Abstract
Escherichia coli lipoproteins are anchored to the inner or outer membrane depending on the residue at position 2. Aspartate at this position makes lipoproteins specific to the inner membrane, whereas other residues cause the release of lipoproteins from the inner membrane in a manner dependent on both ATP binding cassette (ABC) transporter LolCDE and molecular chaperone LolA, followed by LolB-dependent localization in the outer membrane. The function of lipoprotein-sorting signals was examined in proteoliposomes reconstituted from LolCDE and lipoproteins. The release of outer membrane-specific lipoproteins was inhibited on reconstitution with other outer membrane-specific, but not inner membrane-specific, lipoproteins. Outer membrane-specific lipoproteins stimulated ATP hydrolysis by LolCDE whereas inner membrane-specific ones did not. LolA was not required for the stimulation of ATP hydrolysis. These results revealed a previously undocumented function of aspartate at position 2, i.e., lipoproteins having this signal avoid being recognized by LolCDE, thereby remaining in the inner membrane.
Lipoproteins present in various bacteria are synthesized as precursors in the cytoplasm and then translocated across the inner membrane. Subsequent modification reactions sequentially take place on the periplasmic side of the inner membrane, leading to the formation of mature lipoproteins having a lipid-modified cysteine at the N terminus (1, 2). In Escherichia coli, lipoproteins are anchored to the periplasmic side of either the inner or outer membrane through N-terminal lipids, depending on the lipoprotein-sorting signal present at position 2 (3). Five Lol proteins are involved in the sorting and outer membrane localization of lipoproteins (4–9). LolCDE, an ATP binding cassette (ABC) transporter, in the inner membrane releases outer membrane-directed lipoproteins from the inner membrane in an ATP-dependent manner (8, 10), leading to the formation of a water-soluble complex between the lipoprotein and LolA (4). The LolA–lipoprotein complex crosses the periplasm and then interacts with outer membrane receptor LolB, which is essential for the anchoring of lipoproteins to the outer membrane (6, 11). The lipoprotein-sorting signal is recognized at the release step because the inner membrane-specific lipoproteins are not released (4, 8, 11).
Inouye and collaborators first revealed the importance of aspartate at position 2 for the inner membrane localization of E. coli lipoproteins (3), although residues at position 3 were found to affect the aspartate-dependent inner membrane retention (12). Seydel et al. found that aspartate at position 2 was not the sole inner membrane-retention signal, although inner membrane-retention signals other than aspartate have scarcely been found in native lipoproteins (13). By examining LolA-dependent release of lipoproteins, we recently revealed that aspartate at position 2 is critically important for the inner membrane retention of lipoproteins (14). Moreover, it was also found that the strong inner membrane retention because of aspartate at position 2 is possible only with a limited number of species of residues at position 3. Finally, it was concluded that native lipoproteins specific to the inner membrane have residues at positions 2 and 3 that assure inner membrane retention.
Lipoprotein-sorting signals are recognized at the release step involving LolCDE and LolA (8). However, it remains to be clarified whether LolCDE or LolA, or both are responsible for the signal recognition. Moreover, it is not known how the sorting signals determine the membrane specificity. Because apolipoprotein transacylase (15), which catalyzes the last step of lipoprotein maturation, is not found in Bacillus subtilis, despite the high conservation of the other two lipoprotein-processing enzymes, prolipoprotein diacylglyceryl transferase (16) and prolipoprotein signal peptidase (17, 18), it has been suggested to be possible that aspartate at position 2 might inhibit the last step of lipid modification, thereby causing the generation of different species of lipoproteins that are specific to the inner membrane (19). However, so far, we have found no difference in lipid modification between inner membrane-specific and outer membrane-specific lipoproteins (T. Hara, S.M., and H.T., unpublished observations), indicating that the lipoprotein-sorting signals function at the release step but not at the step of lipid modification. To clarify the molecular mechanism underlying this lipoprotein sorting, the release reaction was examined in proteoliposomes reconstituted from purified LolCDE and lipoproteins. We propose here that the inner membrane-retention signal functions as an Lol avoidance signal, thereby causing the retention of lipoproteins in the inner membrane. Sorting signals having such a novel property have not been found in other protein traffic systems.
Materials and Methods
Materials.
LolA (4), Pal (11), Pal(D) (11), L10P (10), LolB (6), and LolB(D) (11) were purified as described. E. coli phospholipids were obtained from Avanti Polar Lipids and washed with acetone as reported (20). Sucrose monocaprate was purchased from Dojindo Laboratories (Kumamoto, Japan).
Construction of Plasmids.
