Abstract
Harmonized neuropsychological assessment for neurocognitive disorders (NCDs) is an urgent priority in clinics. Neuropsychology assessments in NCDs seldom include tests exploring social cognitive skills. In 2022, we launched the SIGNATURE initiative to optimize socio‐cognitive assessment in NCDs. Here, we report findings from the first initiative phase, including consortium creation and evaluation of the state of the art in socio‐cognitive assessment in memory clinics. We developed an ad hoc online survey to explore practices and measures, relevance, and obstacles preventing the use of socio‐cognitive testing in clinics. The survey was distributed within the SIGNATURE network. National coordinators were identified to disseminate the survey to local collaborators and scientific societies active in the field of dementia and/or neuropsychology. Data were analysed in aggregate form and stratified by geographical area and variables of interest. Four hundred and thirteen (413) responses from 10 European and Latin American geographical regions were recorded. Responders were balanced between physicians and psychologists. Seventy‐eight (78) % of respondents reported no/limited experience with socio‐cognitive measures; more than 85% agreed on their relevance in clinics. Ekman‐60 faces was the most well‐known and/or used task, followed by the Faux‐Pas and Reading‐the‐Mind‐in‐the‐Eyes tests. Lack of clinical measures, assessment time, guidelines, and education/training were reported as main obstacles. Real‐life barriers prevent the adoption of socio‐cognitive testing in clinics. Bidirectional collaboration between clinicians and researchers is required to address clinical needs and constraints and facilitate consistent socio‐cognitive assessment.
Keywords: dementia, harmonization, memory clinics, mild cognitive impairment, neurocognitive disorder, social cognition
INTRODUCTION
In the field of neurocognitive disorders (NCDs), the use of cognitive markers has radically changed in the last decade, notably due to the shift of the neurodegenerative disorders (e.g. Alzheimer's disease – AD, frontotemporal dementia – FTD, Parkinson's disease – PD, dementia with Lewy bodies – DLB) definition from a clinico‐pathological to a clinico‐biological framework (Dubois et al., 2010; Kulcsarova et al., 2024; Simuni et al., 2024). Clinical neuropsychological examination alone has shown little accuracy to identify the underlying pathology responsible for NCDs (Beach et al., 2012; Bertoux et al., 2020), while biomarkers can exclude or confirm an aetiology (Dubois et al., 2021; Frisoni et al., 2024). In this context, neuropsychological tests maintain a critical role in confirming the presence of cognitive difficulties, orienting the diagnostic hypothesis, and deciding upon the use of biomarkers, which are potentially invasive or expensive. Neuropsychological tests are also necessary for phenotyping NCDs, designing cognitive interventions, tracking disease progression, and monitoring interventions' efficacy. This is especially relevant as the progression of NCDs is, so far, not fully captured by biomarkers.
Thus far, influential clinical guidelines, such as the latest version of the Diagnostic and Statistical Manual of Mental Disorders (Fifth Edition – DSM‐5) (American Psychiatric Association, 2013), recommend the use of tests assessing social cognition abilities to quantify impairments in suspected major and mild NCDs, as well as in psychiatric and neurodevelopmental disorders, for which no biomarkers are currently available (Henry et al., 2016) or still debated (Massa et al., 2024). Adequate in‐depth cognitive assessment, which includes social cognition among the six core cognitive domains, is necessary not only to effectively diagnose cognitive deficits, but also to ensure proper use of resources for biomarkers that are potentially invasive and/or expansive. On the other hand, socio‐cognitive markers can add information to current neuropsychological testing by detecting crucial cognitive changes (that would otherwise go undetected), such as in the early stages of frontotemporal dementia (Cotter et al., 2018) and providing an additional avenue for differentiating between disorders (Dodich et al., 2017). Furthermore, the routine use of socio‐cognitive tasks may help clinicians to identify clinically relevant socio‐cognitive changes early and, consequently, to apply strategies to improve caregiver empowerment, prevent harmful outcomes in the patient‐caregiver dyad, and implement psychoeducational strategies.
Social cognition is a broad domain and involves any process involved in the understanding of social situations, cues, stimuli, and interactions. Putative components of this domain vary widely from more basic constructs such as attention to social cues, perception and encoding of social stimuli, or representations of socio‐emotional memories, to higher order processes such as affective decision‐making, inference, and attribution of emotions and intentions to others (e.g. theory of mind (ToM) and cognitive empathy), or judgement of moral actions (Happé et al., 2017). Investigations into social cognition are relatively recent in clinical neuropsychology, so is the associated academic and clinical training, and evidence on tests adequate to diagnose socio‐cognitive changes in NCDs is limited (Quesque et al., 2022). However, given the importance and complexity of social interactions in our daily lives, changes in the socio‐cognitive domain may be the first and only symptoms of certain NCDs in their early stages; in addition, such symptoms have considerable impact on patients' and caregivers' quality of life (Brioschi Guevara et al., 2015). For these reasons, detecting socio‐cognitive impairments with adequate neuropsychological tests is an urgent priority in the field of NCD (Ibanez, 2022).
The extent and selectivity of socio‐cognitive disorders in NCDs is a matter of debate (Samtani et al., 2023; Setién‐Suero et al., 2022). Among FTD patients, deficits in emotion recognition, ToM, and empathy are core cognitive markers, especially in the behavioural (Dilcher et al., 2023) and the semantic (Fittipaldi et al., 2019) variants. AD patients may also show emotion recognition and ToM impairments (Bora et al., 2015, 2016) but it is unclear whether they depend on a more general cognitive decline (Dodich et al., 2016) or on selective involvement of socio‐cognitive networks other than in FTD (Le Bouc et al., 2012). Notably, early emotion recognition deficits have been reported even in long‐lasting mild cognitive impairment (Cerami et al., 2018).
With the aim of systematically developing a meaningful, and possibly standardized, socio‐cognitive assessment of patients with suspected NCDs, in February 2022, we launched an open science multi‐centric international initiative on the ‘clinical use of SocIal coGNnition measures for the AssessmenT of neURocognitivE disorders’ (SIGNATURE initiative; https://sites.google.com/unitn.it/signature‐initiative/home). The initiative aims to optimize the clinical use of social cognition tests in NCDs by converging the expertise of methodologists, researchers, clinicians, and caregiver and patient associations. This article presents the initiative, its first phases, and the results of a large‐scale international survey. This survey was clinically focused and investigated the current practices of socio‐cognitive assessment in memory clinics, the best‐known and used tools for assessing social‐cognitive functioning in this context across Europe, the perceived relevance of assessing social cognition in NCDs, and the hurdles envisioned for the use of socio‐cognitive measures in real‐life clinical scenarios.
MATERIALS AND METHODS
SIGNATURE initiative launch and consortium creation
The SIGNATURE initiative was founded in February 2022; it includes members of the previous Geneva Harmonization initiative (Boccardi et al., 2022) and is considered as its continuation (Dodich et al., 2022; Van den Stock, 2022). The roadmap of the SIGNATURE initiative consists of the following phases: Phase 1 – Consortium Creation: creating an inclusive consortium representing memory clinics from heterogeneous locations; Phase 2 – Current Practice Evaluation: identifying clinical practice, experience with socio‐cognitive measures, and perceived obstacles for the use of socio‐cognitive testing in clinics; Phase 3 – Definition of Clinical Recommendations: defining clinical recommendations based on available evidence for the use of socio‐cognitive measures in memory clinics; Phase 4 – New Cross‐cultural Collaborative Projects: defining new cross‐cultural collaborative research studies on socio‐cognitive functioning in NCDs based on phase 3 recommendations and clinical research priorities; Phase 5 – Optimization of Socio‐cognitive Measures Implementation: optimizing socio‐cognitive measures in real‐life scenarios (Figure 1).
