Abstract
Objective
To evaluate whether methylcobalamin could effectively and safely prevent hand-foot syndrome in patients with human epidermal growth factor receptor 2 (HER2) negative early breast cancer receiving adjuvant capecitabine treatment.
Design
Multicentre, double blind, randomised, placebo controlled, phase 3 trial.
Setting
Seven hospitals in China between January 2022 and February 2024.
Participants
Women aged 18-75 years with pathologically confirmed HER2 negative early breast cancer who were scheduled to receive adjuvant capecitabine treatment.
Interventions
Eligible patients were randomly assigned in a 1:1 ratio to receive methylcobalamin at a dose of 0.5 mg orally, three times daily, or a placebo for a maximum of 24 weeks.
Main outcome measures
The primary endpoint was the incidence of grade ≥2 hand-foot syndrome occurring for the first time during capecitabine treatment in the intention-to-treat analysis.
Results
234 patients were randomly assigned to receive methylcobalamin (n=117) or placebo (n=117) and were included in the intention-to-treat and safety analysis. Grade ≥2 hand-foot syndrome occurred in 17 (14.5%) of 117 patients in the methylcobalamin group and 34 (29.1%) of 117 patients in the placebo group (risk difference −14.5%, 95% confidence interval −24.9% to −4.1%; one sided P value=0.003). The rate of reduction or discontinuation of capecitabine treatment because of hand-foot syndrome was 7.7% (9 of 117) in the methylcobalamin group and 13.7% (16 of 117) in the placebo group (risk difference −6.0%, 95% confidence interval −13.9% to 1.9%). The two groups showed similar incidence of any other adverse events (88 (75.2%) in the methylcobalamin group and 95 (81.2%) in the placebo group). No methylcobalamin specific adverse events were observed.
Conclusions
Oral methylcobalamin significantly lowered the severity of hand-foot syndrome by reducing the incidence of grade ≥2 symptoms without unexpected safety concerns in women with HER2 negative early breast cancer who were receiving adjuvant capecitabine treatment. The findings support the use of methylcobalamin to prevent capecitabine associated severe hand-foot syndrome in this patient population.
Trial registration
ClinicalTrials.gov NCT05165069.
Introduction
Patients with human epidermal growth factor receptor 2 (HER2) negative early breast cancer account for about 80-85% of all breast cancers in our clinics.1 Adjuvant capecitabine treatment has improved the prognosis of patients with HER2 negative early breast cancer after chemotherapy.2 3 Hand-foot syndrome, a prevalent dose limiting adverse effect of capecitabine, graded from 1 to 3, occurs in 29-73% of patients.2 3 4 The condition is characterised by erythema, oedema with neuropathic pain, and dysesthesia in the palms and soles, progressing to blistering with desquamation, erosion, and ulceration.5 Although not fatal, grade 2 or 3 hand-foot syndrome substantially impairs quality of life,6 7 and often necessitates dose reduction or discontinuation of capecitabine treatment, which affects dose intensity and overall treatment efficacy.8
Several interventions have been investigated to prevent capecitabine associated hand-foot syndrome, including pyridoxine (vitamin B6), the cyclooxygenase 2 enzyme inhibitor celecoxib, and urea based cream.9 10 11 Unfortunately, pyridoxine did not significantly reduce the risk of hand-foot syndrome,9 and the effect of urea based cream in preventing grade 2 or 3 disease remains uncertain.11 Although celecoxib has been reported as effective in preventing hand-foot syndrome,10 its long term use is limited because of safety concerns about cardiovascular events,12 resulting in a relatively low grade of recommendation.13 Therefore, more effective and safer therapeutic options are urgently needed to prevent the occurrence of grade 2 or 3 hand-foot syndrome in patients with HER2 negative early breast cancer who are undergoing adjuvant capecitabine treatment.
Despite its common occurrence, the pathophysiology of hand-foot syndrome is not well understood. Previous studies identified that small fibre neuropathy might be a potential cause of the pain and palmoplantar dysesthesia in the palms and soles in capecitabine induced hand-foot syndrome.14 15 Methylcobalamin (vitamin B12) has been found to promote neurite outgrowth and neuronal survival by enhancing Erk 1/2 and Akt activities through the methylation cycle in vitro and in vivo,16 17 18 alleviating neuropathic pain and dysesthesia,19 20 and making it a common treatment for neuropathy in clinical settings. We hypothesised that methylcobalamin might decrease the risk of hand-foot syndrome among patients with HER2 negative early breast cancer receiving adjuvant capecitabine treatment. Therefore, we performed this randomised, double blind, phase 3 trial to verify the efficacy and safety of methylcobalamin in preventing grade 2 or 3 hand-foot syndrome compared with placebo among patients with HER2 negative early breast cancer receiving adjuvant capecitabine treatment.
Methods
Study design and patients
This phase 3, multicentre, placebo controlled, double blind, randomised trial (NCT05165069) was conducted at seven hospitals in China (study protocol available in supplementary appendix 1). The study was conducted in accordance with the Declaration of Helsinki. Ethics approval was obtained from the ethics committee of each hospital and all participants gave written informed consent.
Eligible participants were women aged 18-75 years with an ECOG (Eastern Cooperative Oncology Group) score of 0 or 1 and centrally pathologically confirmed HER2 negative early breast cancer. Pathological complete response was not achieved in these patients after standard neoadjuvant chemotherapy or they had lymph node metastasis after upfront surgery, and they were required to receive adjuvant capecitabine treatment and had adequate organ function. Key exclusion criteria included the presence of conditions affecting the accurate assessment of hand-foot syndrome, such as diabetes, dermatomyositis, sclerosis, or lupus erythematosus; and factors that precluded the administration and absorption of the drug, such as an inability to swallow or intestinal obstruction. The full inclusion and exclusion criteria are available in table S1 in supplementary appendix 2.
Randomisation and masking
Eligible patients were randomly assigned in a 1:1 ratio to receive either methylcobalamin (intervention group) or placebo (control group). Randomisation was performed using a computer generated code created with R studio software (version 4.2.2), stratified by hormone receptor status (positive—oestrogen receptor or progesterone receptor ≥1% v negative—oestrogen receptor and progesterone receptor <1%) and study centres with a block size of six. Randomisation details were kept in sequentially numbered, opaque, sealed envelopes prepared by an independent statistician. The participants, investigators, and site staff were masked to treatment allocation.
