Abstract
Although epidemiological evidence indicates that a daily supplement of vitamin E may reduce the risk of prostate cancer, the detailed mechanism underlying this effect remains unclear. Here we demonstrate that α-tocopheryl succinate (VES) can suppress the expression of prostate-specific antigen (PSA), a marker for the progression of prostate cancer. VES can also suppress androgen receptor (AR) expression by means of transcriptional and posttranscriptional modulation, but not ligand binding, nuclear translocation, or AR dimerization. This VES-mediated inhibition of AR is selective because VES does not repress the expression of other nuclear receptors. Cell growth studies further show that VES inhibits the growth of prostate cancer LNCaP cells. In contrast, hydroxyflutamide (HF), an antiandrogen currently used to treat prostate cancer patients, only slightly inhibits LNCaP cell growth. Interestingly, simultaneous addition of HF and VES results in a more significant inhibition of LNCaP cell growth. Moreover, selenomethionine (SM), a prostate cancer treatment adjuvant, shows an inhibitory effect on LNCaP cell growth, yet has no effect on the AR/PSA pathway. Together, our data indicate that VES may suppress androgen/AR-mediated cell growth and PSA expression by inhibiting AR expression at both the transcription and translation levels. This previously undescribed mechanism may explain how VES inhibits the growth of prostate cancer cells and help us to establish new therapeutic concepts for the prevention and treatment of prostate cancer.
Keywords: α-tocopheryl succinate‖selenium‖hydroxyflutamide‖vitamin D receptor
Although vitamin E was identified as an essential nutrient for many decades, the detailed mechanisms of its physiological functions remain unclear (1). Early reports showed that a daily supplement of α-tocopherol (vitamin E) decreased the incidence of prostate cancer from 17.8% to 11.7% in male smokers, whereas β-carotene (another antioxidant) had no effect (2). This was the first large-scale epidemiological study showing that vitamin E may play an important role in the prevention of prostate cancer.
Among vitamin E derivatives used to study the inhibition of cancer cell growth, α-vitamin E succinate (VES) effectively inhibits the growth of several cancer cells (3–6). Previous studies suggested that VES could inhibit the proliferation of prostate cancer cells by arresting DNA synthesis, or by stimulating transforming growth factor beta (TGF-β) (7). The detailed mechanisms by which VES prevents prostate cancer cell proliferation, however, remain largely unknown.
Prostate cancer is the most common noncutaneous cancer and second leading cause of cancer death in American men (8). The androgen receptor (AR) is required for the development of both the normal prostate gland and prostate cancer. In the early stages of prostate cancer, almost all cancer cells are androgen-dependent and highly sensitive to anti-androgens. However, prostate cancer usually recurs after a few years of androgen ablative treatment, and most cancer cells become androgen-independent, rendering antiandrogen therapy useless (9). Reports suggest that mutations in the AR ligand-binding domain, AR coregulators, or receptor phosphorylation may enable the AR to respond to nonandrogen agonists (10–13). Furthermore, the activation of the AR by these factors during androgen ablation therapy may facilitate androgen-independent prostate cancer growth. As androgen-independent prostate tumors are incurable, the prevention of such aberrant AR activation is an attractive therapeutic target. Prostate-specific antigen (PSA) is a key androgen-regulated gene, and is a sensitive and selective marker for prostate cancer screening and assessment (14). Consequently, PSA is used as an indicator of disease progression and response for prostate cancer therapies.
Here we use the androgen-dependent LNCaP human prostate cancer cell line (15) as a cell model to study the potential mechanisms of VES to prevent prostate cancer development and progression. We demonstrate that VES decreases intracellular and secreted levels of PSA in LNCaP cells, which have been cultured either in normal serum or in androgen-stimulated conditions. Furthermore, our results indicate that inhibition of PSA is concomitant with VES-mediated down-regulation of AR protein levels. We have also found that the inhibition of AR protein is not only because of regulation of AR mRNA level but also because VES affects the efficiency of AR protein translation.
Materials and Methods
Chemicals and Reagents.
VES, succinic acid (Suc), selenomethionine (SM), and 5α-dihydrotestosterone (DHT) were purchased from Sigma. HF was a gift from Schering. Antibodies to vitamin D receptor (VDR), peroxisome-proliferator activated receptor α (PPARα), and retinoid X receptor α (RXRα) and β-actin were from Santa Cruz Biotechnology. PSA (clone ER-PR8) antibody was purchased from Dako.
Cell Culture and VES Treatment.
The LNCaP and COS-1 cells were purchased from the American Type Culture Collection (Manassas, VA). Fibroblast cells were primarily cultured from normal prostate tissue. LNCaP cells were grown in phenol red-free RPMI medium 1640 with 8% fetal bovine serum (FBS). The fibroblast cells and COS-1 cells were cultured in phenol red-free Dulbecco's modified Eagle's medium (DMEM) with 10% FBS. The cells were treated with Suc as a control, VES, HF, SM, or DHT at designated concentrations. During the treatment, the medium was changed every 4 days and fresh compounds were added every 2 days.
Cell Counting and Thiazolyl Blue (MTT) Assay.
LNCaP cells (5 × 104) were seeded in each well of 12-well plates. After 36–48 h, the medium was changed to phenol red-free RPMI 1640 medium with 8% FBS or charcoal-stripped FBS (CS-FBS) for another 2, 4, and 6 days, with different treatments. For cell counting, cells were trypsinized, neutralized by medium, and counted on hemocytometers. The MTT assay is a quantitative colorimetric assay for mammalian cell survival and proliferation (13, 16). Fibroblast cells were seeded at 2 × 105 per well in 6-well plates, and cell growth assays were conducted by using the same MTT assay used for LNCaP cells.