FIGURE 1.
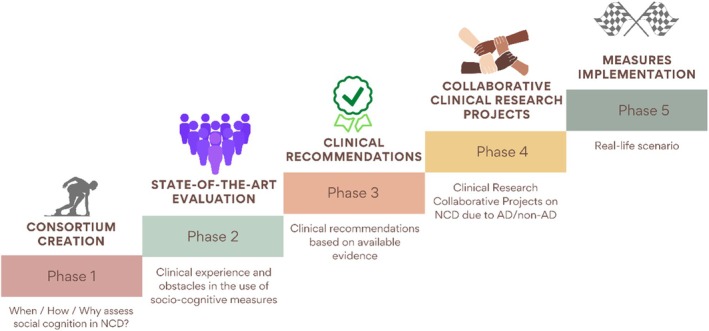
Roadmap of the SIGNATURE Initiative according to the different project phases.
Clinical survey creation and distribution
In late 2022, once the SIGNATURE consortium was created, we launched an anonymous online survey among consortium members (150 members from 98 centres across 19 European and extra‐European countries, see Participant section of SIGNATURE website, https://sites.google.com/unitn.it/signature‐initiative/home). The survey reached neurologists, psychiatrists, geriatricians, psychologists, and neuropsychologists working in memory clinics, to collect local clinical practice, experience with socio‐cognitive measures, the perceived relevance of assessing social cognition, and obstacles to socio‐cognitive assessment in clinics.
The survey was created by a working group (C.C.; A.D.; A.P.; C.M.; G.F.), implemented on Qualtrics, revised by the initiative methodologists (M.B & C.F.), and translated into six languages with the aid of national coordinators. We first distributed the survey link via written invitations to all consortium members. To maximize the survey distribution in each country, we actively involved national coordinators for the dissemination (F.F.O., F.C. & A.I. in Latin America; T.L. in France; AK.S. in Germany; M.T. in Greece; J.M.G. in Spain; M.T.P. in Portugal; G.L. in Italy; E.vd.B. in the Netherlands; C.C., L.S., M.S., in Switzerland; S.MP. in the United Kingdom). They forwarded the survey to local collaborators, clinical federations, and national scientific societies active in the field of dementia and/or neuropsychology. The distribution of the survey was also aided by the harmonization workgroup within the Cognition Professional Interest Area (PIA) of the International Society to Advance Alzheimer's Research and Treatment (ISTAART PIA‐Cognition). This study was approved by the Scuola Universitaria Superiore IUSS Pavia Ethical Committee (IUSS‐University of Pavia; Protocol 104/22) and performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments.
The eligibility criteria for respondents were the ability to provide informed consent and having professional experience with NCDs. At the beginning of the survey, we presented the study objectives and timeline, the survey commitment and estimated duration, as well as information about the research team. We asked potential participants to read and provide their informed consent for anonymous survey data collection by ticking a box. After that, participants were directed to the survey and did not receive any financial incentive to take part.
The survey was structured in five different sections: (1) general information (demographics, working centre, professional qualification, and role, expertise in social cognition); (2) neuropsychology practice in the assessment of persons with major (MNCD) and mild (mNCD) neurocognitive disorders; (3) availability of socio‐cognitive clinical measures in the respondent's geographical region based on a list of 15 tests/questionnaires available for clinical use. Responders were allowed to add additional measures in an open text box; (4) perceived relevance and clinical contribution of socio‐cognitive assessment; and (5) obstacles in the use of socio‐cognitive assessment in NCDs. Only professionals involved in clinical activity (clinicians only or clinical researchers) were asked to complete the entire survey. Those only reporting research activity ended the questionnaire after section 1. The estimated time to complete the entire survey was approximately 10 min, and responses were collected automatically. See Table S1 for details on the survey questions.
Statistical analyses
Survey responses were analysed using descriptive statistics. The response rate was calculated as the ratio of the number of respondents who completed the survey to the total number of potential participants who accessed its first page. Descriptive data were derived for the whole sample and stratified by relevant variables including geographical region; centre profiling (3 levels: academic or research institute, community hospital, local medical centre); professional qualification (physician or psychologist); professional role (clinician only or clinical researcher); and self‐reported ‘expertise in social cognition’ (4 levels: no experience, limited experience, moderate experience, expert in social cognition). Statistical comparisons among these categories were performed using Chi‐square and other non‐parametric statistics (Mann–Whitney U and Kruskal‐Wallis ANOVA).
RESULTS
Description of the survey sample
We collected 413 responses from 10 global geographical regions. The response rate was 83%. Most respondents were female (n = 237, 61%; 42.5 ± 10.4 years of age) and psychologists and/or neuropsychologists (n = 229, 55%; Table 1), especially in the Netherlands (100%), France (71%), Greece (68%), Germany (64%), and Switzerland (62%). Respondents from the Iberian Peninsula (84%) and Brazil (68%) were mostly physicians. Many professionals were clinical researchers (n = 297, 72%). The percentage of clinicians only (vs. clinical researchers) was higher among psychologists and/or neuropsychologists (34%) compared with physicians (21%), χ 2(1) = 9.08, p = .002. Most respondents worked in academic or research institutes (n = 223, 54%), 37% in community hospitals (n = 152), and the remaining 9% in local medical centres (n = 38). A higher rate of respondents from academic or research institutes was reported in Germany (74%), the United Kingdom (70%), Italy (63%), and Brazil (62%). Most respondents (n = 322, 78%) reported no or limited experience in the use of social cognition measures in NCDs, with 15% (n = 62) having moderate experience, and 7% (n = 29) having both clinical experience and a strong research portfolio in the study of social cognition changes in NCDs (i.e., publications, grants). The highest percentages of respondents with moderate‐to‐high experience were found in Spanish‐speaking Latin America (36%), Switzerland (34%), and the Netherlands (31%). In contrast, the highest rates of respondents with no social cognition expertise were in the Iberian Peninsula (59%), Greece (41%), and Germany (41%) (see Table 1 for details on demographics and other variables of interest).
TABLE 1.