Intervention
Patients were given 0.5 mg of methylcobalamin orally three times daily (a dosage used in neuropathy19) or a placebo to be taken for a maximum of 24 weeks. The physical properties of the methylcobalamin and placebo treatment, such as appearance, size, colour, dosage form, weight, taste, and odour, were the same. All patients received oral capecitabine 2000 mg/m2/day, which was divided into two daily doses to be taken in the morning and evening within 30 minutes after a meal over a treatment cycle of 21 days (taken for 14 days and stopped for seven days) for eight cycles continuously.
Clinical follow-up assessments for hand-foot syndrome and adverse events occurred every three weeks (±7 days) until completion of the eighth capecitabine treatment cycle or withdrawal from the study. When patients had grade ≥2 hand-foot syndrome, they were assessed within three days of onset. The hand-foot skin reaction and quality of life daily activity (HF-QoL—18 items of hand-foot syndrome related daily activities rated from 0 (not at all) to 4 (always or extremely)),21 QLQ-C30 (30 items of cancer related health status mostly rated from 1 (not at all) to 4 (very much)),22 and QLQ-BR-23 (23 items of breast cancer related health status rated from 1 (not at all) to 4 (very much))23 questionnaires (available in study protocol in supplementary appendix 1) were completed for all patients at baseline, at the end of cycle 4 of capecitabine treatment, and on completion of capecitabine treatment (cycle 8). Higher scores indicated worse status in these three quality of life questionnaires.
Endpoints
The primary endpoint of the study was the incidence of grade ≥2 hand-foot syndrome that occurred for the first time during capecitabine treatment. At each visit, two experienced dermatologists independently evaluated whether the patient had hand-foot syndrome by assessing the skin condition of their hands and feet, and asking about the presence of any pain or interference with daily activities according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE) version 5.0 (detailed procedure provided in the supplementary methods in supplementary appendix 2). When there were any discrepancies, a third dermatologist was consulted to obtain consistency. The consistency of findings for hand-foot syndrome evaluation was 97% (227/234) between the two dermatologists (table S2 in supplementary appendix 2). According to the NCI-CTCAE 5.0 criteria, hand-foot syndrome is graded from 1 to 3. In this study, grade ≥2 was selected as the primary endpoint because these grades can lead to a reduction or discontinuation of capecitabine treatment and affect patients’ quality of life, whereas grade 1 is generally self-limiting.2 7 Representative images of hand-foot syndrome evaluations for patients are provided in figure S1 in supplementary appendix 2.
Secondary endpoints were rates of reduction or discontinuation of capecitabine that were related to hand-foot syndrome for all the treatment cycles; disease-free survival (the time from randomisation to recurrence, or death from any cause); overall survival (the time from randomisation to death from any cause); and changes in quality of life measures (scores from the HF-QoL daily activity, QLQ-C30, and QLQ-BR-23 questionnaires). The numbers of adverse events and their severity, and the numbers of serious adverse events were reported based on NCI-CTCAE 5.0. The incidence of any grade hand-foot syndrome, or grade 1, 2, 3 separately, the distribution of hand-foot syndrome at each cycle, the median time to develop grade ≥2 hand-foot syndrome, and the total rates of reduction or discontinuation of capecitabine treatment (including those not related to hand-foot syndrome) were analysed as exploratory outcomes.
Sample size
A target sample size of 234 participants was determined based on a grade ≥2 hand-foot syndrome rate of 30% for the placebo group, derived from the results of a trial evaluating celecoxib.10 This trial had 80% power to detect a reduction in hand-foot syndrome rate to 14% in the methylcobalamin group, with a one sided level of significance of 0.025 and a dropout rate of 10%.
Statistical analysis
Primary efficacy analyses were conducted in the intention-to-treat population, which included all randomised patients. Sensitivity analyses for the primary endpoint were performed in the per protocol population, which comprised patients who received the assigned treatment (methylcobalamin or placebo) with at least 80% of the required doses (number of tablets taken/expected number of tablets to be taken×100%) and showed no major deviations from the protocol. Safety analyses were performed in the safety population, which included all patients who had received at least one dose of the trial treatment.
The proportion of patients with grade ≥2 hand-foot syndrome (the primary endpoint) was reported and the absolute risk difference and 95% confidence interval were calculated using the Wald method. The difference between the groups (P value) was evaluated using the Cochran-Mantel-Haensel test, adjusting for the stratification factors (hormone receptor status and study centre). Post hoc log binomial regression analysis with adjustment for the stratification factors was conducted to obtain the adjusted risk difference and corresponding 95% confidence interval. Multivariate log binomial regression analyses were conducted to control for confounders, including age, menopausal status, TNM (tumour node metastasis) stage, hormone receptor status, previous chemotherapy strategy, and capecitabine dosage. Subgroup analyses for the primary endpoint were performed for age, menopausal status, TNM stage, hormone receptor status, previous chemotherapy strategy, and capecitabine dosage using log binomial regression, and the results are presented as risk difference and risk ratio with 95% confidence interval.
Because there was no prespecified plan to adjust the widths of the confidence intervals for the secondary outcomes or for multiple comparisons, the confidence intervals should not be used for causal inference. For prespecified secondary outcomes, rates of reduction or discontinuation of capecitabine treatment that were related to hand-foot syndrome were reported as proportions and absolute risk differences with 95% confidence intervals. Exploratory analyses were conducted to determine the total rates of reduction or discontinuation of capecitabine treatment, and those not related to hand-foot syndrome. Additionally, we examined the distribution of hand-foot syndrome related reductions or discontinuations in each treatment cycle. Kaplan-Meier methods were used to estimate disease-free survival and overall survival with the log rank test. For quality of life, longitudinal analyses were carried out using a linear mixed effect model, which included group (methylcobalamin v placebo) and time intervals (baseline v end of fourth cycle of capecitabine treatment v end of capecitabine treatment) as the fixed effect to test the main effect of group and time. Exploratory comparisons of changes in quality of life scores between the two groups across different time intervals were performed using the Wilcoxon rank sum test. The frequency and severity of adverse events were reported as numbers and percentages.
Post hoc analyses were conducted to compare the rate of any grade or each grade (1, 2, 3) of hand-foot syndrome separately. The Wilcoxon rank sum test was used to compare the difference in the median time to develop grade ≥2 hand-foot syndrome between the two groups. Exploratory analyses were conducted to describe the distribution of hand-foot syndrome in each capecitabine treatment cycle. Unless specified, a two sided P value <0.05 was considered statistically significant. All statistical analyses were performed using R studio software (version 4.2.2).
Patient and public involvement
Lack of funding and expertise restricted our ability to involve patients or the public in the study design, recruitment, conduct, or interpretation of the results. During the recruitment phase, participants were informed about the purpose and content of the trial. Because of privacy concerns and the confidential nature of clinical data, patients did not participate in the subsequent statistical analysis or the writing of the manuscript. For patients who expressed an interest during their clinic visits, the results were communicated to them.
