Abstract
Collective migration of epithelial cells drives diverse tissue remodeling processes. In many cases, a free tissue edge polarizes the cells to promote directed motion, but how edge-free or closed epithelia initiate migration remains unclear. Here, we show that the rotational migration of follicular epithelial cells in the Drosophila egg chamber is a self-organizing process. Combining experiments and theoretical modeling, we identify a positive feedback loop in which the mechanosensitive behavior of the atypical cadherin Fat2 synergizes with the rigid-body dynamics of the egg chamber to both initiate and sustain rotation. Mechanical constraints arising from cell–cell interactions and tissue geometry further align this motion around the egg chamber’s anterior–posterior axis. Our findings reveal a biophysical mechanism — combining Fat2-mediated velocity–polarity alignment, rigid-body dynamics, and tissue geometry — by which a closed epithelial tissue self-organizes into persistent, large-scale rotational migration in vivo, expanding current flocking theories.
Introduction
Collective migration of epithelial cells plays central roles in morphogenesis, intestinal turnover, wound repair, and metastasis [1–4]. To migrate collectively, each epithelial cell must establish a protrusive leading edge and a contractile trailing edge. These individual cell migratory behaviors must also be globally oriented across the tissue plane, allowing the entire epithelium to move in a directed manner [5]. When an epithelium has a free edge, as in a wound healing scenario, this physical asymmetry can work alone or with other external cues to polarize the tissue for directed movement [6–8]. There are cases, however, in which epithelial migration occurs in the absence of external cues. Epithelial cells plated on circular micropatterns can spontaneously break chiral symmetry and undergo rotational migration in either a clockwise or counterclockwise direction [9–13]. Cultured epithelial spheres, [14–21], epithelia grown on cylindrical surfaces, [22], and the spherical alveoli of human mammary organoids also rotate persistently [23]. These examples suggest that the ability to self-organize for rotational migration may be a fundamental property of epithelia that adopt distinct geometries [24], but the mechanisms by which rotation initiates are unknown.
Here, we study a rotational migration that occurs in the Drosophila egg chamber. An egg chamber is an ovarian follicle that consists of a central cluster of germ cells, surrounded by an epithelium of follicle cells [25, 26]. Egg chambers form in a stem cell compartment called the germarium and then progress through 14 developmental stages as they develop into an egg (Fig. 1A). During the early stages of egg chamber development, the follicle cells collectively migrate along the basement membrane (BM) extracellular matrix that encapsulates the tissue [27, 28].
Figure 1: Egg chamber rotation begins within the germarium.
A) Illustration of a transverse section through a developmental array of egg chambers (ovariole). The boxed region highlights the organization of the follicular epithelium. B) Illustration of rotational epithelial migration (arrow), which is driven by follicle cell crawling along the stationary basement membrane. C) Illustration of the basal epithelial surface showing how Fat2 acts in trans from the trailing edge of each cell to stabilize WRC activity at the leading edge of the cell behind. D) Illustrations of transverse sections through stage 1 and 2 egg chambers, highlighting the morphological differences between them. E) Movie stills of a transverse section through one egg chamber that capture the transition from stage 1 to 2. Pink dashed line shows the location of the line-scans used to generate the plot in G. Nuclei marked with SpyDNA. F) Movie stills focused on the follicular epithelium of the same egg chamber as in E. Two different cells are pseudocolored in each row of images to show cell movement over 30 minutes at each of the three time points. G) Line-scans of Col IV-GFP through the anterior-most pole of the follicular epithelium for 5 different rotating egg chambers at stage 1. The bimodal distribution indicates that the BM has not yet formed over the egg chamber’s anterior. H) Quantification of follicle cell migration rates for egg chambers at stage 1. Bar represents mean ± SD. I) Movie stills of stage 1 egg chambers with corresponding kymographs that show either constant migration or the onset of migration (arrowhead). Scale bars, 10μm.