Survey respondents' general information according to geographical region.
| Geographical region | Number of responses | Demographics (male/female/N.A.%) | Centre profiling (academic or research institute/community hospital/local medical centre %) | Professional qualification (physician/psychologist %) | Professional role (clinical researchers/clinicians only %) | Expertise in social cognition (none/limited/moderate/expert %) |
|---|---|---|---|---|---|---|
| Brazil | 37 | 38/62/0 | 62/16/22 | 68/32 | 70/30 | 38/49/14/0 |
| France | 48 | 25/75/0 | 25/69/6 | 29/71 | 42/58 | 19/65/6/10 |
| Germany | 39 | 31/69/0 | 74/23/3 | 36/64 | 79/21 | 41/44/13/3 |
| Greece | 28 | 25/75/0 | 29/67/4 | 32/68 | 82/18 | 43/36/11/10 |
| Iberian Peninsula | 44 | 46/52/2 | 52/41/7 | 84/16 | 89/11 | 59/25/16/0 |
| Italy | 106 | 24/32/44 | 63/24/13 | 49/51 | 77/23 | 41/34/15/10 |
| Spanish‐speaking Latin America | 22 | 36/64/0 | 45/37/18 | 55/45 | 86/14 | 23/41/18/18 |
| The Netherlands | 40 | 7.5/92.5/0 | 55/45/0 | 0/100 | 65/35 | 0/70/23/8 |
| Switzerland | 29 | 48/45/7 | 52/41/7 | 38/62 | 52/48 | 14/52/28/6 |
| United Kingdom | 20 | 55/45/0 | 70/20/10 | 50/50 | 80/20 | 35/55/10/0 |
| Overall sample | 413 | 34/61/5 | 53/38/9 | 44/56 | 72/28 | 31/47/15/7 |
Note: Responses from Argentina, Chile, Colombia, Mexico, and Peru were grouped as ‘Spanish‐speaking Latin America’ and Portugal with Spain as ‘Iberian Peninsula’ (see Methods), as we collected <20 responses from these countries.
Practice in neuropsychological assessment
Almost half of respondents (n = 201, 50%) stated that they use local standard neuropsychological protocols. One hundred sixty‐three of the respondents (40%) stated that they select neuropsychological tests for cognitive assessment based on patients' clinical presentation, France and the United Kingdom having an even higher rate (55%). The use of harmonized protocols was overall minimal (i.e., n = 44, 10% of the whole sample), with Brazil and Spanish‐speaking Latin America reporting the highest percentage (22% each). See Figure S1 for further details on practice in neuropsychological assessment.
Approximately, half of the sample (46% in the case of MNCD, 45% in mNCD) reported not assessing all DSM‐5 core cognitive domains at the first neuropsychological evaluation. Within these groups, 95% and 93%, respectively, of respondents did not assess the social cognition domain (Figure 2).
FIGURE 2.

Coverage of the routine neuropsychological assessment in the respondents' practice. On the left, the percentage of respondents who assess or not (YES/NO) all DSM‐5 core cognitive domains in major (a) and mild (b) NCDs. On the right, the percentage of coverage of individual core cognitive domains in respondents who assess all core cognitive domains.
Psychologists reported assessing social cognition more frequently than physicians in both MNCD (U = 17,991, p = .003) and mNCD (U = 17,721, p = .001). This difference was also true when the analyses were stratified for centre type, χ 2(2) = 8.46, p = .01 for MNCD, χ 2(2) = 8.40, p = .01 for mNCD and professional role (i.e., clinicians only vs. clinical researchers: U = 15,104, p = .02 for MNCD, U = 15,336, p = .04 for mNCD) as well as for expertise in social cognition, χ 2(2) = 96.02, p < .001 for MNCD, χ 2(2) = 87.18, p < .001 for mNCD, with experts in social cognition and clinical researchers assessing this domain more frequently.
Availability of socio‐cognitive measures in clinics
Knowledge and use of socio‐cognitive measures largely differed among respondents based on geographical region (see Figure S1). However, the Ekman‐60 faces (EK‐60F; a well‐known test for recognizing facial affect) test (or its variants; Ekman & Friesen, 1976) was reported as the most well‐known and used task overall (83% of respondents reported to know/use the test), followed by two tests of theory of mind (i.e., the Faux‐Pas (Stone et al., 1998) (72%) and the Reading the Mind in the Eyes (RMET) (Baron‐Cohen et al., 2001) tests (61%) or their variants). Stratifying by geographical region, we took into consideration the top five tests per country. The EK‐60F and the Faux‐Pas test were reported as known/used in 10/10 geographical regions, and the RMET in 8/10 geographical regions. The Mini‐SEA (known/used in 5/10 geographical regions), a test combining facial emotion recognition and a selection of faux‐pas (Bertoux et al., 2012), was the most known/used test in France, while the Story‐based Empathy task (i.e., a test evaluating affective and cognitive theory of mind (Dodich et al., 2015)) was the most known/used test after the EK‐60F in Italy (know/used in 6/10 geographical regions). Finally, the Interpersonal Reactivity Index (Davis, 1980) was reported as the most known/used measure after the RMET (Baron‐Cohen et al., 2001) in Greece.
Perceived relevance and clinical contribution of socio‐cognitive assessment
Survey respondents primarily agreed on the relevance of implementing socio‐cognitive assessment in NCDs for differential (93% agreement) and early (84%) diagnosis, as well as for the detection of new cognitive phenotypes (86%), meaning subgroups of patients with homogeneous cognitive‐behavioural profiles. In this latter case, higher relevance was reported by physicians compared with psychologists (U = 17,809, p = .003) and clinical researchers compared with clinicians only (U = 14,779, p = .013). As expected, socio‐cognitive assessment was considered particularly relevant for the diagnosis of NCD syndromes within the FTLD spectrum and in the context of non‐amnestic mild cognitively impaired patients. However, respondents reported an average relevance >60% for all the considered clinical diagnoses (Figure 3).
FIGURE 3.
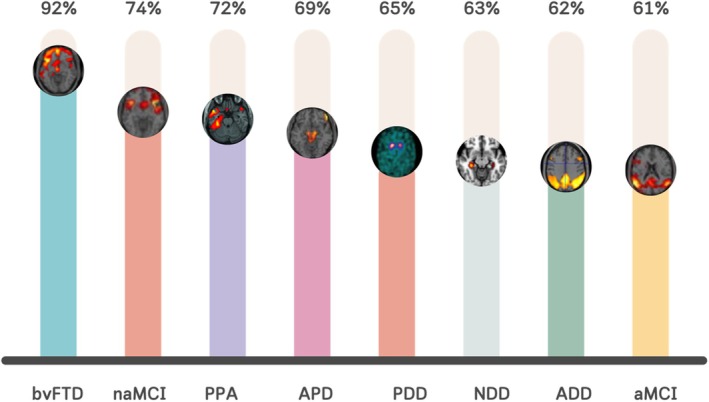
Average scores of perceived relevance of socio‐cognitive assessment for different syndromes. ADD, Alzheimer's disease dementia; aMCI, amnestic mild cognitive impairment; APD, atypical parkinsonisms; bvFTD, behavioural variant of frontotemporal dementia; naMCI, non amnestic mild cognitive impairment; NDD, non‐degenerative dementias; PDD, Parkinson's disease dementia; PPA, primary progressive aphasia.
Obstacles in the use of socio‐cognitive testing in clinics
About 71% of the sample agreed on the presence of crucial obstacles preventing the use of socio‐cognitive testing in memory clinics. This finding was consistent across geographical regions, except Germany, with only 28% agreement. The most relevant obstacles rated by respondents were the lack of standardized clinical neuropsychological measures (86% agreement overall in the sample and >70% in each geographical region) and the lack of clinical guidelines on the use of specific socio‐cognitive tools (79% and >50%). Insufficient time in the clinical setting was also evaluated as a main obstacle (77% overall agreement and >70% geographical‐based agreement), apart from Germany (45%) and the Netherlands (60%). Insufficient education and training on the use of tasks were also reported as an obstacle (67% overall agreement and >50% geographical‐based agreement), except in Germany (45%) and the Netherlands (29%). Most respondents did not consider insufficient neurobiological validation as an obstacle (44% overall agreement), apart from those in the United Kingdom, Spanish‐speaking Latin America, Greece, and the Iberian Peninsula, with an agreement score equal to or higher than 50% (Figure 4 and Figure S1).
