J P, Butters C A, Tobacman L S. J Biol Chem. 1992;267:16106–16113. [PubMed] [Google Scholar]
- 17.McKillop D F, Geeves M A. Biophys J. 1993;65:693–701. doi: 10.1016/S0006-3495(93)81110-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vibert P, Craig R, Lehman W. J Mol Biol. 1997;266:8–14. doi: 10.1006/jmbi.1996.0800. [DOI] [PubMed] [Google Scholar]
- 19.Tobacman L S, Butters C A. J Biol Chem. 2000;275:27587–27593. doi: 10.1074/jbc.M003648200. [DOI] [PubMed] [Google Scholar]
- 20.Whitby F G, Kent H, Stewart F, Stewart M, Xie X, Hatch V, Cohen C, Phillips G N., Jr J Mol Biol. 1992;227:441–452. doi: 10.1016/0022-2836(92)90899-u. [DOI] [PubMed] [Google Scholar]
- 21.Whitby F G, Phillips G N., Jr Proteins. 2000;38:49–59. [PubMed] [Google Scholar]
- 22.Monteiro P B, Lataro R C, Ferro J A, Reinach F C. J Biol Chem. 1994;269:10461–10466. [PubMed] [Google Scholar]
- 23.Otwinowski Z, Minor W. Methods Enzymol. 1997;276:307–326. doi: 10.1016/S0076-6879(97)76066-X. [DOI] [PubMed] [Google Scholar]
- 24.O'Shea E K, Klemm J D, Kim P S, Alber T. Science. 1991;254:539–544. doi: 10.1126/science.1948029. [DOI] [PubMed] [Google Scholar]
- 25.Jones T A, Zou L Y, Cowan S W, Kjeldgaard M. Acta Crystallogr A. 1991;47:110–119. doi: 10.1107/s0108767390010224. [DOI] [PubMed] [Google Scholar]
- 26.Navaza J, Saludjian P. Methods Enzymol. 1997;276:581–594. doi: 10.1016/S0076-6879(97)76079-8. [DOI] [PubMed] [Google Scholar]
- 27.Brünger A T, Adams P D, Clore G M, DeLano W L, Gros P, Grosse-Kunstleve R W, Jian J-S, Kuszewski J, Nilges M, Pannu N S, et al. Acta Crystallogr D. 1998;54:905–921. doi: 10.1107/s0907444998003254. [DOI] [PubMed] [Google Scholar]
- 28.Lupas A, van Dyke M, Stock J. Science. 1991;252:1162–1164. doi: 10.1126/science.252.5009.1162. [DOI] [PubMed] [Google Scholar]
- 29.Lupas A. Methods Enzymol. 1996;266:513–525. doi: 10.1016/s0076-6879(96)66032-7. [DOI] [PubMed] [Google Scholar]
- 30.Berger B, Wilson D B, Wolf E, Tonchev T, Milla M, Kim P S. Proc Natl Acad Sci USA. 1995;92:8259–8263. doi: 10.1073/pnas.92.18.8259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Keller W, Koenig P, Richmond T J. J Mol Biol. 1995;254:657–667. doi: 10.1006/jmbi.1995.0645. [DOI] [PubMed] [Google Scholar]
- 32.Tahirov T H, Sato K, Ichikawa-Iwata E, Sasaki M, Inoue-Bungo T, Shiina M, Kimura K, Takata S, Fujikawa A, Morii H, et al. Cell. 2002;108:57–70. doi: 10.1016/s0092-8674(01)00636-5. [DOI] [PubMed] [Google Scholar]
- 33.Glover J N, Harrison S C. Nature (London) 1995;373:257–261. doi: 10.1038/373257a0. [DOI] [PubMed] [Google Scholar]
- 34.Fujii Y, Shimizu T, Toda T, Yanagida M, Hakoshima T. Nat Struct Biol. 2000;7:889–893. doi: 10.1038/82822. [DOI] [PubMed] [Google Scholar]
- 35.Tahirov T H, Inoue-Bungo T, Morii H, Fujikawa A, Sasaki M, Kimura K, Shiina M, Sato K, Kumasaka T, Yamamoto M, et al. Cell. 2001;104:755–767. doi: 10.1016/s0092-8674(01)00271-9. [DOI] [PubMed] [Google Scholar]