This causes the entire egg chamber to rotate within its surrounding matrix, which remains stationary (Fig. 1B) [27]. The rotational motion changes the mechanical properties of the BM in a way that allows it to channel tissue growth along the anterior-posterior (AP) axis and create the elongated shape of the egg [29, 30]. Rotation always occurs around the AP axis, but it can be either clockwise or counterclockwise for a given egg chamber. Once a direction is chosen, however, the egg chamber rotates persistently in that direction for roughly two days until follicle cell migration stops at stage 8.
Egg chamber rotation is powered, in part, by lamellipodial protrusions that extend from the basal surface of each follicle cell [28]. These protrusions depend on the formation of a local branched actin network, under the control of the WASP family verprolin homolog regulatory complex (WRC), and their tissue-level alignment requires the atypical cadherin Fat2 (Fig. 1C) [31–33]. Fat2 localizes to the trailing edge of each follicle cell [34], where it acts in trans to create a stable domain of protrusive activity at the leading edge of the cell behind, and thereby orients all the cells’ protrusions in a common direction [32, 33]. Without Fat2, protrusions are unstable and unpolarized and rotation does not initiate [33].
Although we have learned a great deal about how Fat2 orients protrusions and other cellular features at the basal surfaces of the follicle cells when rotation is occurring at steady state [31–37], we still know very little about the symmetry-breaking mechanism that allows Fat2 to polarize the tissue for the first time and initiate rotation. Indeed, it has been notoriously difficult to capture the onset of rotation with live imaging, and the exact stage at which rotation begins is debated in the field [28, 37]. The mechanisms that ensure rotation always occurs around the egg chamber’s AP axis are similarly unknown.
In this paper we combine experiments with mathematical modeling to explore how Fat2 mediates chiral symmetry breaking in the follicular epithelium, and how the rotational axis is specified. By employing methods for long-term live imaging and for delaying the onset of rotation, we reveal the dynamic behaviors of the follicle cells as rotation initiates and show that symmetry breaking is a self-organized process. We then use our experimental observations as the basis for a theoretical model and find that a Fat2-based mechanochemical feedback loop and rigid-body dynamics of the egg chamber are sufficient to break chiral symmetry in the follicular epithelium and generate sustained rotation. Finally, we propose that mechanical constraints imposed by cell-cell interactions and tissue geometry define the rotational axis and ensure its stability over time. These findings provide a general framework for understanding how epithelial cells self-organize for rotational migration in the context of a complex, developing tissue.
Results
Egg chamber rotation begins within the germarium
To understand how chiral symmetry is broken in the follicular epithelium, it is essential to know when rotation begins under wild-type conditions. Although it is well accepted that rotation initiates shortly after an egg chamber forms, whether it occurs at stage 1, when the egg chamber resides in the germarium and is attached to the precursors of the stalk cells (pre-stalk), or stage 2 when it is separated from the germarium and fully encased in its own BM (Fig. 1D), has been difficult to discern using standard live imaging approaches. To overcome this barrier, we gently embedded young egg chambers in a fibrinogen-thrombin clot [38], which allowed us to perform continuous live imaging ex vivo for up to nine hours, and we visualized the BM using an endogenous GFP tag on the chain of collagen IV (called Viking in Drosophila, Col IV-GFP) [39]. In all 12 samples examined, rotation was already occurring at an average rate of 0.2 μm/min at stage 1, before a BM had formed around the anterior of the egg chamber, and rotation persisted as the egg chamber transitioned to stage 2 (Fig. 1E–H, S1A,B; Movie 1). In 2 of the 12 samples, we even captured the onset of rotation and found that it reaches a constant rate within minutes after motion is first detected, which is then maintained for many hours (Fig. 1I, S1C; Movie 2). Altogether, these data show that the rotational migration of the follicle cells begins when the egg chamber is still in the germarium and that its onset has switch-like dynamics.