To construct pKM101, pKM201, and pKM301 carrying lolC, lolD, and lolE, respectively, under the control of tacPO and lacIq, the corresponding regions of pJY310 (8) were amplified by PCR using the following oligonucleotide primers: for lolC, C5 (5′-CAGAATTCGAAGGAGATATAAATATGTACCAACCTGTCGCT-3′) and C3 (5′-CGCACTGCAGGAGCTCTTATTCATAACGTAAAGCCTCAGC-3′); for lolD, D5 (5′-GAGCTCGAAGGAGATATAAATATGAATAAGATCCTGTTGCAATGC-3′) and D3 (5′-CACTCTGCAGTTACTCCGCCCCCATCAG-3′); and for lolE, E5 (5′-TAACAGAGCTCGAAGGAGATATAAATATGGCGATGCCTTTATCG-3′) and E3 (5′-AAGCCTGCAGTTTTTGTTCCACCAATATCAAACCC-3′). The amplified DNAs were digested with EcoRI and PstI for lolC, and with SacI and PstI for lolD and lolE, and then respectively inserted into the same restriction site of pTTQ18. To construct pKM401, a 5.2-kbp KpnI-PstI fragment of pKM101 was ligated with a 3.9-kbp KpnI-PstI fragment of pJY301. The resultant plasmid carries lolCDE under the control of tacPO. To construct pKM203 carrying a gene that encodes LolD with a His-tag at its C terminus (LolDHis), PCR was performed with pKM201 as a template and oligonucleotide primers VC5H (5′-ACGATGAGCTCGAAGGAGATATAAATATGAATAAGATCCTGTTGCAATGC-3′) and VC3H (5′-CACTAAGCTTAATGATGATGATGATGATGTTCTAACTCCGCCCCCATCAGGCTCAGTTCCGCC-3′). The amplified DNA was digested with SacI and HindIII, and then cloned into the same site of pMAN885EH (21). To construct pKM402 carrying lolC and lolDHis under PBAD, a 1.7-kbp EcoRI-EcoO651 fragment of pKM401 was ligated with a 4.4-kbp EcoRI-EcoO651 fragment of pKM203. To construct pKM403 encoding an LolDHis derivative having asparagine in place of serine at position 147, pKM402 was mutagenized with a QuikChange site-directed mutagenesis kit (Stratagene) with oligonucleotides S147N-1 (5′-CCATCTGAACTTAATGGCGGCGAAC-3′) and S147N-2 (5′-GTTCGCCGCCATTAAGTTCAGATGG-3′).
Purification of the LolCDE Complex.
E. coli JM83 (21) harboring pKM301 and either pKM402 or pKM403 was grown on LB supplemented with 50 μg/ml ampicillin and 25 μg/ml chloramphenicol at 30°C. When the OD at 660 nm reached 1.0, LolC and LolD-His were induced with 0.2% arabinose. At OD660 = 1.4, expression of LolE was induced with 1 mM isopropyl β-d-thiogalactopyranoside (IPTG), followed by cultivation for 30 min. A membrane fraction was prepared from LolCDE overproducing cells as described (8), and then solubilized with 2% sucrose monocaprate in 50 mM Tris⋅Cl (pH 7.5), containing 2 mM ATP, 5 mM MgSO4, and 2 mM DTT for 10 min on ice. A supernatant was obtained by centrifugation at 100,000 × g for 30 min and then incubated for 45 min at 4°C with 3 ml of Talon resin (CLONTECH), which had been equilibrated with buffer A comprising 50 mM Tris⋅Cl (pH 7.5), 2 mM ATP, 100 mM NaCl, and 2% sucrose monocaprate. The resin was packed into a column, and washed with 60 ml of buffer A and then 30 ml of buffer A supplemented with 10 mM imidazole. The LolCDE complex was eluted with buffer A containing 250 mM imidazole. To stabilize LolCDE, E. coli phospholipids were added to fractions containing LolCDE at 1 mg/ml. The fractions were dialyzed against 50 mM Tris⋅Cl (pH 7.5), containing 2 mM ATP, 1 mM DTT, and 0.5% sucrose monocaprate. LolCDE was further purified on a MonoQ column (Amersham Pharmacia Biotech) equilibrated with 50 mM Tris⋅Cl (pH 7.5), 2 mM ATP, 1 mM DTT, and 0.5% sucrose monocaprate. Elution was performed with a linear gradient of NaCl (0 to 0.5 M). LolCDE was obtained in the fractions corresponding to ≈0.17 M NaCl.
Release of Lipoproteins from Proteoliposomes.