Results
Baseline characteristics
Between January 2022 and February 2024, 234 patients were enrolled from seven hospitals in China (table S3 in supplementary appendix 2). Patients were randomly assigned in a 1:1 ratio to the methylcobalamin group (n=117) and the placebo group (n=117). All patients in both groups received their assigned treatments, and so a total of 234 patients were included in the intention-to-treat and safety analyses (117 patients in each group; fig 1). Of these, 230 (98%) received ≥80% of the methylcobalamin or placebo dose needed (detailed compliance in table S4 in supplementary appendix 2) and were included in the per protocol population (115 patients in each group), while four patients (two in each group) received <80% of the methylcobalamin or placebo dose needed. The last follow-up date for survival outcomes was 22 December 2024.
Fig 1.
CONSORT (consolidated standards of reporting trials) diagram of trial
The median age of the 234 patients at randomisation was 50 years (interquartile range 41-59), and 53.0% were premenopausal. Most patients had TNM stage II tumours (69.7%). A total of 66.7% of patients had triple negative breast cancer, and 94.0% had previously received chemotherapy. Baseline characteristics of patients were generally similar between the two groups (table 1).
Table 1.
Baseline characteristics of patients with HER2 negative early breast cancer
| Characteristics | All patients (n=234) | Methylcobalamin group (n=117) | Placebo group (n=117) |
|---|---|---|---|
| Age (years) | |||
| Median (IQR) | 50 (41-59) | 51 (43-59) | 48 (39-58) |
| <60 | 183 (78.2) | 90 (76.9) | 93 (79.5) |
| ≥60 | 51 (21.8) | 27 (23.1) | 24 (20.5) |
| Menopausal status | |||
| Premenopausal | 124 (53.0) | 58 (49.6) | 66 (56.4) |
| Postmenopausal | 110 (47.0) | 59 (50.4) | 51 (43.6) |
| TNM stage* | |||
| I | 27 (11.5) | 14 (12.0) | 13 (11.1) |
| II | 163 (69.7) | 82 (70.1) | 81 (69.2) |
| III | 44 (18.8) | 21 (17.9) | 23 (19.7) |
| Hormone receptor status | |||
| Positive (ER or PR ≥1%)† | 78 (33.3) | 39 (33.3) | 39 (33.3) |
| Negative (ER and PR <1%) | 156 (66.7) | 78 (66.7) | 78 (66.7) |
| Previous chemotherapy | |||
| Taxane | 17 (7.3) | 7 (6.0) | 10 (8.5) |
| Anthracycline and taxane | 203 (86.8) | 103 (88.0) | 100 (85.5) |
| None | 14 (6.0) | 7 (6.0) | 7 (6.0) |
| Capecitabine starting dosage (mg/day) | |||
| <3000 | 32 (13.7) | 17 (14.5) | 15 (12.8) |
| 3000 | 128 (54.7) | 64 (54.7) | 64 (54.7) |
| >3000 | 74 (31.6) | 36 (30.8) | 38 (32.5) |
Data are numbers (%) unless otherwise stated.
HER2=human epidermal growth factor receptor 2; IQR=interquartile range; ER=oestrogen receptor; PR=progesterone receptor; TNM=tumour node metastasis.
Pathological stage for patients who received upfront surgery and clinical stage for patients who received neoadjuvant chemotherapy.
Seven (6.0%) patients in methylcobalamin group and eight (6.8%) in placebo group had tumours with hormone receptor low positive (ER and PR ≥1% to <10%).
Primary endpoint
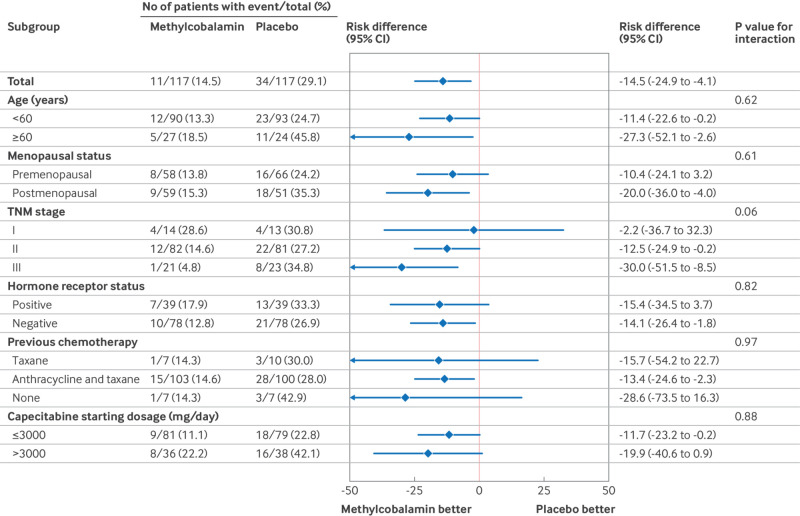
The incidence of grade ≥2 hand-foot syndrome was lower in the methylcobalamin group (17 of 117, 14.5%) than the placebo group (34 of 117, 29.1%; unadjusted risk difference −14.5%, 95% confidence interval −24.9% to −4.1%; Cochran-Mantel-Haensel one sided P value=0.003; table 2). After adjusting for stratification factors (hormone receptor status and study centre), the between group risk difference was −14.1% (−25.0% to −3.2%). Subgroup analyses showed that the prophylactic benefit of methylcobalamin for grade ≥2 hand-foot syndrome was consistent across all patient subgroups, and no significant interactions were observed between the treatment group and subgroup variables (fig 2 and figure S2 in supplementary appendix 2). In exploratory analyses, the incidence of grade ≥2 hand-foot syndrome was lower in the methylcobalamin group than the placebo group across all treatment cycles, with the exception of cycle 6 and cycle 7 (table S5 in supplementary appendix 2).
Table 2.