FIGURE 4.

Percentage of agreement for main obstacles in the use of socio‐cognitive testing in clinics.
A significant difference was found in the sample when stratified by professional qualifications and expertise. A higher number of physicians reported the presence of obstacles compared with psychologists, χ 2(1) = 4.34, p = .037. Physicians emphasized the following obstacles as most relevant: insufficient time in a clinical setting (U = 8116, p = .005); a lack of clinical guidelines (U = 8438, p = .021); and insufficient education and training for the use of tasks (U = 6608, p < .001). Non‐experts in social cognition reported a greater need for education and training, χ 2(3) = 20.13, p < .001 and insufficient time in clinical settings, χ 2(2) = 16.79, p < .001 compared with experts. No significant differences were found based on the centre profile, χ 2(2) = 2.19, p = .33 or the respondent's professional role, χ 2(1) = .52, p = .47.
DISCUSSION
We reviewed current practice in the use of socio‐cognitive testing in NCDs across 10 global geographic regions. We collected information on a large sample of professionals working in clinical and research settings and who are involved in the diagnostic and clinical work‐up of NCD patients. Although most respondents are clinical researchers working in research institutions, 77% of respondents reported having no or limited experience in using social cognition measures in NCDs. This finding confirms the important gap between the considerable amount of experimental research in the field of social cognition and the poor translation of this accumulating evidence into clinics (McDonald & Kelly, 2023; Quesque et al., 2022). Multiple barriers affect the use of neuropsychological measures in clinics (Grazia et al., 2023). Lack of assessment time, guidelines, and validated measures for socio‐cognitive assessment are the major obstacles to the implementation of socio‐cognitive assessments, causing a fragmented international scenario, in which professionals are forced to make a choice based on the few measures available in their own country, without specific training or guidelines to influence their choice. Emerging initiatives are aimed at reducing this gap (e.g. European Consortium on Cross‐Cultural Neuropsychology – ECCroN (Franzen et al., 2021)) between research and clinical practice. However, until now, they are mostly limited to specific fields, such as frontotemporal dementia and psychiatric disorders (e.g. Neuropsychiatric International Consortium Frontotemporal Dementia, NIC‐FTD consortium, https://www.nic‐ftd.com/) (Van den Stock et al., 2022).
Scientific work from the last decade strongly supports the presence of early socio‐cognitive dysfunctions in different NCD syndromes, over and above the FTLD spectrum. Literature demonstrated socio‐cognitive changes in AD (Bora et al., 2015; Stam et al., 2023), PD (Argaud et al., 2018; De Souza et al., 2022; Fittipaldi et al., 2019), DLB (Heitz et al., 2016), and neuropsychiatric and neurodevelopmental disorders (Henry et al., 2016; Morellini et al., 2022). Research also confirms what is stated in the DSM‐5 guidelines that social cognition should be untethered from FTLD and that socio‐cognitive tasks should be included in the assessment of every patient suspected of NCD to improve the detection of deficits and achieve better cognitive phenotyping. Therefore, our initiative is timely based on the extensive evidence that social cognition can decline early in a wide range of NCDs, and that overlooking such deficits may delay patient diagnoses, adding strain and costs for inconclusive examinations to patients and health funders, and possibly increasing the risk of inappropriate medication. To propel such a needed advancement, our initiative supports a bidirectional collaboration between clinicians from centres specialized in NCDs and researchers expert in the field of social cognition. Notably, the current methods in many European and Latin American centres are a heterogeneous scenario characterized by the use of neuropsychological protocols defined internally within each centre, mostly lacking socio‐cognitive testing. This further supports the utility of harmonized batteries and the inclusion of socio‐cognitive testing, both for improving accuracy and optimizing time in neuropsychology services and memory clinics. The use of a standard cognitive assessment in clinics including easy‐to‐administer, quick, and clinically validated measures is thus crucial to avoid repeated cognitive evaluations for patients requiring second opinions or needing to perform follow‐up examinations in different centres. In addition, a standard cognitive assessment is needed for clinicians and researchers to optimize neuropsychological practice and improve data pooling and comparability of clinical research studies.
Among the tests available for clinical use reported in the survey, the EK‐60F and its variants were the best‐known and most widely used tests, followed by the Faux‐Pas test and the RMET. Overall, these measures have shown sufficient evidence of their clinical role in detecting social cognition deficits in different samples of patients with NCD, particularly in patients with the behavioural variant of frontotemporal dementia (see Dodich et al., 2021 for a review). However, these tests present some limitations that may discourage clinician use, including poor psychometric properties (Olderbak et al., 2015) and limited evidence of clinical and ecological validation in this patient population. On the other hand, other tests devised specifically to assess socio‐cognitive dysfunctions in NCDs are still not validated for all languages and cultures, factors that could greatly affect social cognition test performance (Fittipaldi et al., 2024). Methods for handling data heterogeneity post‐assessment in cross‐cultural settings are critically needed (Maito et al., 2023). Among social cognition screening, the mini Social Cognition Emotional Assessment – Mini‐SEA – (Bertoux et al., 2012) was more widely used among French respondents, while the Story‐based Empathy task in those from Italy (Dodich et al., 2015), and the Interpersonal Reactivity Index (Davis, 1980) in Greece. These measures represent useful sources in the clinical diagnosis of behavioural variant frontotemporal dementia, the pathological model of ‘social brain dysfunction’ in NCDs (Schroeter et al., 2014). The next phases of the SIGNATURE project aim to collect ‘bottom‐up data’ from clinicians, patients, and caregivers to inform future developments in socio‐cultural measurement and diagnostic recommendations, in a manner that is based on evidence of both clinical utility and feasibility from a stakeholder perspective.
A key challenge in the clinical application of socio‐cognitive assessment is the cultural appropriateness of the available tests. While our survey highlights the most commonly used measures, it is important to acknowledge that many of these tests were originally developed and validated in specific linguistic and cultural contexts, primarily within Western, educated, industrialized, rich, and democratic (WEIRD) nations (Bourdage et al., 2024; Fujii, 2018). As a result, their applicability to other populations remains largely unknown. To overcome this, some researchers propose the ad hoc development, validation, and standardization of cross‐cultural tests (Franzen et al., 2022). However, the feasibility of this approach remains to be proven, as the socio‐cognitive domain includes abilities that are themselves significantly dependent on socio‐cultural context. Context‐free socio‐emotional stimuli could be thus particularly difficult to implement in some cases (e.g. moral decision‐making or social norms assessment) and probably ineffective in representing the actual socio‐cognitive competence of the individuals. In this context, future international efforts should be devoted to answering these open questions.
Finally, it remains a strong need for better education and training of clinical researchers and clinicians on socio‐cognitive measurement, as noted by the survey respondents, particularly those in the Iberian Peninsula, Greece, Germany, and Italy. This need has been previously documented in two surveys which showed that clinicians feel less confident in assessing social cognition than other cognitive domains (Quesque et al., 2022) and would be in favour of routinely evaluating socio‐cognitive abilities if better prepared (Jarsch et al., 2022). Liaising with the academic and research institutions that provide continuous educational programmes and individual courses would help bridge the gap between academia and the clinical world (Hokkanen et al., 2019). Actions that support academic clinics to advance this are essential to align current clinical practices with the knowledge now widely available through research.