E. coli phospholipids (0.8 mg), LolCDE (4 μg, 28.8 pmol), and the specified lipoproteins were mixed in 100 μl of buffer A for 10 min on ice. The mixture was then diluted 10-fold and dialyzed against 50 mM potassium phosphate (pH 7.5), 5 mM MgSO4, and 100 mM NaCl for 3 h. Proteoliposomes thus formed were collected by centrifugation at 100,000 × g for 1 h, resuspended in 100 μl of buffer B comprising 50 mM potassium phosphate (pH 7.5), 5 mM MgSO4, 100 mM NaCl, and 2 mM ATP, subjected to a cycle of freezing-thawing, and then sonicated briefly as described (8). Aliquots (50 μl) of the proteoliposome suspension containing 2 μg of LolCDE were diluted with 250 μl of buffer B containing 24 μg/ml LolA as specified. Where indicated, ATP in buffer B was replaced by the specified nucleotides. The release reaction was carried out at 30°C for 20 min and terminated with 1 mM ortho-vanadate on ice. The reaction mixture was centrifuged at 100,000 × g for 1 h. Lipoproteins in pellets and supernatants were analyzed by SDS/PAGE and immunoblotting with anti-Pal (5) or anti-Lpp (22) antibodies as described (4). Unless otherwise specified, one-fifth of the pellet material was applied to the gel. To determine the amount of Pal in each fraction, purified Pal was used as a standard. Bands were quantitated with an ATTO densitograph.
ATP Hydrolysis by Proteoliposomes.
Proteoliposomes were reconstituted as described above except that the amount of LolCDE was increased to 20 μg (144 pmol), and that the reconstituted proteoliposomes were suspended in 50 μl of 50 mM Tris⋅Cl (pH 7.5), 100 mM NaCl, and 5 mM MgSO4. To examine ATP hydrolysis, aliquots (20 μl) of the proteoliposome suspensions were diluted with 70 μl of 50 mM Tris⋅Cl (pH 7.5), 100 mM NaCl, and 5 mM MgSO4, with or without 58 μg LolA. ATP hydrolysis was started at 30°C by the addition of 10 μl of 20 mM ATP. The amount of inorganic phosphate was determined spectrophotometrically according to the reported method (23).
Results
ATP-Dependent Release of Lipoproteins from Proteoliposomes Reconstituted with LolCDE.
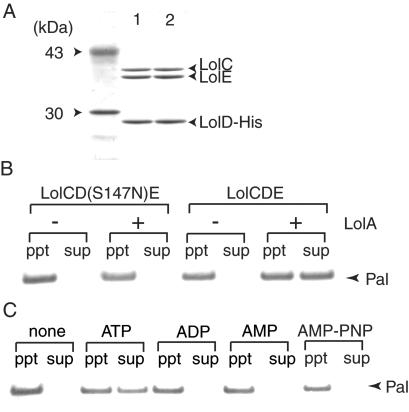
The LolCDE complex has a subunit stoichiometry of C1D2E1 and a molecular mass of 139 kDa (8). To accelerate the purification of the complex, a histidine tag (His-tag) was attached to the C terminus of LolD, and the LolCDE complex was purified on a metal-chelating column (Fig. 1A). A LolD(S147N) mutant possessing a His-tag was also expressed, and the complex containing the mutant LolD was purified. This mutant has a single amino acid substitution in the ABC signature motif (24) and inhibits the growth of E. coli even in the presence of wild-type LolD (8). The mutation did not affect the complex formation, and essentially the same amounts of LolC and LolE were copurified with LolD or LolD(S147N) (Fig. 1A). The LolCD(S147N)E complex was completely inactive as to the release of outer membrane lipoprotein Pal, whereas the wild-type LolCDE complex caused LolA-dependent release (Fig. 1B). ATP was absolutely essential for the LolCDE-dependent release of Pal. Nonhydrolyzable ATP analogue adenosine 5′-[β,γ-imido]triphosphate (AMP-PNP) did not induce the Pal release; neither did ADP or AMP (Fig. 1C).
Figure 1.
Purification and reconstitution of LolCDE. (A) Four-microgram aliquots of purified LolCD(S147N)E (lane 1) and LolCDE (lane 2) were analyzed by SDS/PAGE, followed by staining with Coomassie brilliant blue. The left lane shows molecular weight markers. (B) Proteoliposomes were reconstituted with LolCDE or LolCD(S147N)E and 0.43 μg (23 pmol) Pal, as described under Materials and Methods. The LolA-dependent release of Pal was then examined. (C) The release of Pal from proteoliposomes was examined in the presence of the specified nucleotides.
Lipoproteins with the Inner Membrane-Retention Signal Are Not Substrates for the LolCDE- and LolA-Dependent Release Reaction.