Northern Blot Analysis.
Total RNA was extracted by using Trizol according to the manufacturer's instructions (GIBCO), and 20 μg of total RNA was electrophoresed and transferred to the membrane (17). The fragments of the human AR, PSA, or β-actin cDNAs were labeled with [32P]dCTP. Membranes were prehybridized, hybridized, and washed. The mRNA signals were visualized by using a PhosphorImager (Molecular Dynamics).
In Vivo AR Radioligand Competition Binding Assay.
LNCaP cells were plated into 60-mm dishes and grown to ≈60% confluence. Cells were pretreated with ethanol or 10 μM VES (0.1% vol/vol) for 24 h. Then medium was changed to RPMI 1640 with 8% CS-FBS, and competition ligand binding was performed by using 2.5 nM [3H]R1881, with or without 100-fold excess of unlabeled R1881 (250 nM) (18). After 1-h incubation, cells were harvested by lysis buffer (PBS with 1% Triton X-100). Equal protein amounts of cell extract were subjected to binding assays, which were terminated by adding hydroxylapatite. Each sample was filtered by using a sampling manifold (Millipore) and unbound ligand was removed by washing. Filter papers that contained bound ligand were transferred to counting vials containing 5 ml of liquid scintillation fluid and counted with a multipurpose scintillation counter (Beckman).
[35S]Methionine Labeling of AR.
LNCaP cells were plated into 60-mm dishes and grown to ≈75% confluence. Cells were pretreated with 10 μM VES or ethanol (0.1% vol/vol) for 24 h. Then medium was changed to methionine-free DMEM + 5% dialyzed FCS with or without 10 μM VES at 37°C for 2 h. After 2 h, cells were labeled by incubation with 37°C pulse medium for either (i) 2 h followed by 2-, 6-, and 12-h incubation with chase medium (no [35S]methionine) for protein-stability assay, or (ii) 0.5-, 2-, 6-, and 12-h incubation with no chase period for protein-translation assay. Pulse medium consisted of 100 μCi/ml [35S]methionine (1 Ci = 37 GBq) and 5 μM unlabeled methionine in methionine-free DMEM with 5% dialyzed FBS. To lyse cells, precooled RIPA buffer (1% Nonidet P-40/0.1% SDS/0.5% sodium deoxycholate/1× PBS) plus 1 mM PMSF was added to each dish.
Immunoprecipitation of [35S]Methionine-Labeled Cell Lysate.
Three hundred micrograms of total cellular protein was transferred to new microcentrifuge tubes, and then 3 μl of rabbit anti-AR polyclonal antibody-NH27 (19) and 500 μl of reaction buffer (0.15 M NaCl/0% Triton X-100/20 mM Tris⋅HCl, pH 8.0) was added (20), and incubated for 2 h at 4°C with constant rocking. Twenty-five microliters of protein A/G beads, was added to the solution and incubated for 2 h at 4°C with constant rocking. Samples were centrifuged at 2,500 × g for 3 min at 4°C to collect the beads and then washed three times using ice-cold reaction buffer. Fifty microliters of 1.5× SDS gel-loading buffer was added and boiled for 4 min. Aliquots (25 μl)were subjected to gel electrophoresis, followed by autoradiographic signal quantitation using iqmac software (Molecular Dynamics).
Cell Transfection and Reporter Gene Assay.
For PSA promoter luciferase assay, LNCaP cells were plated in 60-mm dishes until ≈60–70% confluence, and then transfected with 6-kb PSA promoter-linked luciferase reporter (PSA6.0-Luc) by using Superfect (Qiagen, Valencia, CA). Twenty-four hours after transfection, the cells were treated with various compounds for an additional 24 h. For AR N-terminal/C-terminal (N-C) interaction assay, COS-1 cells (1 × 105) were plated on 12-well plates 12 h before being transfected with 0.5 μg of pG5-Luc reporter and other expression vectors depicted in the figure legends. After 24 h transfection, 10 nM DHT and/or 10 μM VES was added for another 24 h. For each transfection, simian virus 40 promoter driven Renilla luciferase (SV40RL) was used as an internal control.
Results
VES Represses the Growth of LNCaP Cells, but Not Prostate Fibroblasts.