Fat2 can mediate symmetry-breaking at multiple developmental stages
We next asked when Fat2 is competent to mediate symmetry-breaking in the follicular epithelium. Fat2’s symmetry-breaking function could be restricted to stage 1 when rotation normally begins, or Fat2 might be capable of mediating symmetry-breaking at multiple developmental stages. Prior work suggested that the former may be true [37]; however, this assertion is at odds with subsequent observations that Fat2 is continuously required to orient cellular protrusions throughout the rotational period [36], and that rotation can initiate at later stages in some mutant conditions [40]. To revisit this question, we delayed the onset of Fat2 expression and asked if it can mediate symmetry-breaking in older egg chambers.
We used two Gal4 drivers to deplete Fat2 with RNAi, traffic jam-Gal4 (tj-Gal4) and 109-30-Gal4, and assessed the level of depletion using an endogenous 3xeGFP tag on Fat2 (Fat2-3xGFP) [32]. tj-Gal4 is expressed in the follicle cells throughout oogenesis [41] (Fig. S1D). Using tj-Gal4 to express fat2-RNAi reduces Fat2 to undetectable levels during the entire migratory period and fully blocks rotation (Fig. 2A–D). By contrast, 109-30-Gal4 is primarily expressed in the follicle cells of the germarium and early egg chambers [42] (Fig. S1D). When 109-30-Gal4 is used to express fat2-RNAi, Fat2 is undetectable through stage 3, begins to be expressed in patches of cells at stage 4 (Fig. S1E), and returns to wild-type levels by stage 7 (Fig. 2A,B). Importantly, the pattern of rotational migration shows a similar temporal progression to that of Fat2 expression, with rotation blocked through stage 3, occurring slowly in some egg chambers at stages 4–5, and reaching normal speed by stage 7 (Fig. 2C,D; Movie 3). Thus, Fat2 can mediate the symmetry-breaking event needed to initiate rotational migration at various developmental stages.
Figure 2: Fat2 can mediate symmetry-breaking at multiple developmental stages.
A) Representative images of Fat2-3xGFP at the basal surface of the follicular epithelium. B) Quantification of Fat2-3xGFP intensity at basal surface cell edges. 109-30>fat2-RNAi depletes Fat2 from the follicular epithelium at stages 2 and 3, but Fat2 levels are equivalent to controls by stage 7. Each data point represents one egg chamber. C) Representative centroid tracks of follicle cell movement over 10 minutes. D) Quantification of follicle cell migration rates over developmental time. 109-30>fat2-RNAi blocks migration at early stages but migration is equivalent to controls by stage 7. Each data point represents one egg chamber. In order on graph, . E) Movie stills of stage 5 egg chambers with corresponding kymographs that show either constant migration or delayed onset of migration (arrowhead). F) Illustration summarizing how 109-30>fat2-RNAi delays the onset of migration. For panels B and D, Two-way ANOVA with Tukey’s multiple comparisons test; ns, p > 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. Bars represent mean ± SD. Scale bars, 10μm.
We next asked if early depletion of other proteins required for symmetry breaking can also delay the onset of rotation. The WRC mediates the formation of cellular protrusions, and is required both to initiate and maintain follicle cell migration [28, 33]. We used 109-30-Gal4 to deplete the WRC component Abelson interacting protein (Abi) by RNAi and found that the onset of migration is delayed to the same extent as when Fat2 is depleted (Fig. S1F). These data show that the ability to initiate migration at a later stage is not exclusive to Fat2 and that it can likely be achieved by delaying the expression of any component of the symmetry-breaking machinery.
Finally, we asked if rotation initiation in these older egg chambers shows the same switch-like behavior seen at stage 1. We embedded ovarioles expressing 109-30>fat2-RNAi in fibrinogen-thrombin clots for long-term live imaging and captured the initiation of rotation in multiple egg chambers that ranged from late stage 4 to early stage 6. Like the natural onset of rotation, this motion quickly reaches a constant rate, which is then maintained, showing that initiation is a rapid process regardless of when it occurs (Fig. 2E; Movie 4).