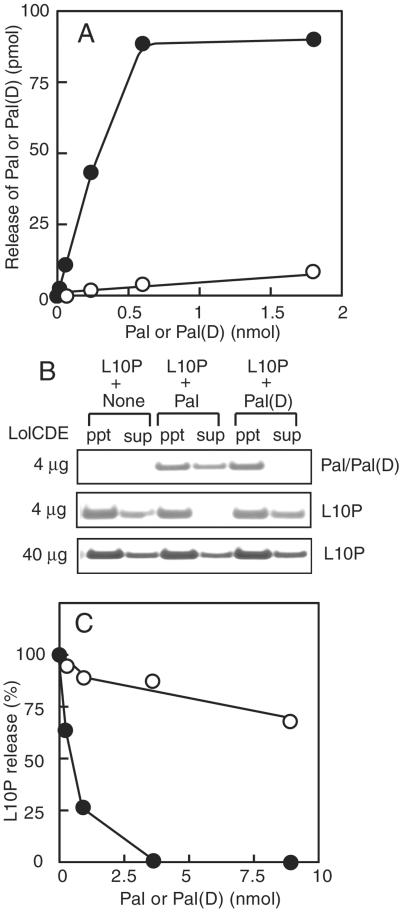
Increasing amounts of Pal or its derivative, Pal(D), having the inner membrane-retention signal, were reconstituted together with a fixed amount of LolCDE into proteoliposomes, and then LolA-dependent release was examined (Fig. 2A). The efficiency of lipoprotein release from proteoliposomes was not high because the orientation of the reconstituted lipoproteins and LolCDE is random, leaving a major fraction of lipoproteins incompetent as to release (8). However, the amount of released Pal increased linearly up to about 0.6 nmol of Pal reconstituted into proteoliposomes. With a saturating amount of Pal, one LolCDE complex was estimated to release about six molecules of Pal. This is an underestimate because only LolCDE having a rightside-out orientation releases Pal into the external medium. In contrast to Pal, Pal(D) was hardly released even when a very large amount of Pal(D) was reconstituted. Because the release reaction was saturable with Pal, we next examined the release of L10P in the presence of a large amount of Pal or Pal(D) (Fig. 2B). L10P, a derivative of major outer membrane lipoprotein Lpp, was released from proteoliposomes when Pal was not reconstituted. When an excess amount of Pal was reconstituted together with L10P, the release of L10P was completely inhibited. In marked contrast, an excess amount of Pal(D) did not inhibit the release of L10P. When the amount of LolCDE reconstituted was increased 10-fold, L10P was released even in the presence of an excess amount of Pal. The release of Pal(D) was hardly detectable even when a large amount was reconstituted. Taken together, these results indicate that Pal, having an outer membrane-sorting signal, competes with L10P for the release reaction catalyzed by LolCDE, whereas Pal(D), having an inner membrane-retention signal, does not. Examination of L10P release in the presence of various amounts of Pal or Pal(D) revealed that the inhibition of L10P release significantly differed between these two lipoproteins (Fig. 2C). We recently found that the N-terminal third residue affects the inner membrane retention caused by aspartate at position 2 (14). The weak inhibition of L10P release by Pal(D) having serine at position 3 seems to be consistent with our observation that the combination of aspartate at position 2 and serine at position 3 is not the strongest inner membrane-retention signal (14).
Figure 2.
Inner membrane-specific lipoproteins do not inhibit the release of outer membrane-specific lipoproteins. (A) Proteoliposomes were reconstituted with the indicated amounts of Pal (filled circles) or Pal(D) (open circles), and then subjected to the release assay. (B) L10P (0.07 nmol), with or without 3.4 nmol Pal or Pal(D), was reconstituted into proteoliposomes. Where indicated, the amount of LolCDE was increased 10-fold. The release of L10P, Pal, and Pal(D) was examined in the presence of LolA. Analysis of Pal and Pal(D) by SDS/PAGE was carried out with 1/750 of the pellet material and 1/30 of the supernatant. (C) The amounts of L10P released in the presence of various amounts of Pal (filled circles) or Pal(D) (open circles) were determined and expressed as percentages, taking the amount of L10P released in the absence of Pal as 100%.
Stimulation of the ATPase Activity of LolCDE by Lipoproteins Having Outer Membrane-Sorting Signals.
