Abstract
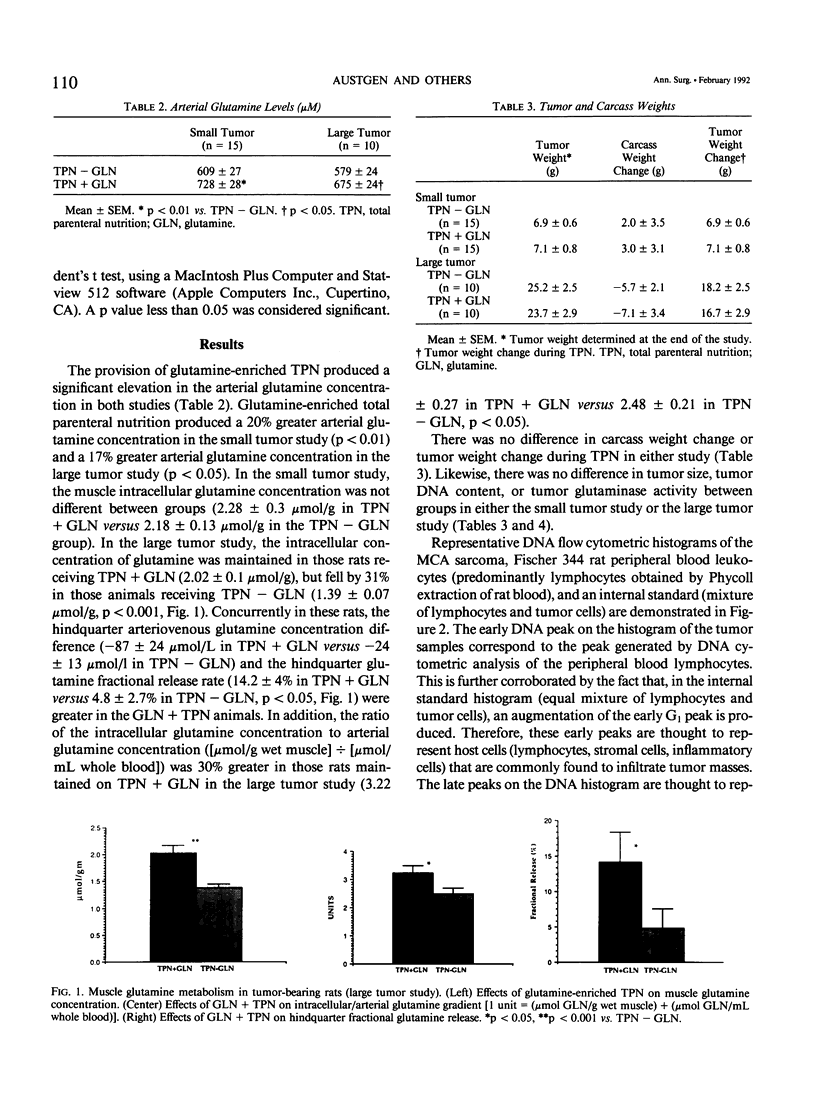
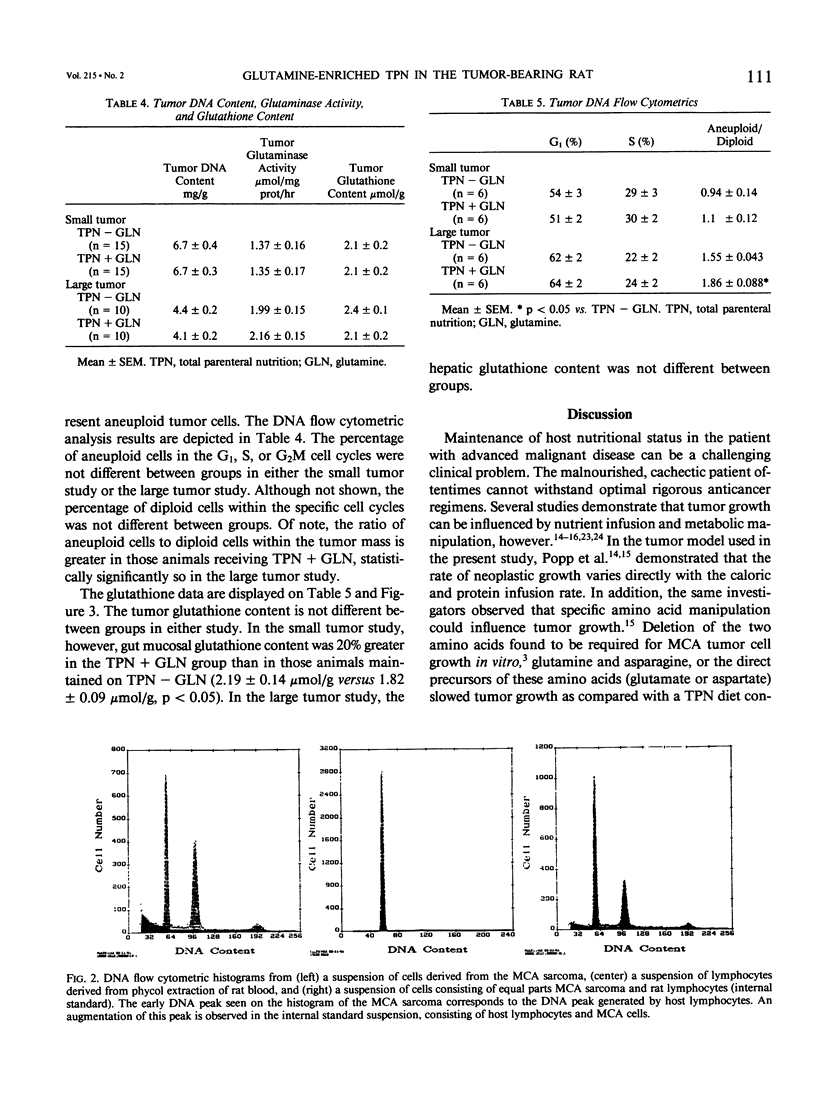
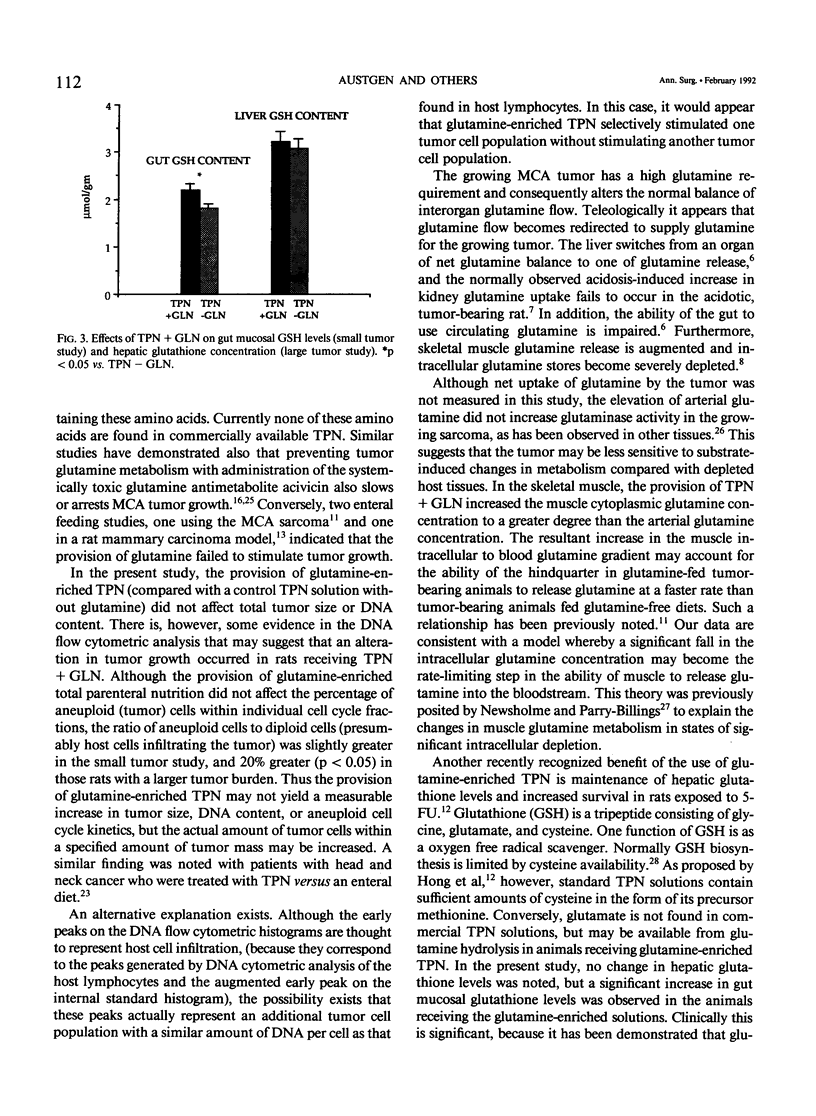
The effects of glutamine-enriched total parenteral nutrition (TPN+GLN) were studied in tumor-bearing rats because glutamine can benefit host tissues but also may stimulate tumor growth. Rats were implanted with the methylcholanthrene-induced fibrosarcoma (MCA sarcoma) and were studied when the tumor constituted less than 5% of carcass weight (small tumor) and when the tumor constituted 10% of carcass weight (large tumor). Provision of 20% of TPN protein as glutamine produced a significant increase in the arterial glutamine level and maintained the skeletal muscle intracellular glutamine concentration (2.02 +/- 0.1 versus 1.39 +/- 0.07 mumol/g, p less than 0.01). Concurrently, hindquarter GLN fractional release increased nearly threefold (p less than 0.05) in the TPN+GLN group. Glutamine-enriched total parenteral nutrition did not affect carcass weight, tumor weight, tumor DNA content, or tumor glutaminase activity. Furthermore, DNA flow cytometric analysis did not demonstrate any difference in percentage of aneuploid tumor cells within the G1, S, or G2M cell cycles. However, the ratio of aneuploid to diploid cells within the tumor mass increased by 20% in animals receiving glutamine. Glutamine-enriched total parenteral nutrition had no effect on tumor glutathione (GSH) levels. No increase in hepatic GSH levels was observed, but gut mucosal GSH levels were 20% greater in the TPN+GLN group (p less than 0.05). The provision of glutamine-enriched TPN may be beneficial to the host by maintaining skeletal muscle glutamine stores and by supporting gut GSH biosynthesis. In this tumor model, TPN+GLN does not appear to increase tumor size, tumor DNA content, or tumor glutamine metabolism, but the ratio of tumor cells to host infiltrating cells within the tumor mass appears to be increased.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baron P. L., Lawrence W., Jr, Chan W. M., White F. K., Banks W. L., Jr Effects of parenteral nutrition on cell cycle kinetics of head and neck cancer. Arch Surg. 1986 Nov;121(11):1282–1286. doi: 10.1001/archsurg.121.11.1282. [DOI] [PubMed] [Google Scholar]