Altogether, these data show that there is no developmental pre-pattern governing when rotation initiates. Instead, it can occur at multiple stages, which suggests that the symmetry-breaking mechanism that polarizes the epithelium for migration is a self-organized process (Fig. 2F).
Fat2 becomes planar polarized concurrent with the onset of rotation
The ability to delay the onset of rotation highlights the robustness of the symmetry-breaking process. It also provides a powerful method to probe the cellular dynamics that drive the transition into rotation. Because the number of follicle cells increases as the egg chamber develops, we can track the behaviors of more cells in a given experiment. With this new method, we first probed the dynamics of Fat2’s planar polarization to the trailing edge of each follicle cell, as it is the localization of Fat2 that orients all the cells’ protrusions in a common direction [33]. We previously proposed that the motion of the tissue itself localizes Fat2, suggesting that this is a mechanosesitive process [32]. However, this proposal came from experiments in which the epithelium was either rotating at steady state or rotation was fully blocked (Fig. S2A). Delaying the onset of migration now lets us ask if Fat2 becomes localized as rotation begins.
We used either tj-Gal4 or 109-30-Gal4 to express Abi-RNAi and assayed the planar polarization of Fat2-3xGFP by determining the ratio of fluorescence intensity along leading-trailing cell edges versus lateral cell edges (Fig. 3A–C). When the epithelium is migrating, Fat2-3xGFP is enriched at leading-trailing edges at all stages examined. Conversely, blocking migration using tj>Abi-RNAi causes Fat2-3xGFP to localize uniformly around all cell edges [32]. When migration was instead delayed using 109-30>Abi-RNAi, we saw a dynamic temporal pattern of Fat2 localization, with Fat2-3xGFP uniformly localized around cell edges at stages 3–4, weakly polarized to leading-trailing edges at stage 5, and robustly polarized by stage 6 (Fig. 3A–C). Hence, Fat2’s tissue-level polarization is undetectable-to-weak during the time when migration normally initiates in these epithelia and robustly polarized only after migration is well underway. The fibrinogen-thrombin mounting method that allows us to capture the migration initiation does not yet allow high-resolution imaging of the basal surface, so we cannot visualize Fat2 polarization in living tissue. Nonetheless, these data suggest that Fat2 functions within a mechanochemical feedback loop to promote rotation: Fat2 acts at the trailing edge of each cell to align the cells’ protrusions, and the resulting epithelial migration, in turn, localizes Fat2 to each cell’s trailing edge.
Figure 3: Fat2 becomes planar polarized concurrent with the onset of rotation.
A) Representative images of Fat2-3xGFP at the basal surface of the follicular epithelium. B) Quantification of Fat2 polarization to leading-trailing cell-cell interfaces. Each data point represents the ratio of Fat2-3xGFP brightness at leading-trailing (horizontal) versus side (vertical) cell edges in one epithelium. Fat2 goes from unpolarized to polarized in 109-30>Abi-RNAi with roughly the same timing as the onset of rotation in this background. Two-way ANOVA with Tukey’s multiple comparisons test; ns, p > 0.05, *p < 0.05, **p < 0.01, ****p < 0.0001. Bars represent mean ± SD. C) Illustration of non-migrating cells with uniformly distributed Fat2, versus migrating follicle cells with polarized Fat2. Scale bar, 10μm.