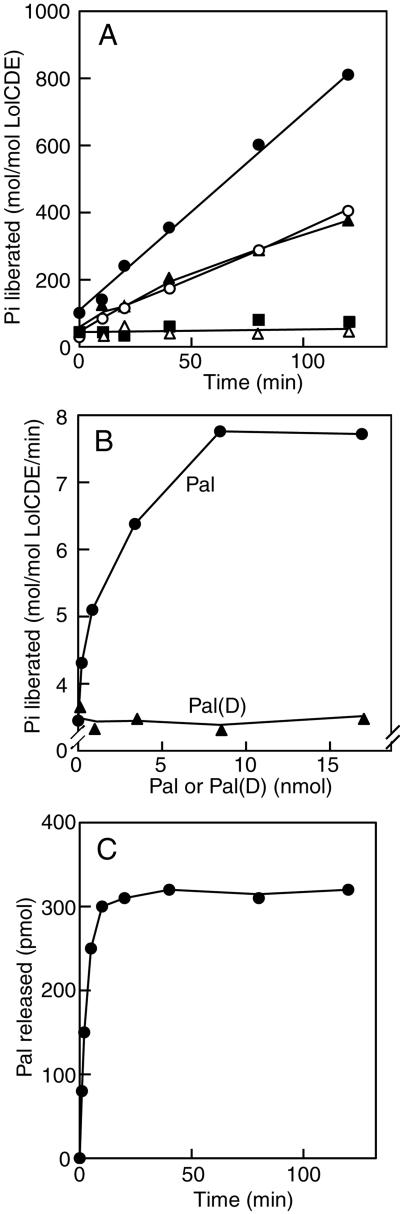
LolCDE reconstituted into proteoliposomes exhibited ATPase activity that was stable at least for 2 hr at 30°C (Fig. 3A). The ATPase activity of LolCDE determined in Triton X-100 or sucrose monocaprate was similar to that determined for proteoliposomes (data not shown). Vanadate, which had previously been shown to inhibit lipoprotein release (10), completely inhibited ATP hydrolysis by LolCDE. The ATPase activity of LolCD(S147N)E, having a mutation in the ABC signature motif, was negligible. Reconstitution of Pal stimulated the ATPase activity of LolCDE by a factor of 2–3 in the absence of LolA. Stimulation of the ATP hydrolysis depended on the amount of Pal reconstituted into proteoliposomes (Fig. 3B). On the other hand, reconstitution of Pal(D) had no stimulatory effect on the ATPase activity. These results, taken together, indicate that lipoproteins with an inner membrane-retention signal are not substrates for LolCDE, and therefore have no effect on the ATPase activity. Stimulation of ATP hydrolysis by Pal decreased in the presence of LolA, suggesting that the decrease in the amount of lipoproteins in proteoliposomes because of the LolA-dependent release accounts for the reduced rate of ATP hydrolysis. In contrast to ATP hydrolysis, which occurred linearly for 2 h even in the presence of LolA, LolA-dependent release of Pal examined under the same conditions leveled off at about 10 min after the start of assaying (Fig. 3C). In contrast, stimulation of ATP hydrolysis by LolA was not detected even when the hydrolysis during the first 8 min was examined in detail (data not shown). These results indicate that the stimulation of ATPase activity by Pal observed here is not dependent on the release reaction.
Figure 3.
ATPase activity of LolCDE reconstituted into proteoliposomes. (A) Proteoliposomes were reconstituted with LolCDE alone (open circles), Pal (9. 2 nmol) and LolCDE (filled circles), or Pal(D) (9.2 nmol) and LolCDE (filled triangles). ATP hydrolysis by these proteoliposomes was determined as described under Materials and Methods. Where specified, LolCD(S147N)E was reconstituted in place of LolCDE (filled squares), or the reaction mixture contained 10 mM ortho-vanadate (open triangles). (B) The ATPase activity of proteoliposomes reconstituted with LolCDE, and various amounts of Pal or Pal(D) was determined. Note that a lower part of the axis is deleted. (C) Proteoliposomes reconstituted with LolCDE and Pal (3.4 nmol) were subjected to the release assay in the presence of LolA.
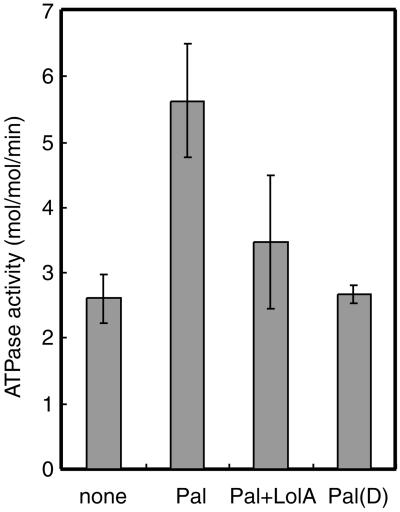
The effects of Pal, Pal(D), and LolA on the ATPase activity of LolCDE were determined at least three times with different proteoliposome preparations, the average values being shown in Fig. 4. The reported ATPase activities of ABC transporters vary significantly between 0.01–20 μmol per min per mg protein (25). The activity of the LolCDE complex in the absence of lipoproteins was determined to be 2.6 mol ATP hydrolyzed per min per mol of complex, which corresponds to 0.02 μmol per min per mg protein. The ATPase activity of LolCDE was within the range of reported values. The activity was increased to 5.63 ± 0.9 mol per min per mol in the presence of Pal.