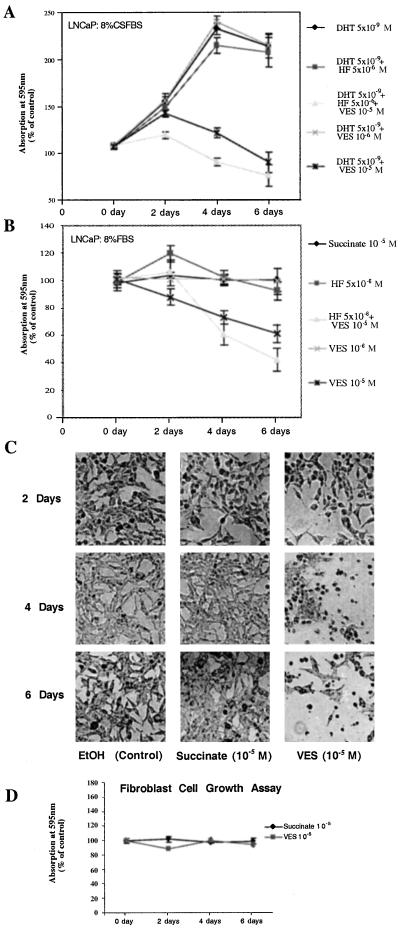
Many prostate tumors progress to a hormone-refractory stage concomitant with the flutamide withdrawal syndrome (21), enabling the tumor to grow in the presence of antiandrogens, such as HF. It is necessary, therefore, to search for more effective antiproliferative reagents to manage prostate cancer. Here, we compare the inhibitory effect of VES with HF in LNCaP cells. Using the MTT assay, Fig. 1A demonstrates that 5 nM DHT can stimulate LNCaP cell growth, and the addition of 5 μM HF fails to repress this DHT-induced cell growth in medium with 8% CS-FBS. In contrast, the addition of 10 μM VES effectively represses DHT-mediated cell growth. Interestingly, addition of both 5 μM HF and 10 μM VES can further repress DHT-mediated cell growth. In addition, when we replace 8% CS-FBS with 8% FBS without DHT, 5 μM HF induces LNCaP cell growth at day 2, with the induction gradually diminishing after day 4 (Fig. 1B). Again, 10 μM VES inhibits LNCaP cell growth and the combination of both 10 μM VES and 5 μM HF could further repress LNCaP cell growth after day 4. Together, results from Fig. 1 A and B demonstrate that 10 μM VES can effectively inhibit LNCaP cell growth, either in FBS or in CS-FBS in the presence of 5 nM DHT. The combination of 10 μM VES and 5 μM HF further represses LNCaP cell growth. At the same time, we also observed a morphologic change in the LNCaP cells during the treatment period with most of the cells dying after VES treatment for 4 days (Fig. 1C).
Figure 1.
VES inhibits the growth of LNCaP cells, but not prostate fibroblast cells. (A) LNCaP cells were cultured in 8% CS-FBS RPMI and treated with DHT (5 nM), Suc (10 μM), HF (5 μM), VES (1 μM or 10 μM), or VES (10 μM) combined with HF (5 μM). Cells were harvested at the time indicated. (B) LNCaP cells were cultured in 8% FBS RPMI and treated with Suc (10 μM), HF (5 μM), VES (1 μM or 10 μM), or VES (10 μM) combined with HF (5 μM). Cells were harvested at the time indicated. (C) Phase-contrast photomicrographs depict representative morphological responses of LNCaP cells at 2, 4, and 6 days of exposure to ethanol, 10 μM Suc, or 10 μM VES. (×100.) (D) Primary cultured prostate fibroblast cells were maintained in 10% FBS DMEM and treated with Suc and VES as indicated. Cell growth was determined by the MTT assay. The control group was cultured in 0.1% (vol/vol) ethanol and was set at 100%. All results were compared with the control group at the same time point.
Surprisingly, when we replaced tumor cells with primary cultured fibroblasts from normal prostate tissue, 10 μM VES had only a marginal inhibitory effect on cell growth (Fig. 1D), suggesting that VES may have selective inhibitory effects on tumor cells that are androgen sensitive. Direct cell-number counting by using a hemocytometer (data not shown) further confirmed these cell growth results.
VES Inhibits the Expression of PSA.
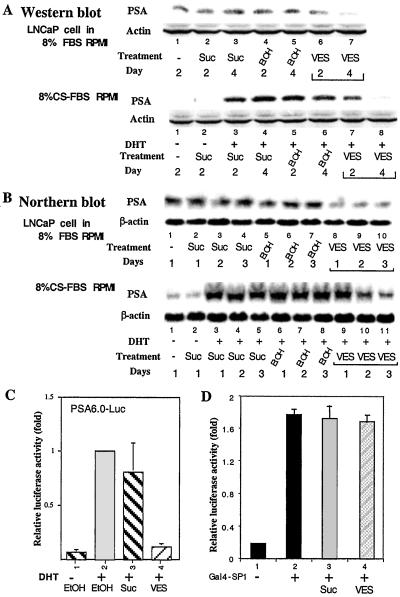
Knowing that VES can inhibit the growth of prostate cancer cells, we were interested in determining whether VES also affects the expression of PSA, a marker used to monitor the progression of prostate cancer (14). As shown in Fig. 2 A and B, using Western blotting and Northern blotting analyses, we found that both mRNA and protein expression of PSA were induced by 5 nM DHT, and the addition of 10 μM VES effectively repressed PSA expression at both the mRNA and protein levels in LNCaP cells cultured under the same conditions as described for Fig. 1 A and B. To further study whether VES-repressed PSA expression occurred at the transcription level, we applied a luciferase reporter linked with the 6.0-kb PSA promoter (PSA6.0-Luc) to assay the VES effect. As shown in Fig. 2C, 5 nM DHT induced PSA6.0-Luc activity, and the addition of 10 μM VES, but not Suc, repressed DHT induced-PSA6.0-Luc activity. To test whether the VES-mediated inhibition of PSA promoter is specific, we examined the effect of VES on the transactivation of SP1 by testing GAL4 DNA-binding domain (DBD) fused SP1, which can bind to and activate GAL4 binding site-linked luciferase reporter, pG5-Luc. Our results indicate that 10 μM VES did not significantly inhibit GAL4-SP1 transcription activity (Fig. 2D). Together, our data show that 10 μM VES not only represses DHT-mediated cell growth, but also selectively represses DHT-induced PSA expression in LNCaP cells.
Figure 2.