CONCLUSION
This work has strengths and limitations. Due to our recruitment modalities, we collected a greater proportion of individuals identified as clinical researchers rather than clinicians. This underscores existing hurdles that must be overcome and the potential for bias in some of the findings. The number of responses in single countries is also unbalanced, potentially leading to findings that do not reflect the true extent of the use of relevant tests in specific contexts. Findings may not fully represent the different local scenarios and require future confirmatory evidence to be entirely capable of describing the complexity of country‐based clinical settings. Future research should thus aim to include larger, more representative samples across countries to provide a more comprehensive understanding of testing practices worldwide. A broader view on this will undoubtedly be needed and could also benefit from the input of other groups and countries not represented in the current work. A goal of the SIGNATURE initiative, in collaboration with the ISTAART‐PIA‐Cognition harmonization workgroup, is to evaluate the leading approaches used in other geographical regions not covered by the present work (e.g. North America).
On the other hand, the main strengths of this work lie in the open science approach of the Initiative that results in a proactive spontaneous participation of several European and non‐European clinicians, providing a global perspective on the worldwide interest in the field. In addition, the Initiative offers an insightful clinical perspective complementary to that of experimental research, bridging the gap between theoretical advances and practical applications. Harmonized practice within clinical neuropsychology services should integrate the best available research evidence, clinical expertise, and individual patient‐caregiver needs (Hokkanen et al., 2019). However, developing and validating fine‐grained socio‐cognitive tests free of culture‐based effects in terms of diversity, education, and principles is a significant challenge (Franzen et al., 2021). Collaborative international initiatives and concrete attempts to extend participation to more diverse stakeholders may help to overcome these issues.
AUTHOR CONTRIBUTIONS
Chiara Cerami: Conceptualization; methodology; writing – original draft; writing – review and editing; funding acquisition; supervision; project administration. Marina Boccardi: Methodology; conceptualization; writing – review and editing. Claudia Meli: Formal analysis; data curation; writing – review and editing. Andrea Panzavolta: Formal analysis; writing – review and editing; data curation. Giulia Funghi: Formal analysis; writing – review and editing; data curation. Cristina Festari: Methodology; writing – review and editing. Stefano F. Cappa: Writing – review and editing. Thanos Chatzikostopoulos: Writing – review and editing. Christian Chicherio: Writing – review and editing. Florencia Clarens: Writing – review and editing. Fabricio Ferreira de Oliveira: Writing – review and editing. Francesco Di Lorenzo: Writing – review and editing. Marco Filardi: Writing – review and editing. Agustin Ibanez: Writing – review and editing. Nicola Girtler: Writing – review and editing. Thibaud Lebouvier: Writing – review and editing. Giancarlo Logroscino: Writing – review and editing. Antonella Luca: Writing – review and editing. Sarah E. MacPherson: Writing – review and editing. Jordi A. Matias‐Guiu: Writing – review and editing. Tommaso Piccoli: Writing – review and editing. Olivier Piguet: Writing – review and editing. Simone Pomati: Writing – review and editing. Mirella Russo: Writing – review and editing. Leonardo Sacco: Writing – review and editing. Ann‐Katrin Schild: Writing – review and editing. Stefano L. Sensi: Writing – review and editing. Steven D. Shirk: Writing – review and editing. Marc Sollberger: Writing – review and editing. Miguel Tábuas‐Pereira: Writing – review and editing. Magda Tsolaki: Writing – review and editing. Esther van den Berg: Writing – review and editing. Maxime Bertoux: Writing – review and editing. Fiona Kumfor: Writing – review and editing. Jan Van den Stock: Writing – review and editing. Kathleen A. Welsh‐Bohmer: Writing – review and editing. Alessandra Dodich: Conceptualization; methodology; writing – original draft; writing – review and editing; project administration; funding acquisition.
CONFLICT OF INTEREST STATEMENT
Chiara Cerami has been granted for consultancy by Biogen Italy, Newel Health, LinkForMed, Ethos srl. Stefano F. Cappa has received speaker honoraria from Biogen, Roche and Nutricia and is member of the Scientific Advisory Board of Brain Control. Cristina Festari has received funding through her institution from the Alzheimer's Association and Italian Ministry of Health. Giancarlo Logroscino has served as investigator for clinical trials sponsored by Biogen Pharmaceuticals, Axovant, Alector, Denali, Roche, Eisai, Genentech, Amylyx, Piam Farmaceutici SpA and has been granted for speech and consultancy by EISAI, Roche, Lilly, Piam Farmaceutici Spa, Biogen. Jordi Matias‐Guiu has been granted for speech and consultancy by Almirall, Alter, Fujirebio, Esteve, KRKA and Schwabbe, Araclon, Eisai, and Schwabbe and is supported by grants from Instituto de Salud Carlos III and Fundacion Conocimiento Madrid. Fabricio Ferreira de Oliveira has been granted for consultancy by Gerson Lehrman Group, Atheneum Partners, Guidepoint, Lionbridge. He is supported by FAPESP—The State of São Paulo Research Foundation (Grant No. #2015/10109‐5) and is a board member of AAN Global Strategies Subcommittee, Awards Committee of the International Parkinson and Movement Society, ISTAART PIA Biofluid Based Biomarkers working group, ISTAART PIA Neuropsychiatric Syndromes and ESF Committee of Experts. Leonardo Sacco has served as investigator for clinical trials sponsored by Biogen and was member of the Advisory Board of Roche and Eisai. Marc Sollberger has been granted for speech and consultancy by Lilly, Eisai, OM Pharma. Katheen Welsh‐Bohmer is a consultant to WCG, Senaptec, Jigsawdio. She serves on the U.S. POINTER data safety monitoring board and is unpaid board member for the nonprofit Dementia Alliance of NC (DANC). She is also funded through US Federal Grants (NIA/NIH), NC DHHS state funds, and private foundational support (National Philanthropic Trust/Gates to the Alzheimer's Data Discovery Initiative ADDI). The other authors do not declare any conflict of interest.
Supporting information
Appendix S1.
ACKNOWLEDGEMENTS
This manuscript was facilitated by the Alzheimer's Association International Society to Advance Alzheimer's Research and Treatment (ISTAART), through the Cognition professional interest area (PIA). The views and opinions expressed by authors in this publication represent those of the authors and do not necessarily reflect those of the PIA membership, ISTAART, or the Alzheimer's Association. The study was supported by the Joint Programme of Neurodegenerative Disorders (JPND) Working Group Grant 2022.
Cerami, C. , Boccardi, M. , Meli, C. , Panzavolta, A. , Funghi, G. , Festari, C. , Cappa, S. F. , Chatzikostopoulos, T. , Chicherio, C. , Clarens, F. , de Oliveira, F. F. , Di Lorenzo, F. , Filardi, M. , Ibanez, A. , Girtler, N. , Lebouvier, T. , Logroscino, G. , Luca, A. , MacPherson, S. E. , … Dodich, A. ; (2025). Understanding barriers and optimizing socio‐cognitive assessment in the diagnosis of neurocognitive disorders. Journal of Neuropsychology, 19, 603–618. 10.1111/jnp.12431
DATA AVAILABILITY STATEMENT
The datasets generated and/or analysed during the current study are available upon request to the corresponding author.