Figure 4.
Effects of lipoproteins and LolA on ATP hydrolysis by LolCDE. The ATPase activities of proteoliposomes reconstituted with LolCDE were determined under the indicated conditions, as in Fig. 3A. The results represent the averages of at least three determinations.
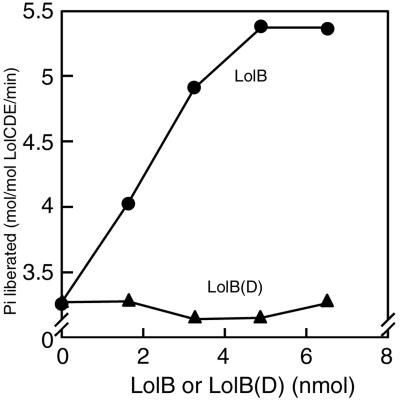
LolB is an essential lipoprotein (7) that functions as an outer membrane receptor for lipoproteins (6). We previously showed that the release and outer membrane localization of LolB are dependent on LolA and LolB, respectively (11). Although a significant portion of LolB was released in the absence of external LolA, this apparent LolA-independent release was completely abolished with LolB(D), which possesses aspartate instead of serine at position 2 (11), suggesting that LolB, but not LolB(D), is a substrate for LolCDE. The ATPase activities of proteoliposomes reconstituted with LolCDE and various amounts of LolB or LolB(D) clearly indicate that aspartate at position 2 also functions as an Lol avoidance signal for LolB (Fig. 5).
Figure 5.
LolB also stimulates ATP hydrolysis by LolCDE. Proteoliposomes were reconstituted with LolCDE and the indicated amounts of LolB or LolB(D). The ATP hydrolysis by these proteoliposomes was examined as in Fig. 3.
Discussion
We previously reported that among 20 residues only aspartate at position 2 causes the retention of lipoproteins in the inner membrane (14). We showed here that lipoproteins having aspartate at position 2 neither compete with outer membrane-specific lipoproteins for the release reaction nor stimulate ATP hydrolysis by LolCDE. These results strongly indicate that lipoproteins with aspartate at position 2 are not substrates for LolCDE. Thus, the mechanism underlying the aspartate-dependent retention of lipoproteins in the inner membrane was found to be simple, although it is not known at present why aspartate at position 2 specifically functions as a Lol avoidance signal.
It has been believed that the residue at position 2 positively functions as a lipoprotein-sorting signal. However, our results suggest that this is not always the case. More correctly, any residue other than aspartate at position 2 allows the recognition of lipoproteins by LolCDE; namely, the second residue is not the active lipoprotein-sorting signal. We speculate that aspartate at position 2 actively prevents the recognition of lipoproteins by LolCDE through an unknown mechanism. We recently isolated an LolCDE mutant that is able to recognize and release lipoproteins having a strong inner membrane-retention signal (S. Narita, K. Kanamaru, S.M., and H.T., unpublished observations). This mutant seems to be useful for clarifying the specific function of aspartate at position 2. Moreover, it is of great interest to determine whether or not the nonnative inner membrane-retention signals found by Seydel et al. (13) also function as Lol avoidance signals.