VES inhibits the expression of PSA. (A) VES inhibits PSA expression at the protein level. LNCaP cells were cultured in 8% FBS RPMI or 8% CS-FBS RPMI plus 5 nM DHT and treated with ethanol, 10 μM Suc, or 10 μM VES (0.1% vol/vol) for 2 and 4 days. Cells without treatment were harvested on day 2 and used as a control. Western blotting was used to detect the expression of PSA protein. Actin served as an internal control. (B) VES inhibits PSA expression at the mRNA level. LNCaP cells were treated with 10 μM VES, 10 μM Suc, or ethanol (0.1% vol/vol), respectively. Cells were harvested on days 1, 2, and 3 for Northern blotting analysis. β-Actin served as an internal control. (C) VES inhibits the expression of PSA gene at the transcription level. A transient transfection assay was performed in LNCaP cells using the PSA6.0-Luc plasmid with treatment of 10 μM Suc, 10 μM VES, or ethanol (0.1% vol/vol). The histogram represents the level of luciferase activity normalized to simian virus 40 activities and expressed as the fold of the PSA-promoter activity without VES treatment in the presence of DHT. (D) VES has no effect on the transactivation activity of SP1. In COS-1 cells, 1 μg of Gal4-DBD-fused SP1 (Gal4-SP1) was cotransfected with 1 μg of pG5-Luc and 5 ng of SV40RL in the presence or absence of 10 μM VES as indicated. The transfections were performed at least three times and presented as an average ± SD.
VES Affects AR mRNA and Protein Expression.
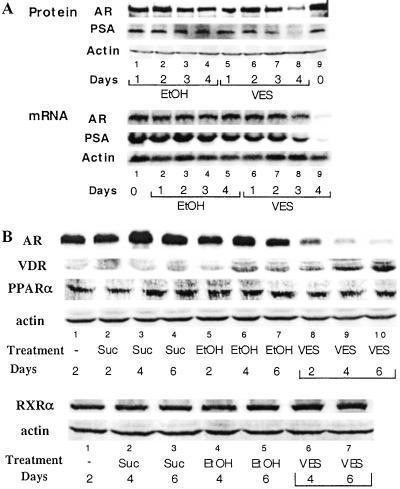
As AR has been demonstrated to play essential roles for the induction of PSA expression (22), we were interested in determining the potential influence of VES on AR functions. As shown in Fig. 3A, Northern blotting data indicate that VES inhibits AR mRNA and protein expression; however, PSA mRNA and protein levels begin to decrease at earlier times.
Figure 3.
VES differentially regulates the protein level of AR, VDR, PPARα, and RXRα. (A) VES down-regulates AR at the transcription and posttranscription level. LNCaP cells were cultured in 8% FBS RPMI and treated with 10 μM VES, or ethanol (0.1% vol/vol). Cells were harvested at different time points. Twenty-five micrograms of RNA and 50 μg of protein collected from the same culture dish were applied for Northern blotting and Western blotting assays, respectively (45). The amount of actin is shown as a control. (B) LNCaP cells were treated with 10 μM Suc, 10 μM VES, or ethanol (0.1% vol/vol). LNCaP cells without treatment were harvested on day 2 and used as a control. Whole-cell lysates were subjected to Western blotting assay using primary antibodies for AR, VDR, PPARα, or RXRα.
As androgen/AR play major roles in the induction of PSA and the down-regulation of AR mRNA is a later event than the down-regulation of PSA activity (Fig. 3), we were interested in knowing whether the VES mediated-suppression of PSA occurs by other mechanisms to influence the androgen/AR function rather than regulating AR protein and mRNA level.
VES Does Not Affect the Ligand-Binding, N-C Dimerization, or Nuclear Translocation of AR.
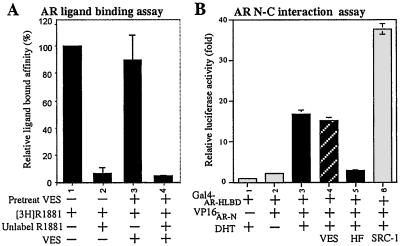
After binding to androgen(s), AR will form a dimer (23), translocate from the cytoplasm to the nucleus (24), and activate its target genes by recognition of androgen-response elements (25). First, we used competition radioligand-binding assay to examine whether VES would affect AR-ligand-binding ability. Results show that unlabeled R1881 can compete for 95% of the specific binding, and VES treatment has little influence on AR ligand binding (Fig. 4A). Next, we examined whether VES affects the N-C interaction of AR, which has been suggested to play an important role in AR transactivation (26). We used a mammalian two-hybrid system, which included the hinge and ligand-binding domain of AR fused with the GAL4-DBD (GAL-ARHLBD), the N terminus of AR fused with VP16 (VP16-ARN), and a pG5-Luc reporter (23). Our results show that 10 nM DHT triggers the AR N-C interaction and addition of 10 μM VES has little influence on the AR N-C interaction (Fig. 4B, lane 3 vs. 4). We also examined whether VES could influence translocation of AR. Although VES has little influence on the AR distribution between cytosol and nucleus, the total AR-staining intensity is reduced, suggesting that VES may affect AR protein expression (data not shown). These immunostaining results not only confirm our earlier Northern and Western blotting assays, but also indicate that VES may function via a posttranscription pathway to down-regulate AR protein function.
Figure 4.