Primary and secondary efficacy endpoints
| Variable | All patients (n=234) | Methylcobalamin group (n=117) | Placebo group (n=117) | Risk difference (95% CI) | P value |
|---|---|---|---|---|---|
| Primary endpoint | |||||
| Incidence of grade ≥2 hand-foot syndrome | 51 (21.8) | 17 (14.5) | 34 (29.1) | −14.5 (−24.9 to−4.1); −14.1 (−25.0 to−3.2)* |
0.003† |
| Secondary endpoints | |||||
| Hand-foot syndrome related reduction or discontinuation of capecitabine | |||||
| Total | 25 (10.7) | 9 (7.7) | 16 (13.7) | −6.0 (−13.9 to 1.9) | 0.14‡ |
| Reduction | 21 (17.9) | 8 (6.8) | 13 (11.1) | −4.3 (−11.6 to 3.0) | 0.25‡ |
| 25% | 13 (5.6) | 6 (5.1) | 7 (6.0) | −0.9 (−6.7 to 5.0) | — |
| 50% | 8 (3.4) | 2 (1.7) | 6 (5.1) | −3.4 (−8.1 to 1.2) | — |
| Discontinuation | 4 (1.7) | 1 (0.9) | 3 (2.6) | −1.7 (−5.0 to 1.6) | 0.31‡ |
| Hand-foot syndrome grade§ | |||||
| Any | 127 (54.3) | 60 (51.3) | 67 (57.3) | −6.0 (−18.7 to 6.8) | 0.36‡ |
| 1 | 76 (32.5) | 43 (36.8) | 33 (28.2) | 8.5 (−3.4 to 20.5) | 0.16‡ |
| 2 | 43 (18.4) | 14 (12.0) | 29 (24.8) | −12.8 (−22.6 to−3.0) | 0.01‡ |
| 3 | 8 (3.4) | 3 (2.6) | 5 (4.3) | −1.7 (−6.4 to 2.9) | 0.47‡ |
| Initiation of grade ≥2 hand-foot syndrome, median (range)¶ | |||||
| Week | 8 (2-23) | 8 (2-20) | 8 (2-23) | — | 0.93** |
| Cycle | 3 (1-8) | 3 (1-7) | 3 (1-8) | — | — |
Data are numbers (%) unless otherwise stated.
CI=confidence interval.
From log-binomial regression adjusting for stratification factors (hormone receptor status and study centre).
One sided P value from Cochran-Mantel-Haensel test adjusting for stratification factors (hormone receptor status and study centre).
From Pearson’s χ2 test.
Based on highest grade of hand-foot syndrome that occurred for first time during capecitabine treatment.
Based on patients who developed grade ≥2 hand-foot syndrome.
From Wilcoxon rank sum test.
Fig 2.
Subgroup analyses of incidence of grade ≥2 hand-foot syndrome. CI=confidence interval; TNM=tumour node metastasis
The results of the sensitivity analyses for the incidence of grade ≥2 hand-foot syndrome in the per protocol population were similar to those in the intention-to-treat analysis (table S6 in supplementary appendix 2). Additionally, after adjusting for age, menopausal status, TNM stage, hormone receptor status, previous chemotherapy strategy, and capecitabine dosage, a reduced risk of grade ≥2 hand-foot syndrome was still observed with methylcobalamin treatment (risk difference −15.7%, 95% confidence interval −26.4% to−5.0%; risk ratio 0.47, 95% confidence interval 0.28 to 0.81; P=0.007; table S7 in supplementary appendix 2).
Secondary endpoints
Eight patients (6.8%) in the methylcobalamin group and 13 (11.1%) in the placebo group reduced capecitabine because of hand-foot syndrome (table 2), whereas one patient (0.9%) in the methylcobalamin group and three (2.6%) in the placebo group discontinued capecitabine because of hand-foot syndrome. Additionally, two patients (6.8%) in the methylcobalamin group and three (11.1%) in the placebo group reduced capecitabine owing to other adverse events, while one patient (0.9%) in the methylcobalamin group discontinued capecitabine because of distant metastasis (table S8 in supplementary appendix 2). Overall, reduction or discontinuation of capecitabine occurred in 12 patients (10.3%) in the methylcobalamin group and 19 (16.2%) in the placebo group. In exploratory analyses, a lower proportion of patients in the methylcobalamin group reduced or discontinued capecitabine because of hand-foot syndrome compared with the placebo group across cycles 3-8, while no dose adjustment occurred in either group during cycles 1 and 2 (table S9 in supplementary appendix 2).
With median survival follow-up time of 24 months (interquartile range 18-27) in the methylcobalamin group and 25 months (19-27) in the placebo group, the Kaplan-Meier survival curves showed that there were no significant differences in disease-free survival (three and five events, respectively; P=0.49) or overall survival (one and three events, respectively; P=0.32) between the two groups (figure S3 in supplementary appendix 2).
In the post hoc analysis, hand-foot syndrome of any grade occurred in 60 and 67 patients in the methylcobalamin and placebo groups, respectively (risk difference −6.0%, 95% confidence interval −18.7% to 6.8%; table 2). Grade 1 hand-foot syndrome occurred more commonly (43/117 v 33/117; risk difference 8.5%, 95% confidence interval −3.4% to 20.5%) in the methylcobalamin group, but without statistical significance. Grade 2 hand-foot syndrome occurred in 14 patients in the methylcobalamin group and 29 patients in the placebo group (−12.8%, −22.6% to −3.0%), and grade 3 was reported in three patients in the methylcobalamin group and five patients in the placebo group (−1.7%, −6.4% to 2.9%). The median time to develop grade ≥2 hand-foot syndrome was cycle 3 or eight weeks in both groups with no significant difference (P=0.93; table 2). The cumulative hazard plot indicated that the most grade ≥2 hand-foot syndrome events occurred within the first four cycles (figure S4 in supplementary appendix 2).
Quality of life outcomes
The quality of life questionnaires were completed at baseline, at the end of cycle 4 of capecitabine treatment, and on completion of capecitabine treatment by 114 patients (97.4%) in the methylcobalamin group and 116 (99.1%) in the placebo group. Figure S5 and table S10 (in supplementary appendix 2) present the median and mean scores for HF-QoL daily activity, QLQ-C30, and QLQ-BR23. The linear mixed effect model showed that no significant treatment effects were observed for HF-QoL daily activity, QLQ-C30, and QLQ-BR23 scores (table S11 in supplementary appendix 2).
Among the exploratory analyses, the increase in HF-QoL daily activity scores from baseline to the end of cycle 4 of capecitabine treatment was significantly lower in the methylcobalamin group than the placebo group (median 6.0 (interquartile range 4.0-9.0) v 8.0 (4.3-13.8); P=0.005; figure S6 in supplementary appendix 2). However, there was no difference in scores on completion of capecitabine treatment. Additionally, the changes in QLQ-C30 and QLQ-BR23 total scores across different time intervals were comparable between the two groups.