Based on our observations, we can clearly explain the complex behavior of PulA, a lipoprotein of Klebsiella oxitoca, expressed in E. coli. To understand the mechanism by which PulA is localized to the outer surface of the outer membrane of K. oxitoca, Pugsley and collaborators extensively examined the membrane localization of PulA in E. coli with or without other pul genes (2). Wild-type PulA having aspartate at position 2 was localized on the outer surface of the outer membrane when expressed with other pul genes comprising the Type II secretion pathway. In contrast, PulA was exclusively localized on the periplasmic surface of the inner membrane in the absence of other pul genes, indicating that aspartate at position 2 functions as a Lol avoidance signal in the absence of other pul genes. Substitution of aspartate with another residue resulted in the localization of PulA on both the periplasmic surface and outer surface of the outer membrane when other pul genes were present. However, this PulA derivative was exclusively localized on the periplasmic surface of the outer membrane in the absence of other pul genes (2). Taken together, these observations and our findings indicate that PulA expressed in E. coli should have a Lol avoidance signal to be efficiently translocated to the outer surface of the outer membrane through the Type II pathway; otherwise the Lol system causes the localization of PulA to the periplasmic surface of the outer membrane. It seems likely that essentially the same mechanism functions in K. oxitoca because highly similar Lol homologs are present in Klebsiella strains. Furthermore, a similar mechanism may function in other bacteria having lipoproteins on the outer surface of the outer membrane. For example, Borrelia burgdorferi, the Lyme disease spirochete, has been reported to possess more than 100 lipoproteins, some of which are on the outer surface of the outer membrane (26). Because this bacterium also possesses Lol homologs, lipoproteins on the outer surface of the outer membrane might have a Lol avoidance signal, which may not be aspartate in this bacterium. Alternatively, the Lol system may not be complete because a LolB homolog has not been found in this bacterium. In any event, because spirochetal lipoproteins have been reported to cause an immunoresponse of host cells (27), it is important to clarify the mechanism underlying the sorting of more than 100 lipoproteins in B. burgdorferi.
LolA did not stimulate but rather decreased the Pal-dependent stimulation of ATP hydrolysis by LolCDE (Fig. 4). Because the stimulation of ATP hydrolysis depended on the amount of Pal in proteoliposomes (Fig. 3B), the decrease in the amount of Pal in proteoliposomes because of its release most likely accounts for the LolA-dependent decrease in ATP hydrolysis. It was difficult to reconstitute higher amounts of Pal than those used in this study. Therefore, we could not determine ATPase activity under conditions where the amount of Pal in proteoliposomes was still saturating even in the presence of the release reaction. Under such conditions, the ATPase activity might be higher than the value shown in Fig. 4. If a single E. coli cell is assumed to possess ≈102 LolCDE molecules (unpublished observation) and ≈5 × 105 Lpp molecules (28), which should be released during a doubling time of 50 min at 30°C through stoichiometric ATP hydrolysis, the ATPase activity of LolCDE would be 100 mol per min per mol, which is significantly higher than the ATPase activity determined in the presence of Pal (Fig. 4). E. coli possesses at least 90 lipoprotein species, most of which seem to be localized in the outer membrane (S.M., K. Tanaka, and H.T., unpublished observations). The number of molecules in a single cell is expected to be fewer than 103 for each lipoprotein except Lpp and Pal, the number of molecules of the latter having been reported to be at ≈104 molecules (29). Therefore, the ATPase activity required for the release of many minor lipoproteins seems to be only marginal. ATPase activities have been determined with various ABC transporters (25). Because the substrates for all these ABC transporters are water soluble, LolCDE is the first example exhibiting elevated ATP hydrolysis depending on substrates exclusively localized in the lipid bilayer. On the other hand, as found for LolCDE, both the strong inhibition of ATP hydrolysis by vanadate and the importance of the ABC signature for ATP hydrolysis have been reported for a number of ABC transporters (24, 25).
Acknowledgments
We thank R. Ishihara for technical support. This work was supported by grants to H.T. from Core Research for Evolutional Science and Technology (CREST) of the Japan Science and Technology Corporation, and the Ministry of Education, Science, Sports and Culture of Japan.