VES cannot affect the ligand binding and N-C dimerization of AR. (A) LNCaP cells cultured in 8% CS-FBS RPMI were treated with 2.5 nM [3H]R1881, with or without 100-fold excess of unlabeled R1881. Cells were harvested and washed, and the radioactivity was measured. [3H]R1881-binding without competition was set at 100%. Data were presented as means ± SD and from the values of at least three independent experiments. (B) AR N-C dimerization. COS-1 cells without endogenous AR were cotransfected with GAL4-DBD-fused AR-HLBD (Gal4-AR-HLBD), VP16-fused AR-N (VP16-AR-N), or pSG5-SRC-1 in the presence or absence of 10 nM DHT and/or 10 μM VES. HF was added as a control to block DHT-mediated AR N-C interaction. SRC-1, a steroid receptor coactivator, was applied as a positive control to enhance N-C interaction (26).
Together, our data suggest that VES cannot influence the ligand-binding, N-C dimerization, and nuclear translocation of AR. Instead, VES reduces the overall AR-staining intensity, suggesting that VES may affect AR expression at the transcriptional or translational level.
VES Inhibits AR Protein Translation in LNCaP Cells.
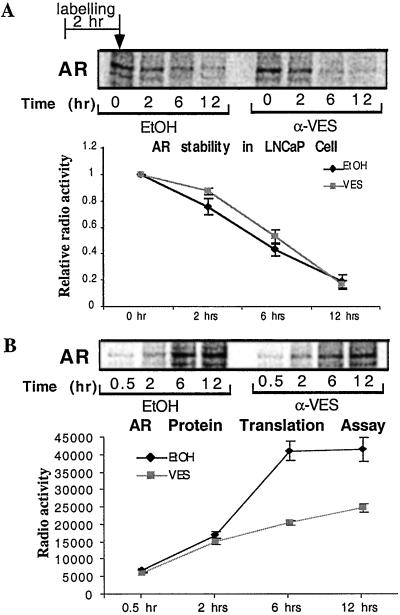
To determine the possible mechanism involved in the regulation of AR expression at the posttranscriptional level, a pulse-chase labeling was applied to characterize whether VES affects AR-protein-translation efficiency or stability (27). Although the intensity of signal is different at the starting point, the degradation rates of the AR are similar in the absence or presence of VES. Our data indicate that VES has little effect on AR-protein stability (Fig. 5A). On the other hand, after treatment with VES, AR-protein synthesis is much slower compared with that of the control group (Fig. 5B). These results suggest that VES may regulate AR protein level through inhibition of protein translation rather than influencing stability.
Figure 5.
VES has no effect on AR protein stability, but reduces AR translation. (A) For the stability assay, after pretreatment with ethanol or 10 μM VES (0.1% vol/vol) for 24 h, LNCaP cells were labeled with [35S]methionine. After 2-h labeling, cells were washed and supplied with fresh medium, and then were harvested at time points of 0, 2, 6, and 12 h. (B) For the AR-translation assay, LNCaP cells were cultured in methionine-free medium for 2 h, then 100 μCi/ml of [35S]methionine was added and remained in the medium until harvesting at 0.5, 2, 6, and 12 h. After cell lysis, 300 μg of total protein was subjected to immunopreciptitation by anti-AR NH27 antibody, resolved on an SDS/8% PAGE gel, and the autoradiographic signal was quantitated by using iqmac software (Molecular Dynamics).
VES Differentially Regulates the Expression of AR, VDR, PPARα, and RXRα.
To test whether the VES-mediated down-regulation of AR function is specific, we examined the expression level of other nuclear receptors under the same conditions. When antibodies for AR, VDR, PPARα, and RXRα were used, our results indicated that 10 μM VES, but not 10 μM Suc, could suppress AR protein level. This VES-mediated AR repression is selective as 10 μM VES showed little effect on the PPARα and RXRα expression (Fig. 3B) and, in contrast, increased the expression of VDR (Fig. 3B).
VES, but Not Selenium, Affects AR and PSA Expression.
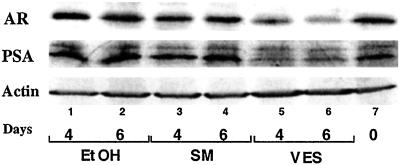
In previous research, selenium has been combined with vitamin E to study their antitumor activity, especially in epidemiological studies (4, 28). Therefore, we were interested in testing whether selenium could function similarly to vitamin E, which inhibits AR expression in LNCaP cells. SM is known to be the major source of selenium in the diet. In the current study, we used 10 μM SM, which has been reported to inhibit LNCaP cell growth (29). Although we observed the SM-mediated growth inhibition in LNCaP cells after 4 days treatment (data not shown), our Western blot data suggest that SM has no effect on AR and PSA expression (Fig. 6). Together, our results suggest that VES, but not selenium, down-regulates the expression of AR and PSA. The VES-mediated growth inhibition of prostate cancer cells may be partly due to down-regulated AR expression, and SM may function through other mechanisms to inhibit the growth of prostate cancer cells.
Figure 6.
SM has no effect on AR and PSA expression. LNCaP cells were cultured in 8% FBS RPMI and treated with 10 μM SM, 10 μM VES, or ethanol (0.1% vol/vol). Protein harvested from cells without treatment on the first day (day 0) was used as a control. Fifty micrograms of whole-cell lysate was subjected to Western blotting assay.
Discussion
VES Differentially Inhibits the Growth of Cancer Cells and Primary Fibroblast Cultures.