REFERENCES
- American Psychiatric Association . (2013). Diagnostic and statistical manual of mental disorders. American Psychiatric Publishing. 10.1176/appi.books.9780890425596 [DOI] [Google Scholar]
- Argaud, S. , Vérin, M. , Sauleau, P. , & Grandjean, D. (2018). Facial emotion recognition in Parkinson's disease: A review and new hypotheses. Movement Disorders, 33, 554–567. 10.1002/mds.27305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron‐Cohen, S. , Wheelwright, S. , Hill, J. , Raste, Y. , & Plumb, I. (2001). The “Reading the mind in the eyes” test revised version: A study with normal adults, and adults with Asperger syndrome or high‐functioning autism. Journal of Child Psychology and Psychiatry, 42, 241–251. [PubMed] [Google Scholar]
- Beach, T. G. , Monsell, S. E. , Phillips, L. E. , & Kukull, W. (2012). Accuracy of the clinical diagnosis of Alzheimer disease at National Institute on Aging Alzheimer disease centers, 2005–2010. Journal of Neuropathology and Experimental Neurology, 71, 266–273. 10.1097/NEN.0b013e31824b211b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertoux, M. , Cassagnaud, P. , Lebouvier, T. , Lebert, F. , Sarazin, M. , le Ber, I. , Dubois, B. , NeuroCEB Brain Bank , Auriacombe, S. , Hannequin, D. , Wallon, D. , Ceccaldi, M. , Maurage, C. A. , Deramecourt, V. , & Pasquier, F. (2020). Does amnesia specifically predict Alzheimer's pathology? A neuropathological study. Neurobiology of Aging, 95, 123–130. 10.1016/j.neurobiolaging.2020.07.011 [DOI] [PubMed] [Google Scholar]
- Bertoux, M. , Delavest, M. , De Souza, L. C. , Funkiewiez, A. , Lépine, J. P. , Fossati, P. , Dubois, B. , & Sarazin, M. (2012). Social cognition and emotional assessment differentiates frontotemporal dementia from depression. Journal of Neurology, Neurosurgery, and Psychiatry, 83, 411–416. 10.1136/jnnp-2011-301849 [DOI] [PubMed] [Google Scholar]
- Boccardi, M. , Monsch, A. U. , Ferrari, C. , Altomare, D. , Berres, M. , Bos, I. , Buchmann, A. , Cerami, C. , Didic, M. , Festari, C. , Nicolosi, V. , Sacco, L. , Aerts, L. , Albanese, E. , Annoni, J. M. , Ballhausen, N. , Chicherio, C. , Démonet, J. F. , Descloux, V. , … the Consortium for the Harmonization of Neuropsychological Assessment for Neurocognitive Disorders . (2022). Harmonizing neuropsychological assessment for mild neurocognitive disorders in Europe. Alzheimer's & Dementia, 18, 29–42. 10.1002/alz.12365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bora, E. , Velakoulis, D. , & Walterfang, M. (2016). Meta‐analysis of facial emotion recognition in behavioral variant frontotemporal dementia. Journal of Geriatric Psychiatry and Neurology, 29, 205–211. 10.1177/0891988716640375 [DOI] [PubMed] [Google Scholar]
- Bora, E. , Walterfang, M. , & Velakoulis, D. (2015). Theory of mind in behavioural‐variant frontotemporal dementia and Alzheimer's disease: A meta‐analysis. Journal of Neurology, Neurosurgery and Psychiatry, 86, 714–719. 10.1136/jnnp-2014-309445 [DOI] [PubMed] [Google Scholar]
- Bourdage, R. , Narme, P. , Neeskens, R. , Papma, J. , & Franzen, S. (2024). An evaluation of cross‐cultural adaptations of social cognition testing: A systematic review. Neuropsychology Review, 34, 1048–1094. 10.1007/s11065-023-09616-0 [DOI] [PubMed] [Google Scholar]
- Brioschi Guevara, A. , Knutson, K. M. , Wassermann, E. M. , Pulaski, S. , Grafman, J. , & Krueger, F. (2015). Theory of mind impairment in patients with behavioural variant fronto‐temporal dementia (bv‐FTD) increases caregiver burden. Age and Ageing, 44, 891–895. 10.1093/ageing/afv059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerami, C. , Dodich, A. , Iannaccone, S. , Magnani, G. , Santangelo, R. , Presotto, L. , Marcone, A. , Gianolli, L. , Cappa, S. F. , & Perani, D. (2018). A biomarker study in long‐lasting amnestic mild cognitive impairment. Alzheimer's Research & Therapy, 10, 42. 10.1186/s13195-018-0369-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotter, J. , Granger, K. , Backx, R. , Hobbs, M. , Looi, C. Y. , & Barnett, J. H. (2018). Social cognitive dysfunction as a clinical marker: A systematic review of meta‐analyses across 30 clinical conditions. Neuroscience & Biobehavioral Reviews, 84, 92–99. 10.1016/j.neubiorev.2017.11.014 [DOI] [PubMed] [Google Scholar]
- Davis, M. H. (1980). A multidimensional approach to individual differences in empathy. JSAS Catalog of Selected Documents in Psychology, 10, 85. [Google Scholar]
- De Souza, L. C. , Bertoux, M. , Radakovic, R. , Hornberger, M. , Mariano, L. I. , Resende, E. P. F. , Quesque, F. , Guimarães, H. C. , Gambogi, L. B. , Tumas, V. , Camargos, S. T. , Cardoso, F. E. C. , Teixeira, A. L. , & Caramelli, P. (2022). I'm looking through you: Mentalizing in frontotemporal dementia and progressive supranuclear palsy. Cortex, 155, 373–389. 10.1016/j.cortex.2022.07.015 [DOI] [PubMed] [Google Scholar]
- Dilcher, R. , Malpas, C. B. , O'Brien, T. J. , & Vivash, L. (2023). Social cognition in behavioral variant frontotemporal dementia and pathological subtypes: A narrative review. Journal of Alzheimer's Disease, 94, 19–38. 10.3233/JAD-221171 [DOI] [PubMed] [Google Scholar]
- Dodich, A. , Boccardi, M. , Sacco, L. , Monsch, A. U. , Démonet, J. F. , Filardi, M. , Logroscino, G. , Salmon, D. P. , Weinbtraub, S. , Dubois, B. , Cappa, S. F. , Cerami, C. , & the Consortium for the Harmonization of Neuropsychological Assessment for Neurocognitive Disorders . (2022). Answer to “social cognition assessment for mild neurocognitive disorders”. Alzheimers Dement, 18, 1441–1442. 10.1002/alz.12664 [DOI] [PubMed] [Google Scholar]
- Dodich, A. , Cerami, C. , Canessa, N. , Crespi, C. , Iannaccone, S. , Marcone, A. , Realmuto, S. , Lettieri, G. , Perani, D. , & Cappa, S. F. (2015). A novel task assessing intention and emotion attribution: Italian standardization and normative data of the story‐based empathy task. Neurological Sciences, 36, 1907–1912. 10.1007/s10072-015-2281-3 [DOI] [PubMed] [Google Scholar]
- Dodich, A. , Cerami, C. , Cappa, S. F. , Marcone, A. , Golzi, V. , Zamboni, M. , Giusti, M. C. , & Iannaccone, S. (2017). Combined socio‐behavioral evaluation improves the differential diagnosis between the behavioral variant of frontotemporal dementia and Alzheimer's disease: In search of neuropsychological markers. Journal of Alzheimer's Disease, 61, 761–772. 10.3233/JAD-170650 [DOI] [PubMed] [Google Scholar]