Abbreviation
- ABC
ATP binding cassette
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Hayashi S, Wu H C. J Bioenerg Biomembr. 1990;22:451–471. doi: 10.1007/BF00763177. [DOI] [PubMed] [Google Scholar]
- 2.Pugsley A P. Microbiol Rev. 1993;57:50–108. doi: 10.1128/mr.57.1.50-108.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yamaguchi K, Yu F, Inouye M. Cell. 1988;53:423–432. doi: 10.1016/0092-8674(88)90162-6. [DOI] [PubMed] [Google Scholar]
- 4.Matsuyama S, Tajima T, Tokuda H. EMBO J. 1995;14:3365–3372. doi: 10.1002/j.1460-2075.1995.tb07342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tajima T, Yokota N, Matsuyama S, Tokuda H. FEBS Lett. 1998;439:51–54. doi: 10.1016/s0014-5793(98)01334-9. [DOI] [PubMed] [Google Scholar]
- 6.Matsuyama S, Yokota N, Tokuda H. EMBO J. 1997;16:6947–6955. doi: 10.1093/emboj/16.23.6947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tanaka K, Matsuyama S, Tokuda H. J Bacteriol. 2001;183:6538–6542. doi: 10.1128/JB.183.22.6538-6542.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yakushi T, Masuda K, Narita S, Matsuyama S, Tokuda H. Nat Cell Biol. 2000;2:212–218. doi: 10.1038/35008635. [DOI] [PubMed] [Google Scholar]
- 9.Narita S, Tanaka K, Matsuyama S, Tokuda H. J Bacteriol. 2002;184:1417–1422. doi: 10.1128/JB.184.5.1417-1422.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yakushi T, Yokota N, Matsuyama S, Tokuda H. J Biol Chem. 1998;273:32576–32581. doi: 10.1074/jbc.273.49.32576. [DOI] [PubMed] [Google Scholar]
- 11.Yokota N, Kuroda T, Matsuyama S, Tokuda H. J Biol Chem. 1999;274:30995–30999. doi: 10.1074/jbc.274.43.30995. [DOI] [PubMed] [Google Scholar]
- 12.Gennity J M, Inouye M. J Biol Chem. 1991;266:16458–16464. [PubMed] [Google Scholar]
- 13.Seydel A, Gounon P, Pugsley A P. Mol Microbiol. 1999;34:810–821. doi: 10.1046/j.1365-2958.1999.01647.x. [DOI] [PubMed] [Google Scholar]
- 14.Terada M, Kuroda T, Matsuyama S, Tokuda H. J Biol Chem. 2001;276:47690–47694. doi: 10.1074/jbc.M109307200. [DOI] [PubMed] [Google Scholar]
- 15.Gupta S D, Gan K, Schmid M B, Wu H C. J Biol Chem. 1993;268:16551–16556. [PubMed] [Google Scholar]
- 16.Sankaran K, Wu H C. J Biol Chem. 1994;269:19701–19706. [PubMed] [Google Scholar]
- 17.Yu F, Yamada H, Daishima K, Mizushima S. FEBS Lett. 1984;173:264–268. doi: 10.1016/0014-5793(84)81060-1. [DOI] [PubMed] [Google Scholar]
- 18.Innis M A, Tokunaga M, Williams M E, Loranger J M, Chang S Y, Chang S, Wu H C. Proc Natl Acad Sci USA. 1984;81:3708–3712. doi: 10.1073/pnas.81.12.3708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tjalsma H, Kontinen V P, Prágai Z, Wu H, Meima R, Veneman G, Bron S, Sarvas M, van Dijl J M. J Biol Chem. 1999;274:1698–1707. doi: 10.1074/jbc.274.3.1698. [DOI] [PubMed] [Google Scholar]
- 20.Tokuda H, Shiozuka K, Mizushima S. Eur J Biochem. 1990;192:583–589. doi: 10.1111/j.1432-1033.1990.tb19264.x. [DOI] [PubMed] [Google Scholar]
- 21.Yakushi T, Tajima T, Matsuyama S, Tokuda H. J Bacteriol. 1997;179:2857–2862. doi: 10.1128/jb.179.9.2857-2862.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hussain M, Ichihara S, Mizushima S. J Biol Chem. 1980;255:3707–3712. [PubMed] [Google Scholar]
- 23.Chifflet S, Torriglia A, Chiesa R, Tolosa S. Anal Biochem. 1988;168:1–4. doi: 10.1016/0003-2697(88)90002-4. [DOI] [PubMed] [Google Scholar]
- 24.Holland I B, Blight M A. J Mol Biol. 1999;293:381–399. doi: 10.1006/jmbi.1999.2993. [DOI] [PubMed] [Google Scholar]
- 25.Schneider E, Hunke S. FEMS Microbiol Rev. 1998;22:1–20. doi: 10.1111/j.1574-6976.1998.tb00358.x. [DOI] [PubMed] [Google Scholar]
- 26.Fraser C M, Casjens S, Huang W M, Sutton G G, Clayton R, Lathigra R, White O, Ketchum K A, Dodson R, Hickey E K, et al. Nature (London) 1997;390:580–586. doi: 10.1038/37551. [DOI] [PubMed] [Google Scholar]
- 27.Scragg I G, Kwiatkowski D, Vidal V, Reason A, Paxton T, Panico M, Dell A, Morris H. J Biol Chem. 2000;275:937–941. doi: 10.1074/jbc.275.2.937. [DOI] [PubMed] [Google Scholar]
- 28.Braun V. Biochim Biophys Acta. 1975;415:335–377. doi: 10.1016/0304-4157(75)90013-1. [DOI] [PubMed] [Google Scholar]
- 29.Cascales E, Bernadac A, Gavioli M, Lazzaroni J C, Lloubes R. J Bacteriol. 2002;184:754–759. doi: 10.1128/JB.184.3.754-759.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]