The LNCaP cell line is derived from lymph node prostate cancer metastasis (15), and is one of the best in vitro models for human prostate cancer studies, as it represents a hormone-refractory prostate carcinoma, and its growth is responsive to androgen. In addition, LNCaP cells express a functional mutant AR, and produce PSA, which is a sensitive and specific tumor marker for prostate cancer screening and assessment (22, 30–32). Whereas both the wild-type AR and the LNCaP mutant respond to androgen, estrogenic compounds and some androgens bind to the LNCaP mutant AR with higher affinity, and more effectively stimulate AR-transcriptional activity and PSA expression (12, 33). Our growth assay results indicated that VES could effectively inhibit the growth of LNCaP cells. HF, a popular antiandrogen, cannot effectively suppress the growth of LNCaP cells, which is consistent with the results from previous reports. However, the combination of VES and HF more effectively inhibits LNCaP cell growth than VES alone (Fig. 1). This combination may be a potential application for clinical treatment and could warrant further study.
VES Regulates the Expression of AR and PSA in Prostate Cancer.
AR is a critical factor in the development and differentiation of the prostate gland and prostate cancer. In the later stages of prostate cancer, more than 80% of prostate cancer tissues remain positive for AR staining (34). Overall, these observations indicate the importance of the AR in the initiation and progression of prostate cancer. In the present study, our results indicate that VES could effectively down-regulate the protein level of AR, which could be one of the major reasons accounting for VES-mediated growth inhibition in the prostate cancer cells.
Many factors may influence AR function, including interrupting production of the AR at the mRNA or protein levels, ligand binding, dimerization, nuclear translocation, the presence of AR-associated proteins, etc. Our data indicate that VES has a delayed and marginal inhibiting effect on the transcription of AR (Fig. 3), with no obvious effects on ligand binding, nuclear translocation, and the interaction between the N terminus and the C terminus of AR (Fig. 4). VES, however, influences AR function by down-regulating the efficiency of its translation in LNCaP cells (Fig. 5). This could represent a previously unreported mechanism for regulating AR function.
The Antioxidant Effects of Vitamin E and Other Possible Factors for VES-Mediated Functions in Prostate Cells.
One possible mechanism for VES to suppress prostate tumor growth and AR expression may be through its antioxidant effects. Although this is possible, our unpublished data suggest that ascorbic acid, which has antioxidant activity, has little effect on the LNCaP growth and AR-protein expression (S.Y., Y.Z., and J.N., unpublished observation). In addition, VES, an esterified vitamin E analog, has little antioxidant activity. Therefore, it is insufficient to hypothesize that the antioxidant activity is the major factor contributing to VES-mediated suppression of AR and PSA expression. Moreover, the minimal effect VES had on cultured prostate fibroblasts (Fig. 1D) and on other non-androgen-stimulated prostate cancer cell (data not shown) would support the concept that the major inhibition of prostate cancer cell growth by VES may be achieved through its effects on AR expression and function.
Recently, a 46-kDa tocopherol-associated protein (TAP) has been identified from the cytosol of bovine liver (35). In the followup study, a human TAP (hTAP) was isolated, and the recombinant hTAP was capable of binding to vitamin E with a Kd of 460 nM. Since Northern blotting assays indicate that higher levels of hTAP mRNA are found in the liver, brain, and prostate, this molecule may be important for and predictive of functional roles of vitamin E in prostate cancer management (36).
In addition, reports indicated that LNCaP and PC-3 cells were sensitive to VES-induced apoptosis, with 100%, and 60% of cells, respectively, undergoing apoptosis after 3 days of treatment with 20 μM of VES, respectively. However, prostate epithelial cells were resistant to VES-induced apoptosis (37). Whether VES interruption of AR function has any correlation with VES-mediated cell apoptosis, however, remains interesting yet unclear. Further experiments, using microarrays to characterize the downstream biological targets of VES, may help obtain better insight into the functions of VES in prostate cancer cells.
VES, VDR, and Prostate Cancer.
It is well documented that 10 nM 1α,25-dihydroxyvitamin D3 (vitamin D3) can inhibit prostate cancer growth (38). Recently, data from several clinical trials using vitamin D3 to treat prostate cancer patients suggested that vitamin D3 could decrease the PSA levels in patients' sera (39). We found that 10 μM VES could induce VDR expression (Fig. 3B). Whether increased VDR expression contributes to vitamin D3-mediated suppression of prostate cancer growth, however, is not known. Nevertheless, this provides another possible explanation for VES' suppression of prostate cancer cell growth, and the possible molecular mechanisms for VES-regulated VDR expression could be an interesting project for future study.
Vitamin E, Selenium, and Prostate Cancer.
In addition to vitamin E, a growing body of evidence suggests that a higher serum level of dietary supplemental selenium substantially reduces the incidence of lung, colon, or prostate cancer (40, 41). Other studies also reported that the selenium compounds inhibit the growth of prostate cancer cells through the induction of apoptosis. However, it is unclear whether vitamin E and selenium function through similar mechanisms to inhibit LNCaP-cell growth.
Because SM is the major component of the selenium in our daily diet (42), we have applied SM to compare the vitamin E-mediated down-regulation of AR and PSA. Although SM can inhibit LNCaP cell growth, we found no significant effect of SM on AR/PSA protein expression. Therefore, it is likely that SM and vitamin E function via different pathways to inhibit LNCaP cell growth.
Various Compounds Inhibit the AR at Different Transcription or Translation Steps.
AR is a key player in the initiation and progression of prostate cancer. If the combination of different compounds can elicit more profound effects on AR-mediated prostate cancer growth, a greater potential for cancer prevention and chemotherapy is possible.