- Brehe J. E., Burch H. B. Enzymatic assay for glutathione. Anal Biochem. 1976 Jul;74(1):189–197. doi: 10.1016/0003-2697(76)90323-7. [DOI] [PubMed] [Google Scholar]
- Bump E. A., Yu N. Y., Brown J. M. Radiosensitization of hypoxic tumor cells by depletion of intracellular glutathione. Science. 1982 Aug 6;217(4559):544–545. doi: 10.1126/science.7089580. [DOI] [PubMed] [Google Scholar]
- Cameron I. L., Pavlat W. A. Stimulation of growth of a transplantable hepatoma in rats by parenteral nutrition. J Natl Cancer Inst. 1976 Mar;56(3):597–602. doi: 10.1093/jnci/56.3.597. [DOI] [PubMed] [Google Scholar]
- Chance W. T., Cao L., Fischer J. E. Insulin and acivicin improve host nutrition and prevent tumor growth during total parenteral nutrition. Ann Surg. 1988 Oct;208(4):524–531. doi: 10.1097/00000658-198810000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chance W. T., Cao L., Kim M. W., Nelson J. L., Fischer J. E. Reduction of tumor growth following treatment with a glutamine antimetabolite. Life Sci. 1988;42(1):87–94. doi: 10.1016/0024-3205(88)90627-3. [DOI] [PubMed] [Google Scholar]
- Chance W. T., van Lammeren F. M., Chen M. H., Chen W. J., Murphy R. F., Joffe S. N., Fischer J. E. Plasma and brain cholecystokinin levels in cancer anorexia. J Surg Res. 1984 May;36(5):490–498. doi: 10.1016/0022-4804(84)90131-8. [DOI] [PubMed] [Google Scholar]
- Deneke S. M., Fanburg B. L. Regulation of cellular glutathione. Am J Physiol. 1989 Oct;257(4 Pt 1):L163–L173. doi: 10.1152/ajplung.1989.257.4.L163. [DOI] [PubMed] [Google Scholar]