Safety
Overall, 183 (78.2%) patients experienced other treatment related adverse events of any grade: 88 (75.2%) in the methylcobalamin group and 95 (81.2%) in the placebo group (table 3). The most common adverse events were grade 1 or 2 hematologic toxicities in both groups. Grade ≥3 adverse events occurred in 12 (10.3%) and 18 (15.4%) patients in the methylcobalamin and placebo groups, respectively, with lymphopenia being the most common grade ≥3 adverse event. One patient with a grade 3 non-hematologic adverse event, stomatitis, was reported in the methylcobalamin group. All adverse events were reversible with observation or symptomatic treatment, and no patient discontinued capecitabine treatment because of these events. No patient experienced serious adverse events, and no deaths caused by adverse events were reported.
Table 3.
Other treatment related adverse events
| Event* | Methylcobalamin group (n=117) | Placebo group (n=117) | P value for difference in any grade† | |||
|---|---|---|---|---|---|---|
| Any grade | Grade 3-4 | Any grade | Grade 3-4 | |||
| Any | 88 (75.2) | 12 (10.3) | 95 (81.2) | 18 (15.4) | 0.27 | |
| Lymphopenia | 46 (39.3) | 10 (8.5) | 55 (47.0) | 13 (11.1) | 0.23 | |
| Leukopenia | 35 (29.9) | 4 (3.4) | 35 (29.9) | 3 (2.6) | 1.00 | |
| Anaemia | 33 (28.2) | 0 | 36 (30.8) | 0 | 0.67 | |
| Neutropenia | 33 (28.2) | 3 (2.6) | 33 (28.2) | 6 (5.1) | 1.00 | |
| ALT increased | 25 (21.4) | 0 | 20 (17.1) | 0 | 0.41 | |
| ALP increased | 23 (19.7) | 0 | 18 (15.4) | 0 | 0.39 | |
| AST increased | 20 (17.1) | 0 | 24 (20.5) | 0 | 0.50 | |
| Platelet count decreased | 20 (17.1) | 0 | 25 (21.4) | 1 (0.9) | 0.41 | |
| Diarrhoea | 18 (15.4) | 0 | 15 (12.8) | 0 | 0.57 | |
| Stomatitis | 17 (14.5) | 1 (0.9) | 12 (10.3) | 0 | 0.32 | |
| Bilirubin level increased | 15 (12.8) | 0 | 20 (17.1) | 0 | 0.36 | |
| Fatigue | 12 (10.3) | 0 | 13 (11.1) | 0 | 0.83 | |
| Nausea | 8 (6.8) | 0 | 12 (10.3) | 0 | 0.35 | |
| Creatinine increased | 4 (3.4) | 0 | 3 (2.6) | 0 | 0.70 | |
| Vomiting | 3 (2.6) | 0 | 6 (5.1) | 0 | 0.31 | |
Data are numbers (%).
ALP=alkaline phosphatase; ALT=alanine aminotransferase; AST=aspartate aminotransferase.
Each patient was counted once for highest grade of each adverse event that occurred.
From Pearson’s χ2 test.
Discussion
In this trial, we found that among women with HER2 negative early breast cancer who received adjuvant capecitabine treatment, the addition of oral methylcobalamin led to less severe hand-foot syndrome—fewer patients had grade ≥2 hand-foot syndrome in the methylcobalamin group than in the placebo group without added safety issues. Additionally, we found that oral methylcobalamin had the potential to reduce the rate of dose modification of capecitabine. The evidence indicates that capecitabine treatment offers survival advantages for patients with advanced breast cancer and for those with HER2 negative early breast cancer.2 3 24 Given the widespread use of capecitabine in cancer treatment, these findings provide important clinical implications for oncologists to prevent severe hand-foot syndrome complications and maintain treatment adherence.
Comparison with other studies
Currently, strategies to prevent capecitabine induced hand-foot syndrome remain limited. One effective approach is the use of the cyclooxygenase 2 enzyme inhibitor celecoxib (200 mg twice daily), which reduced the incidence of grade ≥2 hand-foot syndrome from 29.6% to 14.7% (P<0.05) in an open label trial involving 139 patients.10 However, the increased risk of myocardial infarction associated with celecoxib (hazard ratio 2.26, 95% confidence interval 1.0 to 5.1) might limit its clinical use.12 Similarly, the D-TORCH trial showed that the topical cyclooxygenase 2 enzyme inhibitor diclofenac significantly decreased the incidence of grade ≥2 hand-foot syndrome in 264 patients compared with placebo (3.8% v 15.0%; P=0.003).25 The lower rate of grade ≥2 hand-foot syndrome in the D-TORCH trial might be attributed to the limited assessment of only the hands and the relatively short duration (four cycles), which might not fully capture the condition’s progression. Additionally, the long term safety of topical diclofenac remains a concern because it might be absorbed through damaged skin on the hands and feet.26
Another effective treatment is urea based cream, which reduced the incidence of hand-foot syndrome of any grade in an open label trial of 152 patients compared with an antioxidant cream (22.4% v 39.5%; P=0.02).11 However, its effectiveness in lowering the risk of grade 2 or 3 hand-foot syndrome is uncertain. Furthermore, topical treatments might be inconvenient for patients. Although there is a hypothesis that pyrimidine analogues might interfere with pyridoxal phosphate, thereby reducing pyridoxine levels and potentially leading to acrodynia (a condition similar to hand-foot syndrome) in rats,27 28 pyridoxine has proven ineffective in preventing grade ≥2 hand-foot syndrome.9 In contrast, although the rates of hand-foot syndrome of any grade were comparable, our trial showed that oral methylcobalamin is a safe, simple, and convenient option, and is associated with reduced incidence of grade 2 or 3 hand-foot syndrome, which is of greater concern to clinicians and patients. These findings highlight the clinical significance of using methylcobalamin in women with HER2 negative early breast cancer receiving adjuvant capecitabine treatment.
Potential causes of capecitabine induced hand-foot syndrome proposed in the literature include raised levels of thymidine phosphorylase in the palms and soles,29 upregulation of the cyclooxygenase 2 enzyme,30 and small fibre neuropathy.14 Of these, small fibre neuropathy is considered a probable cause of the neuropathic pain, dysesthesias, paraesthesia, and temperature intolerance associated with capecitabine induced hand-foot syndrome.14 Accordingly, methylcobalamin was reported to enhance Erk 1/2 and Akt activities through the methylation cycle to promote nerve regeneration16 and alleviate symptoms of neuropathic pain, paraesthesia, and heaviness.9 31 The precise underlying mechanism of methylcobalamin preventing capecitabine induced hand-foot syndrome requires further confirmation.