Our data indicate that, unlike other natural products that also showed an inhibitory effect on AR expression at either the transcription (43) or nuclear translocation level (44), vitamin E could be a natural product that inhibits prostate cancer growth by influencing the translation of AR. This newly discovered mechanism could provide an opportunity for the combination of vitamin E with other natural products to coordinately suppress AR function and prevent prostate tumor progression.
In sum, our results may contribute new knowledge to understand the vitamin E-mediated suppression of prostate tumor growth, which may help to design a better therapeutic treatment for prostate cancer patients.
Acknowledgments
We thank Dr. Chawnshang Chang for helpful discussion and plasmid support, and Karen Wolf, Erik Sampson, and Brenna Simons for assistance in manuscript preparation. This research was partially supported by National Institutes of Health Grant DK60912.
Abbreviations
- AR
androgen receptor
- PSA
prostate-specific antigen
- DHT
5α-dihydrotestosterone
- Suc
succinic acid
- VES
α-tocopheryl succinate (vitamin E succinate)
- HF
hydroxyflutamide
- N-C
N-terminal/C-terminal
- VDR
vitamin D receptor
- PPAR
peroxisome proliferator-activated receptor
- RXR
retinoid X receptor
- Luc
luciferase
- MTT
thiazolyl blue [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide]
- CS-FBS
charcoal-stripped fetal bovine serum
- SM
selenomethionine
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Traber M G, Packer L. Am J Clin Nutr. 1995;62:1501S–1509S. doi: 10.1093/ajcn/62.6.1501S. [DOI] [PubMed] [Google Scholar]
- 2.The Alpha-Tocopherol Beta Carotene Cancer Prevention Study Group. N Engl J Med. 1994;330:1029–1035. [Google Scholar]
- 3.Giovannucci E. J Natl Cancer Inst. 2000;92:1966–1967. doi: 10.1093/jnci/92.24.1966. [DOI] [PubMed] [Google Scholar]
- 4.Helzlsouer K J, Huang H Y, Alberg A J, Hoffman S, Burke A, Norkus E P, Morris J S, Comstock G W. J Natl Cancer Inst. 2000;92:2018–2023. doi: 10.1093/jnci/92.24.2018. [DOI] [PubMed] [Google Scholar]
- 5.Neuzil J, Weber T, Schroder A, Lu M, Ostermann G, Gellert N, Mayne G C, Olejnicka B, Negre-Salvayre A, Sticha M, et al. FASEB J. 2001;15:403–415. doi: 10.1096/fj.00-0251com. [DOI] [PubMed] [Google Scholar]
- 6.Neuzil J, Weber T, Terman A, Weber C, Brunk U T. Redox Rep. 2001;6:143–151. doi: 10.1179/135100001101536247. [DOI] [PubMed] [Google Scholar]
- 7.Israel K, Sanders B G, Kline K. Nutr Cancer. 1995;24:161–169. doi: 10.1080/01635589509514404. [DOI] [PubMed] [Google Scholar]
- 8.Landis S H, Murray T, Bolden S, Wingo P A. CA Cancer J Clin. 1999;49:8–31. doi: 10.3322/canjclin.49.1.8. [DOI] [PubMed] [Google Scholar]
- 9.Sogani P C, Whitmore W F., Jr Cancer Treat Res. 1988;39:131–145. doi: 10.1007/978-1-4613-1731-9_9. [DOI] [PubMed] [Google Scholar]
- 10.Fenton M A, Shuster T D, Fertig A M, Taplin M E, Kolvenbag G, Bubley G J, Balk S P. Clin Cancer Res. 1997;3:1383–1388. [PubMed] [Google Scholar]
- 11.Yeh S, Miyamoto H, Shima H, Chang C. Proc Natl Acad Sci USA. 1998;95:5527–5532. doi: 10.1073/pnas.95.10.5527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grigoryev D N, Long B J, Njar V C, Brodie A H. J Steroid Biochem Mol Biol. 2000;75:1–10. doi: 10.1016/s0960-0760(00)00131-x. [DOI] [PubMed] [Google Scholar]
- 13.Yeh S, Lin H K, Kang H Y, Thin T H, Lin M F, Chang C. Proc Natl Acad Sci USA. 1999;96:5458–5463. doi: 10.1073/pnas.96.10.5458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vernon S E, Williams W D. J Urol. 1983;130:95–98. doi: 10.1016/s0022-5347(17)50977-5. [DOI] [PubMed] [Google Scholar]
- 15.Horoszewicz J S, Leong S S, Kawinski E, Karr J P, Rosenthal H, Chu T M, Mirand E A, Murphy G P. Cancer Res. 1983;43:1809–1818. [PubMed] [Google Scholar]
- 16.Mosmann T. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 17.Sambrook J, Russell D W. Molecular Cloning: A Laboratory Manual. 3rd Ed. Plainview, NY: Cold Spring Harbor Lab. Press; 2001. [Google Scholar]
- 18.Ekman P, Barrack E R, Greene G L, Jensen E V, Walsh P C. J Clin Endocrinol Metab. 1983;57:166–176. doi: 10.1210/jcem-57-1-166. [DOI] [PubMed] [Google Scholar]