- Dodich, A. , Cerami, C. , Crespi, C. , Canessa, N. , Lettieri, G. , Iannaccone, S. , Marcone, A. , Cappa, S. F. , & Cacioppo, J. T. (2016). Differential impairment of cognitive and affective mentalizing abilities in neurodegenerative dementias: Evidence from behavioral variant of frontotemporal dementia, Alzheimer's disease, and mild cognitive impairment. Journal of Alzheimer's Disease, 50, 1011–1022. 10.3233/JAD-150605 [DOI] [PubMed] [Google Scholar]
- Dodich, A. , Crespi, C. , Santi, G. C. , Cappa, S. F. , & Cerami, C. (2021). Evaluation of discriminative detection abilities of social cognition measures for the diagnosis of the behavioral variant of frontotemporal dementia: A systematic review. Neuropsychology Review, 31, 251–266. 10.1007/s11065-020-09457-1 [DOI] [PubMed] [Google Scholar]
- Dubois, B. , Feldman, H. H. , Jacova, C. , Cummings, J. L. , DeKosky, S. T. , Barberger‐Gateau, P. , Delacourte, A. , Frisoni, G. , Fox, N. C. , Galasko, D. , Gauthier, S. , Hampel, H. , Jicha, G. A. , Meguro, K. , O'Brien, J. , Pasquier, F. , Robert, P. , Rossor, M. , Salloway, S. , … Scheltens, P. (2010). Revising the definition of Alzheimer's disease: A new lexicon. Lancet Neurology, 9, 1118–1127. 10.1016/S1474-4422(10)70223-4 [DOI] [PubMed] [Google Scholar]
- Dubois, B. , Villain, N. , Frisoni, G. B. , Rabinovici, G. D. , Sabbagh, M. , Cappa, S. , Bejanin, A. , Bombois, S. , Epelbaum, S. , Teichmann, M. , Habert, M. O. , Nordberg, A. , Blennow, K. , Galasko, D. , Stern, Y. , Rowe, C. C. , Salloway, S. , Schneider, L. S. , Cummings, J. L. , & Feldman, H. H. (2021). Clinical diagnosis of Alzheimer's disease: Recommendations of the international working group. Lancet Neurology, 20, 484–496. 10.1016/S1474-4422(21)00066-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekman, P. , & Friesen, W. (1976). Pictures of facial affect. Consulting Psychologists Press. [Google Scholar]
- Fittipaldi, S. , Ibanez, A. , Baez, S. , Manes, F. , Sedeno, L. , & Garcia, A. M. (2019). More than words: Social cognition across variants of primary progressive aphasia. Neuroscience and Biobehavioral Reviews, 100, 263–284. 10.1016/j.neubiorev.2019.02.020 [DOI] [PubMed] [Google Scholar]
- Fittipaldi, S. , Legaz, A. , Maito, M. , Hernandez, H. , Altschuler, F. , Canziani, V. , Moguilner, S. , Gillan, C. M. , Castillo, J. , Lillo, P. , Custodio, N. , Avila‐Funes, J. A. , Cardona, J. F. , Slachevsky, A. , Henriquez, F. , Fraile‐Vazquez, M. , Cruz de Souza, L. , Borroni, B. , Hornberger, M. , … Ibanez, A. (2024). Heterogeneous factors influence social cognition across diverse settings in brain health and age‐related diseases. Nature Mental Health, 2, 63–75. 10.1038/s44220-023-00164-3 [DOI] [Google Scholar]
- Franzen, S. , European Consortium on Cross‐Cultural Neuropsychology (ECCroN) , Watermeyer, T. J. , Pomati, S. , Papma, J. M. , Nielsen, T. R. , Narme, P. , Mukadam, N. , Lozano‐Ruiz, Á. , Ibanez‐Casas, I. , Goudsmit, M. , Fasfous, A. , Daugherty, J. C. , Canevelli, M. , Calia, C. , van den Berg, E. , & Bekkhus‐Wetterberg, P. (2022). Cross‐cultural neuropsychological assessment in Europe: Position statement of the European consortium on cross‐cultural neuropsychology (ECCroN). Clinical Neuropsychology, 36, 546–557. 10.1080/13854046.2021.1981456 [DOI] [PubMed] [Google Scholar]
- Franzen, S. , Papma, J. M. , van den Berg, E. , & Nielsen, T. R. (2021). Cross‐cultural neuropsychological assessment in the European Union: A Delphi expert study. Archives of Clinical Neuropsychology, 36, 815–830. 10.1093/arclin/acaa083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisoni, G. B. , Festari, C. , Massa, F. , Cotta Ramusino, M. , Orini, S. , Aarsland, D. , Agosta, F. , Babiloni, C. , Borroni, B. , Cappa, S. F. , Frederiksen, K. S. , Froelich, L. , Garibotto, V. , Haliassos, A. , Jessen, F. , Kamondi, A. , Kessels, R. P. C. , Morbelli, S. D. , O'Brien, J. T. , … Nobili, F. (2024). European intersocietal recommendations for the biomarker‐based diagnosis of neurocognitive disorders. Lancet Neurology, 23, 302–312. 10.1016/S1474-4422(23)00447-7 [DOI] [PubMed] [Google Scholar]
- Fujii, D. E. M. (2018). Developing a cultural context for conducting a neuropsychological evaluation with a culturally diverse client: The ECLECTIC framework. The Clinical Neuropsychologist, 32, 1356–1392. 10.1080/13854046.2018.1435826 [DOI] [PubMed] [Google Scholar]
- Grazia, A. , Altomare, D. , Preis, L. , Monsch, A. U. , Cappa, S. F. , Gauthier, S. , Frölich, L. , Winblad, B. , Welsh‐Bohmer, K. A. , Teipel, S. J. , Boccardi, M. , & For the Consortium for the Harmonization of Neuropsychological Assessment . (2023). Feasibility of a standard cognitive assessment in European academic memory clinics. Alzheimers Dement, 19, 2276–2286. 10.1002/alz.12830 [DOI] [PubMed] [Google Scholar]
- Happé, F. , Cook, J. L. , & Bird, G. (2017). The structure of social cognition: In(ter)dependence of Sociocognitive processes. Annual Review of Psychology, 68, 243–267. 10.1146/annurev-psych-010416-044046 [DOI] [PubMed] [Google Scholar]
- Heitz, C. , Noblet, V. , Phillipps, C. , Cretin, B. , Vogt, N. , Philippi, N. , Kemp, J. , de Petigny, X. , Bilger, M. , Demuynck, C. , Martin‐Hunyadi, C. , Armspach, J. P. , & Blanc, F. (2016). Cognitive and affective theory of mind in dementia with Lewy bodies and Alzheimer's disease. Alzheimer's Research & Therapy, 8, 10. 10.1186/s13195-016-0179-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry, J. D. , von Hippel, W. , Molenberghs, P. , Lee, T. , & Sachdev, P. S. (2016). Clinical assessment of social cognitive function in neurological disorders. Nature Reviews. Neurology, 12, 28–39. 10.1038/nrneurol.2015.229 [DOI] [PubMed] [Google Scholar]
- Hokkanen, L. , Lettner, S. , Barbosa, F. , Constantinou, M. , Harper, L. , Kasten, E. , Mondini, S. , Persson, B. , Varako, N. , & Hessen, E. (2019). Training models and status of clinical neuropsychologists in Europe: Results of a survey on 30 countries. The Clinical Neuropsychologist, 33, 32–56. 10.1080/13854046.2018.1484169 [DOI] [PubMed] [Google Scholar]