In this trial, 67% of enrolled patients had triple negative breast cancer and 33% had HER2 negative and hormone receptor positive early breast cancer and received adjuvant capecitabine. Patients with hormone receptor positive early breast cancer had either not achieved pathological complete response after standard neoadjuvant chemotherapy according to the CREATE-X trial2 and were not candidates for CDK (cyclin dependent kinase) 4 or 6 inhibitors, or they were much older (≥65 years) and had not received previous chemotherapy according to the CALGB 49907 trial (table S12 in supplementary appendix 2).32 33 For patients who had received previous chemotherapy without pathological complete response, adjuvant capecitabine treatment can give improved survival, while for older patients who had not received previous chemotherapy, it can offer similar overall survival and better quality of life compared with intravenous chemotherapy. Subgroup data showed the efficacy of methylcobalamin in preventing capecitabine associated hand-foot syndrome was consistent for patients with hormone receptor positive and hormone receptor negative early breast cancer.
In this trial, no apparent safety issues were reported by adding oral methylcobalamin for patients receiving adjuvant capecitabine treatment. The overall reported incidence of adverse events in this study was similar to that of the CREATE-X trial and the SYSUCC-001 trial,2 3 both of which enrolled patients to receive adjuvant capecitabine treatment. The addition of methylcobalamin did not increase the toxicity of capecitabine treatment.
Our results showed that the need to reduce the dose or discontinue capecitabine treatment was relatively lower in the methylcobalamin group than the placebo group, although without statistical significance, and so methylcobalamin could potentially maintain the efficacy of capecitabine treatment in women with HER2 negative early breast cancer. However, because of the short follow-up period with few events in this study, we did not observe a significant difference in survival outcomes between the two groups. This might suggest that methylcobalamin does not increase cancer risk, a finding supported by previous studies,34 and its application during chemotherapy does not appear to influence long term survival outcomes for patients with breast cancer.35 Nevertheless, it remains unclear whether methylcobalamin diminishes the cytotoxic effects of capecitabine.
In this trial, methylcobalamin showed a potential improvement in hand-foot syndrome related quality of life at the end of cycle 4 of capecitabine treatment and the improvement disappeared after further treatment. This finding is consistent with that of the D-TORCH trial, which reported improved hand-foot syndrome related quality of life within the first four cycles of capecitabine treatment when using topical diclofenac gel.25 This could be because the majority of grade ≥2 hand-foot syndrome events occurred within the first four cycles, with fewer severe events that impair quality of life occurring in subsequent cycles.
Limitations of this study
This study has several limitations. Our trial only included Chinese participants. Considering there are known differences in the pharmacokinetic profile of capecitabine for Asians and non-Asians,36 caution is required when generalising our findings to patients from other geographical regions and different racial or ethnic backgrounds. Additionally, the lack of patient and public involvement in the trial led to the omission of patient preference information. Furthermore, the use of opaque sealed envelopes might not be an optimal allocation process, with the potential of introducing selection bias. A rigorous randomisation process with identical properties and packages, labelled only numerically, was performed in this study to ensure allocation concealment and masking, minimising the risk of selection bias to some extent.
Biomarkers that could predict the incidence of grade ≥2 hand-foot syndrome, including dihydropyrimidine dehydrogenase status,37 serum folate, and red blood cell folate,38 were scarce. Furthermore, patients did not have skin biopsies, resulting in an unclear understanding of the exact mechanism by which methylcobalamin prevents hand-foot syndrome. Finally, the long term impact of methylcobalamin on the prevention of capecitabine associated hand-foot syndrome and patient survival remains to be explored during ongoing follow-up studies.
Future direction
Despite the efficacy of methylcobalamin found in this trial, some patients still developed grade ≥2 hand-foot syndrome during capecitabine treatment. Future studies could be conducted to identify patients who might benefit from the administration of methylcobalamin, enabling a more targeted treatment regimen.
Conclusions
Oral methylcobalamin, compared with placebo, significantly lowered the severity of hand-foot syndrome by reducing the incidence of grade ≥2 hand-foot syndrome with excellent safety among women with HER2 negative early breast cancer who received adjuvant capecitabine treatment. These findings support the use of methylcobalamin to prevent capecitabine associated severe hand-foot syndrome in women with HER2 negative early breast cancer receiving adjuvant capecitabine treatment.
What is already known on this topic
Hand-foot syndrome is a prevalent dose limiting adverse effect of capecitabine treatment and grade 2 or 3 symptoms significantly impair patients’ adherence to capecitabine treatment and quality of life
Current preventive strategies to reduce the severity of capecitabine induced hand-foot syndrome are insufficient
What this study adds
This study compared oral methylcobalamin with a placebo in preventing grade 2 or 3 hand-foot syndrome among patients with HER2 (human epidermal growth factor receptor 2) negative early breast cancer receiving adjuvant capecitabine treatment
Methylcobalamin significantly lowered the severity of hand-foot syndrome by reducing the incidence rate of grade ≥2 symptoms with an excellent safety profile
Acknowledgments
The authors thank the patients and their families, nurses, statisticians, and research staff who participated in this trial. The support provided by Yangtze River Pharmaceutical Group for supplying the placebo used in this study is appreciated.
Web extra.