- 19.Lin H K, Yeh S, Kang H Y, Chang C. Proc Natl Acad Sci USA. 2001;98:7200–7205. doi: 10.1073/pnas.121173298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang C R, Leskov K, Hosley-Eberlein K, Criswell T, Pink J J, Kinsella T J, Boothman D A. Proc Natl Acad Sci USA. 2000;97:5907–5912. doi: 10.1073/pnas.97.11.5907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Crawford E D, Eisenberger M A, McLeod D G, Spaulding J T, Benson R, Dorr F A, Blumenstein B A, Davis M A, Goodman P J. N Engl J Med. 1989;321:419–424. doi: 10.1056/NEJM198908173210702. [DOI] [PubMed] [Google Scholar]
- 22.Berns E M, de Boer W, Mulder E. Prostate. 1986;9:247–259. doi: 10.1002/pros.2990090305. [DOI] [PubMed] [Google Scholar]
- 23.Ikonen T, Palvimo J J, Janne O A. J Biol Chem. 1997;272:29821–29828. doi: 10.1074/jbc.272.47.29821. [DOI] [PubMed] [Google Scholar]
- 24.Yeh S, Chang C. Proc Natl Acad Sci USA. 1996;93:5517–5521. doi: 10.1073/pnas.93.11.5517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chang C, Saltzman A, Yeh S, Young W, Keller E, Lee H J, Wang C, Mizokami A. Crit Rev Eukaryotic Gene Expression. 1995;5:97–125. doi: 10.1615/critreveukargeneexpr.v5.i2.10. [DOI] [PubMed] [Google Scholar]
- 26.Ting H J, Yeh S, Nishimura K, Chang C. Proc Natl Acad Sci USA. 2002;99:661–666. doi: 10.1073/pnas.022469899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bouteille M. Exp Cell Res. 1971;69:135–147. doi: 10.1016/0014-4827(71)90319-3. [DOI] [PubMed] [Google Scholar]
- 28.Fleshner N E, Kucuk O. Urology. 2001;57:90–94. doi: 10.1016/s0090-4295(00)00949-3. [DOI] [PubMed] [Google Scholar]
- 29.Menter D G, Sabichi A L, Lippman S M. Cancer Epidemiol Biomarkers Prev. 2000;9:1171–1182. [PubMed] [Google Scholar]
- 30.Veldscholte J, Voorhorst-Ogink M M, Bolt-de Vries J, van Rooij H C, Trapman J, Mulder E. Biochim Biophys Acta. 1990;1052:187–194. doi: 10.1016/0167-4889(90)90075-o. [DOI] [PubMed] [Google Scholar]
- 31.Brinkmann A O, Kuiper G G, Ris-Stalpers C, van Rooij H C, Romalo G, Trifiro M, Mulder E, Pinsky L, Schweikert H U, Trapman J. J Steroid Biochem Mol Biol. 1991;40:349–352. doi: 10.1016/0960-0760(91)90201-f. [DOI] [PubMed] [Google Scholar]
- 32.Horoszewicz J S, Kawinski E, Murphy G P. Anticancer Res. 1987;7:927–935. [PubMed] [Google Scholar]
- 33.Yeh S, Miyamoto H, Chang C. Lancet. 1997;349:852–853. doi: 10.1016/S0140-6736(05)61756-4. [DOI] [PubMed] [Google Scholar]
- 34.Sadi M V, Walsh P C, Barrack E R. Cancer. 1991;67:3057–3064. doi: 10.1002/1097-0142(19910615)67:12<3057::aid-cncr2820671221>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 35.Stocker A, Zimmer S, Spycher S E, Azzi A. IUBMB Life. 1999;48:49–55. doi: 10.1080/713803478. [DOI] [PubMed] [Google Scholar]
- 36.Zimmer S, Stocker A, Sarbolouki M N, Spycher S E, Sassoon J, Azzi A. J Biol Chem. 2000;275:25672–25680. doi: 10.1074/jbc.M000851200. [DOI] [PubMed] [Google Scholar]
- 37.Israel K, Yu W, Sanders B G, Kline K. Nutr Cancer. 2000;36:90–100. doi: 10.1207/S15327914NC3601_13. [DOI] [PubMed] [Google Scholar]
- 38.Zhao X Y, Feldman D. Steroids. 2001;66:293–300. doi: 10.1016/s0039-128x(00)00164-1. [DOI] [PubMed] [Google Scholar]
- 39.Beer T M, Hough K M, Garzotto M, Lowe B A, Henner W D. Semin Oncol. 2001;28:49–55. doi: 10.1016/s0093-7754(01)90155-1. [DOI] [PubMed] [Google Scholar]
- 40.Clark L C, Combs G F, Jr, Turnbull B W, Slate E H, Chalker D K, Chow J, Davis L S, Glover R A, Graham G F, Gross E G, et al. J Am Med Assoc. 1996;276:1957–1963. [PubMed] [Google Scholar]
- 41.Clark L C, Cantor K P, Allaway W H. Arch Environ Health. 1991;46:37–42. doi: 10.1080/00039896.1991.9937427. [DOI] [PubMed] [Google Scholar]
- 42.Schrauzer G N. J Am Coll Nutr. 2001;20:1–4. doi: 10.1080/07315724.2001.10719007. [DOI] [PubMed] [Google Scholar]
- 43.Xing N, Chen Y, Mitchell S H, Young C Y. Carcinogenesis. 2001;22:409–414. doi: 10.1093/carcin/22.3.409. [DOI] [PubMed] [Google Scholar]
- 44.Zhu W, Zhang J S, Young C Y. Carcinogenesis. 2001;22:1399–1403. doi: 10.1093/carcin/22.9.1399. [DOI] [PubMed] [Google Scholar]
- 45.Chomczynski P. BioTechniques. 1993;15:532–537. [PubMed] [Google Scholar]