- Ibanez, A. (2022). The mind's golden cage and cognition in the wild. Trends in Cognitive Sciences, 26, 1031–1034. 10.1016/j.tics.2022.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarsch, M. , Semenkova, A. , Monsch, A. , Kressig, R. W. , & Sollberger, M. (2022). Eine Lücke, die es zu schließen gilt: Die Untersuchung sozial‐kognitiver Fähigkeiten an deutschsprachigen Memory‐Kliniken A gap that needs to be closed: The assessment of social‐cognitive abilities in German‐speaking memory clinics. Zeitschrift für Neuropsychologie, 33, 129–137. 10.1024/1016-264X/a000358 [DOI] [Google Scholar]
- Kulcsarova, K. , Skorvanek, M. , Postuma, R. B. , & Berg, D. (2024). Defining Parkinson's disease: Past and future. Journal of Parkinson's Disease, 14, S257–S271. 10.3233/JPD-230411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bouc, R. , Lenfant, P. , Delbeuck, X. , Ravasi, L. , Lebert, F. , Semah, F. , & Pasquier, F. (2012). My belief or yours? Differential theory of mind deficits in frontotemporal dementia and Alzheimer's disease. Brain, 135, 3026–3038. 10.1093/brain/aws237 [DOI] [PubMed] [Google Scholar]
- Maito, M. A. , Santamaría‐García, H. , Moguilner, S. , Possin, K. L. , Godoy, M. E. , Avila‐Funes, J. A. , Behrens, M. I. , Brusco, I. L. , Bruno, M. A. , Cardona, J. F. , Custodio, N. , García, A. M. , Javandel, S. , Lopera, F. , Matallana, D. L. , Miller, B. , de Oliveira, M. O. , Pina‐Escudero, S. D. , Slachevsky, A. , … Ibañez, A. (2023). Classification of Alzheimer's disease and frontotemporal dementia using routine clinical and cognitive measures across multicentric underrepresented samples: A cross sectional observational study. Lancet Regional Health. Americas, 17, 100387. 10.1016/j.lana.2022.100387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massa, F. , Villain, N. , Ramusino, M. C. , & Frisoni, G. B. (2024). Clinical versus biomarker‐based diagnosis of neurocognitive disorders – Authors' reply. The Lancet Neurology, 23, 766–767. 10.1016/S1474-4422(24)00275-8 [DOI] [PubMed] [Google Scholar]
- McDonald, S. , & Kelly, M. (2023). Evaluating social cognition. In American Psychological Association (Ed.), APA handbook of neuropsychology: Neuroscience and neuromethods, Vol. 2 (pp. 293–312). American Psychological Association. [Google Scholar]
- Morellini, L. , Ceroni, M. , Rossi, S. , Zerboni, G. , Rege‐Colet, L. , Biglia, E. , Morese, R. , & Sacco, L. (2022). Social cognition in adult ADHD: A systematic review. Frontiers in Psychology, 13, 940445. 10.3389/fpsyg.2022.940445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olderbak, S. , Wilhelm, O. , Olaru, G. , Geiger, M. , Brenneman, M. W. , & Roberts, R. D. (2015). A psychometric analysis of the reading the mind in the eyes test: Toward a brief form for research and applied settings. Frontiers in Psychology, 6, 1503. 10.3389/fpsyg.2015.01503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quesque, F. , Nivet, M. , Etchepare, A. , Wauquiez, G. , Prouteau, A. , Desgranges, B. , & Bertoux, M. (2022). Social cognition in neuropsychology: A nationwide survey revealing current representations and practices. Applied Neuropsychology. Adult, 31, 689–702. 10.1080/23279095.2022.2061859 [DOI] [PubMed] [Google Scholar]
- Samtani, S. , Meka, A. , & Siette, J. (2023). Beyond memory: Exploring the value of social cognition for older adults with neurocognitive disorders. Frontiers in Psychiatry, 14, 1209745. 10.3389/fpsyt.2023.1209745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeter, M. L. , Laird, A. R. , Chwiesko, C. , Deuschl, C. , Schneider, E. , Bzdok, D. , Eickhoff, S. B. , & Neumann, J. (2014). Conceptualizing neuropsychiatric diseases with multimodal data‐driven meta‐analyses – The case of behavioral variant frontotemporal dementia. Cortex, 57, 22–37. 10.1016/j.cortex.2014.02.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setién‐Suero, E. , Murillo‐García, N. , Sevilla‐Ramos, M. , Abreu‐Fernández, G. , Pozueta, A. , & Ayesa‐Arriola, R. (2022). Exploring the relationship between deficits in social cognition and neurodegenerative dementia: A systematic review. Frontiers in Aging Neuroscience, 14, 778093. 10.3389/fnagi.2022.778093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simuni, T. , Chahine, L. M. , Poston, K. , Brumm, M. , Buracchio, T. , Campbell, M. , Chowdhury, S. , Coffey, C. , Concha‐Marambio, L. , Dam, T. , DiBiaso, P. , Foroud, T. , Frasier, M. , Gochanour, C. , Jennings, D. , Kieburtz, K. , Kopil, C. M. , Merchant, K. , Mollenhauer, B. , … Marek, K. (2024). A biological definition of neuronal α‐synuclein disease: Towards an integrated staging system for research. Lancet Neurology, 23, 178–190. 10.1016/S1474-4422(23)00405-2 [DOI] [PubMed] [Google Scholar]
- Stam, D. , Rosseel, S. , De Winter, F.‐L. , Van den Bossche, M. J. A. , Vandenbulcke, M. , & Van den Stock, J. (2023). Facial expression recognition deficits in frontotemporal dementia and Alzheimer's disease: A meta‐analytic investigation of effects of phenotypic variant, task modality, geographical region and symptomatic specificity. Journal of Neurology, 270, 5731–5755. 10.1007/s00415-023-11927-4 [DOI] [PubMed] [Google Scholar]
- Stone, V. E. , Baron‐Cohen, S. , & Knight, R. T. (1998). Frontal lobe contributions to theory of mind. Journal of Cognitive Neuroscience, 10, 640–656. 10.1162/089892998562942 [DOI] [PubMed] [Google Scholar]
- Van den Stock, J. (2022). Social cognition assessment for mild neurocognitive disorders. Alzheimers Dement, 18, 1439–1440. 10.1002/alz.12475 [DOI] [PubMed] [Google Scholar]
- Van den Stock, J. , Bertoux, M. , Diehl‐Schmid, J. , Piguet, O. , Rankin, K. P. , Pasquier, F. , Ducharme, S. , Pijnenburg, Y. , & Kumfor, F. (2022). Current potential for clinical optimization of social cognition assessment for frontotemporal dementia and primary psychiatric disorders. Neuropsychology Review, 33, 544–550. 10.1007/s11065-022-09554-3 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1.
Data Availability Statement
The datasets generated and/or analysed during the current study are available upon request to the corresponding author.