Extra material supplied by authors
Web appendix 1: Study protocol
Web appendix 2: Supplementary methods, figures, and tables
Web appendix 3: Study code
Contributors: CG, YX, YZ, and YY conceived and designed this study. LL and QL improved the study design. CG, JY, WT, WW, JZ, LZ, and AZ recruited and treated patients. YX, JY, WT, WW, JZ, LZ, and AZ collected data. CG, YX, YZ, FX, YY, and ZC contributed to the data analysis. CG, YX, YZ, LL, FX, YY, and ZC contributed to the data interpretation. ES contributed to valuable suggestions in manuscript revisions. All authors were involved in the preparation and critically reviewed the manuscript. All authors had full access to the raw data and approved the final version of manuscript for submission. YX, YZ, LL, FX, and YY contributed equally to this study. CG is the guarantor. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Funding: This work was supported by grants from the National Science and Technology Major Project for the Prevention and Treatment of Cancer, Cardiovascular, Respiratory, and Metabolic Diseases (grant No 2024ZD0519800), Noncommunicable Chronic Diseases-National Science and Technology Major Project of China (2023ZD0501100), Fundamental Research Funds for the Central Universities, Sun Yat-sen University, Sun Yat-sen University Clinical Research 5010 Program (2022005), National Natural Science Foundation of China (82371739, 82304120, 82003311, 81872139, 82072907, U1911204, and 51861125203), Science and Technology Projects in Guangzhou (2025A04J7152), Guangdong Science and Technology Department (2020B1212060018 and 2020B1212030004), Project of The Beijing Xisike Clinical Oncology Research Foundation (Y-Roche2019/2-0078 and Y-pierrefabre202102-0107), Department of Natural Resources of Guangdong Province (GDNRC(2021)51), the Science and Technology Planning Project of Guangdong Province (2023B1212060013), Guangzhou Science and Technology Program (202102010272), High-tech, Major and Characteristic Technology Projects in Guangzhou Area (2023-2025) (2023P-ZD14), Guangdong Provincial Clinical Research Centre for Breast Diseases (2023B110005), and National Key R&D Program of China (2021YFC3001000). The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of this report
Competing interests: All authors have completed the ICMJE uniform disclosure form at https://www.icmje.org/disclosure-of-interest/ and declare: support from National Science and Technology Major Project for the Prevention and Treatment of Cancer, Cardiovascular, Respiratory, and Metabolic Diseases, Noncommunicable Chronic Diseases-National Science and Technology Major Project of China, Fundamental Research Funds for the Central Universities, Sun Yat-sen University, Sun Yat-sen University Clinical Research 5010 Program, National Natural Science Foundation of China, Science and Technology Projects in Guangzhou, Guangdong Science and Technology Department, Project of The Beijing Xisike Clinical Oncology Research Foundation, Department of Natural Resources of Guangdong Province, the Science and Technology Planning Project of Guangdong Province, Guangzhou Science and Technology Program, High-tech, Major and Characteristic Technology Projects in Guangzhou Area, Guangdong Provincial Clinical Research Centre for Breast Diseases, and National Key R&D Program of China for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
Transparency: The corresponding author affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Dissemination to participants and related patient and public communities: Results were communicated to the participating sites and were clearly communicated to study participants who expressed an interest during clinic visits. The results and conclusion will be disseminated through patient education, press releases, academic conferences, and social media.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Ethical approval
Ethics approval was obtained from the ethics committee of each hospital and all participants signed written informed consent. The study was conducted in accordance with the Declaration of Helsinki.
Data availability statement
The code used to analyse the data in the paper can be found in supplementary appendix 3. The raw database underlying the study is openly and publicly available (https://doi.org/10.5061/dryad.gmsbcc31k). The data sharing statement is given in table S13 in supplementary appendix 2.
References
- 1. Waks AG, Winer EP. Breast cancer treatment: a review. JAMA 2019;321:288-300. 10.1001/jama.2018.19323. [DOI] [PubMed] [Google Scholar]
- 2. Masuda N, Lee SJ, Ohtani S, et al. Adjuvant capecitabine for breast cancer after preoperative chemotherapy. N Engl J Med 2017;376:2147-59. 10.1056/NEJMoa1612645. [DOI] [PubMed] [Google Scholar]
- 3. Wang X, Wang SS, Huang H, et al. South China Breast Cancer Group (SCBCG) . Effect of capecitabine maintenance therapy using lower dosage and higher frequency vs observation on disease-free survival among patients with early-stage triple-negative breast cancer who had received standard treatment: the SYSUCC-001 randomized clinical trial. JAMA 2021;325:50-8. 10.1001/jama.2020.23370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hong RX, Xu F, Xia W, et al. Metronomic capecitabine plus aromatase inhibitor as initial therapy in patients with hormone receptor-positive, human epidermal growth factor receptor 2-negative metastatic breast cancer: the phase III MECCA trial. J Clin Oncol 2025;43:1314-24. 10.1200/JCO.24.00938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Janusch M, Fischer M, Marsch W, et al. The hand-foot syndrome—a frequent secondary manifestation in antineoplastic chemotherapy. Eur J Dermatol 2006;16:494-9. [PubMed] [Google Scholar]
- 6. Haley AC, Calahan C, Gandhi M, et al. Skin care management in cancer patients: an evaluation of quality of life and tolerability. Support Care Cancer 2011;19:545-54. 10.1007/s00520-010-0851-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sibaud V, Dalenc F, Chevreau C, et al. HFS-14, a specific quality of life scale developed for patients suffering from hand-foot syndrome. Oncologist 2011;16:1469-78. 10.1634/theoncologist.2011-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bonadonna G, Valagussa P, Moliterni A, Zambetti M, Brambilla C. Adjuvant cyclophosphamide, methotrexate, and fluorouracil in node-positive breast cancer: the results of 20 years of follow-up. N Engl J Med 1995;332:901-6. 10.1056/NEJM199504063321401. [DOI] [PubMed] [Google Scholar]
- 9. Heilfort L, Kutschan S, Dörfler J, et al. A systematic review of the benefit of B-vitamins as a complementary treatment in cancer patients. Nutr Cancer 2023;75:33-47. 10.1080/01635581.2022.2098348. [DOI] [PubMed] [Google Scholar]
- 10. Zhang RX, Wu XJ, Wan DS, et al. Celecoxib can prevent capecitabine-related hand-foot syndrome in stage II and III colorectal cancer patients: result of a single-center, prospective randomized phase III trial. Ann Oncol 2012;23:1348-53. 10.1093/annonc/mdr400. [DOI] [PubMed] [Google Scholar]