- Hammarqvist F., Wernerman J., Ali R., von der Decken A., Vinnars E. Addition of glutamine to total parenteral nutrition after elective abdominal surgery spares free glutamine in muscle, counteracts the fall in muscle protein synthesis, and improves nitrogen balance. Ann Surg. 1989 Apr;209(4):455–461. doi: 10.1097/00000658-198904000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimberg V. S., Salloum R. M., Kasper M., Plumley D. A., Dolson D. J., Hautamaki R. D., Mendenhall W. R., Bova F. C., Bland K. I., Copeland E. M., 3rd Oral glutamine accelerates healing of the small intestine and improves outcome after whole abdominal radiation. Arch Surg. 1990 Aug;125(8):1040–1045. doi: 10.1001/archsurg.1990.01410200104017. [DOI] [PubMed] [Google Scholar]
- Klimberg V. S., Souba W. W., Salloum R. M., Plumley D. A., Cohen F. S., Dolson D. J., Bland K. I., Copeland E. M., 3rd Glutamine-enriched diets support muscle glutamine metabolism without stimulating tumor growth. J Surg Res. 1990 Apr;48(4):319–323. doi: 10.1016/0022-4804(90)90066-b. [DOI] [PubMed] [Google Scholar]
- Knox W. E., Horowitz M. L., Friedell G. H. The proportionality of glutaminase content to growth rate and morphology of rat neoplasms. Cancer Res. 1969 Mar;29(3):669–680. [PubMed] [Google Scholar]
- Kovacević Z., Morris H. P. The role of glutamine in the oxidative metabolism of malignant cells. Cancer Res. 1972 Feb;32(2):326–333. [PubMed] [Google Scholar]
- Labarca C., Paigen K. A simple, rapid, and sensitive DNA assay procedure. Anal Biochem. 1980 Mar 1;102(2):344–352. doi: 10.1016/0003-2697(80)90165-7. [DOI] [PubMed] [Google Scholar]
- Lash L. H., Hagen T. M., Jones D. P. Exogenous glutathione protects intestinal epithelial cells from oxidative injury. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4641–4645. doi: 10.1073/pnas.83.13.4641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller T. J., Franco R. S., Chance W. T., Martelo O. J., Popp M. B. Amino acid requirements of a rat sarcoma as determined by a stem cell assay. JPEN J Parenter Enteral Nutr. 1987 May-Jun;11(3):223–228. doi: 10.1177/0148607187011003223. [DOI] [PubMed] [Google Scholar]