- 36.Freymann D, Down J, Carrington M, Roditi I, Turner M, Wiley D. J Mol Biol. 1990;216:141–160. doi: 10.1016/S0022-2836(05)80066-X. [DOI] [PubMed] [Google Scholar]
- 37.Burkhard P, Kammerer R A, Steinmetz M O, Bourkenkov G P, Aebi U. Structure (London) 2000;8:223–230. doi: 10.1016/s0969-2126(00)00100-3. [DOI] [PubMed] [Google Scholar]
- 38.Day C L, Alber T. J Mol Biol. 2000;301:147–156. doi: 10.1006/jmbi.2000.3895. [DOI] [PubMed] [Google Scholar]
- 39.Lupas A. Trends Biochem Sci. 1996;21:375–382. [PubMed] [Google Scholar]
- 40.Lees-Miller J P, Helfman D M. BioEssays. 1991;13:429–437. doi: 10.1002/bies.950130902. [DOI] [PubMed] [Google Scholar]
- 41.Jones D T. J Mol Biol. 1999;292:195–202. doi: 10.1006/jmbi.1999.3091. [DOI] [PubMed] [Google Scholar]
- 42.Cuff J A, Barton G J. Proteins. 1999;34:508–519. doi: 10.1002/(sici)1097-0134(19990301)34:4<508::aid-prot10>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 43.Rost B. Methods Enzymol. 1996;266:525–539. doi: 10.1016/s0076-6879(96)66033-9. [DOI] [PubMed] [Google Scholar]
- 44.Flicker P F, Phillips G N, Jr, Cohen C. J Mol Biol. 1982;162:495–501. doi: 10.1016/0022-2836(82)90540-x. [DOI] [PubMed] [Google Scholar]
- 45.Mak A S, Smillie L B. J Mol Biol. 1981;149:541–550. doi: 10.1016/0022-2836(81)90486-1. [DOI] [PubMed] [Google Scholar]
- 46.Pearlstone J R, Smillie L B. J Biol Chem. 1982;257:10587–10592. [PubMed] [Google Scholar]
- 47.Hammell R L, Hitchcock-DeGregori S E. J Biol Chem. 1997;272:22409–22416. doi: 10.1074/jbc.272.36.22409. [DOI] [PubMed] [Google Scholar]
- 48.Pearlstone J R, Smillie L B. J Biol Chem. 1983;258:2534–2542. [PubMed] [Google Scholar]
- 49.Willadsen K A, Butters C A, Hill L E, Tobacman L S. J Biol Chem. 1992;267:23746–23752. [PubMed] [Google Scholar]
- 50.Tobacman L S, Lin D, Butters C, Landis C, Back N, Pavlov D, Homsher E. J Biol Chem. 1999;274:28363–28370. doi: 10.1074/jbc.274.40.28363. [DOI] [PubMed] [Google Scholar]
- 51.Palm T, Graboski S, Hitchcock-DeGregori S E, Greenfield N J. Biophys J. 2001;81:2827–2837. doi: 10.1016/S0006-3495(01)75924-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Oliveira D M, Nakaie C R, de Sousa A D, Farah C S, Reinach F C. J Biol Chem. 2000;275:27513–27519. doi: 10.1074/jbc.M002735200. [DOI] [PubMed] [Google Scholar]
- 53.Sellers J R. J Biol Chem. 1981;256:9274–9278. [PubMed] [Google Scholar]
- 54.Lehman W, Szent-Györgyi A G. J Gen Physiol. 1975;66:1–30. doi: 10.1085/jgp.66.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McLachlan A D, Stewart M. J Mol Biol. 1975;98:293–304. doi: 10.1016/s0022-2836(75)80119-7. [DOI] [PubMed] [Google Scholar]
- 56.Greenfield N J, Montelione G T, Farid R S, Hitchcock-DeGregori S E. Biochemistry. 1998;37:7834–7843. doi: 10.1021/bi973167m. [DOI] [PubMed] [Google Scholar]
- 57.Cho Y J, Hitchcock-DeGregori S E. Proc Natl Acad Sci USA. 1991;88:10153–10157. doi: 10.1073/pnas.88.22.10153. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.