- 11. Hofheinz RD, Gencer D, Schulz H, et al. Mapisal versus urea cream as prophylaxis for capecitabine-associated hand-foot syndrome: a randomized phase III trial of the AIO Quality of Life Working Group. J Clin Oncol 2015;33:2444-9. 10.1200/JCO.2014.60.4587. [DOI] [PubMed] [Google Scholar]
- 12. Caldwell B, Aldington S, Weatherall M, Shirtcliffe P, Beasley R. Risk of cardiovascular events and celecoxib: a systematic review and meta-analysis. J R Soc Med 2006;99:132-40. 10.1177/014107680609900315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lacouture ME, Sibaud V, Gerber PA, et al. ESMO Guidelines Committee. Electronic address: clinicalguidelines@esmo.org . Prevention and management of dermatological toxicities related to anticancer agents: ESMO Clinical Practice Guidelines. Ann Oncol 2021;32:157-70. 10.1016/j.annonc.2020.11.005. [DOI] [PubMed] [Google Scholar]
- 14. Stubblefield MD, Custodio CM, Kaufmann P, Dickler MN. Small-fiber neuropathy associated with capecitabine (Xeloda)-induced hand-foot syndrome: a case report. J Clin Neuromuscul Dis 2006;7:128-32. 10.1097/01.cnd.0000211401.19995.a2. [DOI] [PubMed] [Google Scholar]
- 15. Miller KK, Gorcey L, McLellan BN. Chemotherapy-induced hand-foot syndrome and nail changes: a review of clinical presentation, etiology, pathogenesis, and management. J Am Acad Dermatol 2014;71:787-94. 10.1016/j.jaad.2014.03.019. [DOI] [PubMed] [Google Scholar]
- 16. Okada K, Tanaka H, Temporin K, et al. Methylcobalamin increases Erk1/2 and Akt activities through the methylation cycle and promotes nerve regeneration in a rat sciatic nerve injury model. Exp Neurol 2010;222:191-203. 10.1016/j.expneurol.2009.12.017. [DOI] [PubMed] [Google Scholar]
- 17. Akaike A, Tamura Y, Sato Y, Yokota T. Protective effects of a vitamin B12 analog, methylcobalamin, against glutamate cytotoxicity in cultured cortical neurons. Eur J Pharmacol 1993;241:1-6. 10.1016/0014-2999(93)90925-8. [DOI] [PubMed] [Google Scholar]
- 18. Yamazaki K, Oda K, Endo C, Kikuchi T, Wakabayashi T. Methylcobalamin (methyl-B12) promotes regeneration of motor nerve terminals degenerating in anterior gracile muscle of gracile axonal dystrophy (GAD) mutant mouse. Neurosci Lett 1994;170:195-7. 10.1016/0304-3940(94)90272-0. [DOI] [PubMed] [Google Scholar]
- 19. Zhang YF, Ning G. Mecobalamin. Expert Opin Investig Drugs 2008;17:953-64. 10.1517/13543784.17.6.953. [DOI] [PubMed] [Google Scholar]
- 20. Didangelos T, Karlafti E, Kotzakioulafi E, et al. Vitamin B12 supplementation in diabetic neuropathy: a 1-year, randomized, double-blind, placebo-controlled trial. Nutrients 2021;13:395. 10.3390/nu13020395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Anderson RT, Keating KN, Doll HA, Camacho F. The Hand-Foot Skin Reaction and Quality of Life Questionnaire: an assessment tool for oncology. Oncologist 2015;20:831-8. 10.1634/theoncologist.2014-0219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 1993;85:365-76. 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 23. Sprangers MA, Groenvold M, Arraras JI, et al. The European Organization for Research and Treatment of Cancer breast cancer-specific quality-of-life questionnaire module: first results from a three-country field study. J Clin Oncol 1996;14:2756-68. 10.1200/JCO.1996.14.10.2756. [DOI] [PubMed] [Google Scholar]
- 24. Natori A, Ethier JL, Amir E, Cescon DW. Capecitabine in early breast cancer: a meta-analysis of randomised controlled trials. Eur J Cancer 2017;77:40-7. 10.1016/j.ejca.2017.02.024. [DOI] [PubMed] [Google Scholar]
- 25. Santhosh A, Sharma A, Bakhshi S, et al. D-TORCH Trial Investigators . Topical Diclofenac for Prevention of Capecitabine-Associated Hand-Foot Syndrome: a double-blind randomized controlled trial. J Clin Oncol 2024;42:1821-9. 10.1200/JCO.23.01730. [DOI] [PubMed] [Google Scholar]
- 26. US Food and Drug Administration. Draft Guidance on Diclofenac Sodium October 2022. https://www.accessdata.fda.gov/drugsatfda_docs/psg/PSG_022122.pdf.
- 27. Vukelja SJ, Lombardo FA, James WD, Weiss RB. Pyridoxine for the palmar-plantar erythrodysesthesia syndrome. Ann Intern Med 1989;111:688-9. 10.7326/0003-4819-111-8-688. [DOI] [PubMed] [Google Scholar]
- 28. Fabian CJ, Molina R, Slavik M, Dahlberg S, Giri S, Stephens R. Pyridoxine therapy for palmar-plantar erythrodysesthesia associated with continuous 5-fluorouracil infusion. Invest New Drugs 1990;8:57-63. 10.1007/BF00216925. [DOI] [PubMed] [Google Scholar]
- 29. Milano G, Etienne-Grimaldi MC, Mari M, et al. Candidate mechanisms for capecitabine-related hand-foot syndrome. Br J Clin Pharmacol 2008;66:88-95. 10.1111/j.1365-2125.2008.03159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lin E, Morris JS, Ayers GD. Effect of celecoxib on capecitabine-induced hand-foot syndrome and antitumor activity. Oncology (Williston Park) 2002;16(Suppl No 14):31-7. [PubMed] [Google Scholar]
- 31. Zhang M, Han W, Hu S, Xu H. Methylcobalamin: a potential vitamin of pain killer. Neural Plast 2013;2013:424651. 10.1155/2013/424651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Muss HB, Polley MC, Berry DA, et al. Randomized trial of standard adjuvant chemotherapy regimens versus capecitabine in older women with early breast cancer: 10-year update of the CALGB 49907 trial. J Clin Oncol 2019;37:2338-48. 10.1200/JCO.19.00647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kornblith AB, Lan L, Archer L, et al. Quality of life of older patients with early-stage breast cancer receiving adjuvant chemotherapy: a companion study to cancer and leukemia group B 49907. J Clin Oncol 2011;29:1022-8. 10.1200/JCO.2010.29.9859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Van de Roovaart HJ, Stevens MM, Goodridge AE, et al. Safety and efficacy of vitamin B in cancer treatments: a systematic review. J Oncol Pharm Pract 2024;30:451-63. 10.1177/10781552231178686 [DOI] [PubMed] [Google Scholar]
- 35. Ambrosone CB, Zirpoli GR, Hutson AD, et al. Dietary supplement use during chemotherapy and survival outcomes of patients with breast cancer enrolled in a cooperative group clinical trial (SWOG S0221). J Clin Oncol 2020;38:804-14. 10.1200/JCO.19.01203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Haller DG, Cassidy J, Clarke SJ, et al. Potential regional differences for the tolerability profiles of fluoropyrimidines. J Clin Oncol 2008;26:2118-23. 10.1200/JCO.2007.15.2090. [DOI] [PubMed] [Google Scholar]
- 37. van Kuilenburg AB. Dihydropyrimidine dehydrogenase and the efficacy and toxicity of 5-fluorouracil. Eur J Cancer 2004;40:939-50. 10.1016/j.ejca.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 38. Yap YS, Kwok LL, Syn N, et al. Predictors of hand-foot syndrome and pyridoxine for prevention of capecitabine-induced hand-foot syndrome: a randomized clinical trial. JAMA Oncol 2017;3:1538-45. 10.1001/jamaoncol.2017.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Web appendix 1: Study protocol
Web appendix 2: Supplementary methods, figures, and tables
Web appendix 3: Study code
Data Availability Statement
The code used to analyse the data in the paper can be found in supplementary appendix 3. The raw database underlying the study is openly and publicly available (https://doi.org/10.5061/dryad.gmsbcc31k). The data sharing statement is given in table S13 in supplementary appendix 2.