- Mårtensson J., Jain A., Meister A. Glutathione is required for intestinal function. Proc Natl Acad Sci U S A. 1990 Mar;87(5):1715–1719. doi: 10.1073/pnas.87.5.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newsholme E. A., Parry-Billings M. Properties of glutamine release from muscle and its importance for the immune system. JPEN J Parenter Enteral Nutr. 1990 Jul-Aug;14(4 Suppl):63S–67S. doi: 10.1177/014860719001400406. [DOI] [PubMed] [Google Scholar]
- Popp M. B., Enrione E. B., Wagner S. C., Chance W. T. Influence of total nitrogen, asparagine, and glutamine on MCA tumor growth in the Fischer 344 rat. Surgery. 1988 Aug;104(2):152–160. [PubMed] [Google Scholar]
- Popp M. B., Wagner S. C., Brito O. J. Host and tumor responses to increasing levels of intravenous nutritional support. Surgery. 1983 Aug;94(2):300–308. [PubMed] [Google Scholar]
- Rennie M. J., MacLennan P. A., Hundal H. S., Weryk B., Smith K., Taylor P. M., Egan C., Watt P. W. Skeletal muscle glutamine transport, intramuscular glutamine concentration, and muscle-protein turnover. Metabolism. 1989 Aug;38(8 Suppl 1):47–51. doi: 10.1016/0026-0495(89)90140-6. [DOI] [PubMed] [Google Scholar]
- Sauer L. A., Stayman J. W., 3rd, Dauchy R. T. Amino acid, glucose, and lactic acid utilization in vivo by rat tumors. Cancer Res. 1982 Oct;42(10):4090–4097. [PubMed] [Google Scholar]
- Souba W. W., Strebel F. R., Bull J. M., Copeland E. M., Teagtmeyer H., Cleary K. Interorgan glutamine metabolism in the tumor-bearing rat. J Surg Res. 1988 Jun;44(6):720–726. doi: 10.1016/0022-4804(88)90106-0. [DOI] [PubMed] [Google Scholar]
- Vindeløv L. L., Christensen I. J., Nissen N. I. A detergent-trypsin method for the preparation of nuclei for flow cytometric DNA analysis. Cytometry. 1983 Mar;3(5):323–327. doi: 10.1002/cyto.990030503. [DOI] [PubMed] [Google Scholar]
- Wolf C. R., Lewis A. D., Carmichael J., Adams D. J., Allan S. G., Ansell D. J. The role of glutathione in determining the response of normal and tumor cells to anticancer drugs. Biochem Soc Trans. 1987 Aug;15(4):728–730. doi: 10.1042/bst0150728. [DOI] [PubMed] [Google Scholar]