Fat2 promotes local motility at the basal surface before rotation begins
We next used our delayed migration assay to visualize the dynamic movements of the follicle cells just before rotation begins and to determine how Fat2 influences these movements. For these experiments, we focused on stage 5, which is a ~5-hr period [43] during which some 109-30>fat2-RNAi epithelia are rotating, and some have not yet initiated rotation (pre-rotation) (Fig. 2D). For each epithelium, we took one 30-minute movie near the apical surface and one at the basal surface (Fig. 4C), and tracked the positions of cell centroids at both locations over time (Movie 5,Movie 6). In rotating 109-30>fat2-RNAi epithelia, the apical and basal centroids of each cell move processively and in concert with one another, as expected. By contrast, in the pre-rotation 109-30>fat2-RNAi epithelia, the apical cell centroids are largely static, while the basal centroids are highly dynamic (Fig. 4A,B; S2B,C; Movie 6). This local movement of the basal surface appears to be generated by the same protrusive activity that will eventually drive the rotational migration, with individual protrusions acting both cell-autonomously and non-autonomously to move the centroids of other cells (Movie 5). Notably, epithelia bearing the null mutation fat2N103-2, which never migrate, show little centroid movement at their basal surfaces (Fig. 4A, S2B). Altogether, these data suggest that local motility at the basal epithelial surface may be a key feature of how rotation initiates and that Fat2 is required for this motility.
Figure 4: Fat2 promotes local motility at the basal surface before rotation begins.
A) Representative images of a 109-30>fat2-RNAi epithelium that is rotating, a 109-30>fat2-RNAi epithelium pre-rotation, and a fat2N103-2 epithelium that will never rotate. Panels on the left show the overlay of the first (green) and last (magenta) frame of a 30-min movie (Movie 6). Panels on the right show cell centroid tracks from the same movie. B) Quantification of cell centroid displacement at apical versus basal surfaces in 109-30>fat2-RNAi epithelia pre-rotation or that are already rotating . Local motility at the basal surface precedes the onset of rotation. Each data point represents the average of cell displacements in one epithelium. Solid lines connect measurements from the same egg chamber. Dotted line at represents the average cell displacements from fat2N103-2 samples (Fig. S2). Paired t-test; ns, p > 0.05, **p < 0.01. C) Diagram denoting apical and basal imaging planes. D) Representative cell centroid tracks taken at the basal surfaces of five epithelia. The polar order parameter increases from left to right. E) Quantification of the polar order parameter for basal surfaces tracks of 109-30>fat2-RNAi epithelia that are either rotating (red, open circles; ) or pre-rotation (red, closed circles; ), compared to fat2N103-2 epithelia (yellow circles; ). Some pre-rotation epithelia have polar order parameters that exceed those of rotating epithelia, showing that the follicle cells can align their basal surface movements before rotation begins. Scale bars, 10μm.
We next investigated whether the local movements of the follicle cells’ basal surfaces become aligned before rotation begins by computing a polar order parameter for each tissue (see methods). A polar order parameter of corresponds to all cell movements being perfectly aligned, while corresponds to cell movements that are isotropic. Pre-rotation 109-30>fat2-RNAi epithelia showed a range of tissue-level order, with some epithelia being largely isotropic, like fat2N103-2 epithelia, and others showing varying degrees of alignment (Fig. 4D,E). Notably, in the 109-30>fat2-RNAi condition, the extent of cell alignment in three pre-rotation epithelia exceeded that of some epithelia that were already rotating (Fig. 4E). We conclude that the follicle cells can align their basal surface movements before rotation begins and further speculate that each 109-30>fat2-RNAi epithelium we analyzed represents a snapshot of the developmental trajectory a given epithelium takes from a disordered to an ordered state as it transitions into rotation.
It has long been known that the apical surfaces of the follicle cells adhere to the germ cells at the center of the egg chamber. This adhesion causes the germ cells to rotate in concert with the follicle cells [27] and likely limits neighbor exchange between the follicle cells over short time scales. In this way, the egg chamber rotates as a rigid body in which the primary motive forces are restricted to the basal epithelial surface where Fat2 is active. Our finding that Fat2-dependent cell motility is restricted to the basal surface before rotation begins suggests that this is also true during the symmetry-breaking process, which provides key insight into the mechanical state of the egg chamber when rotation initiates.
Rigid-body dynamics and the mechanosensitive behavior of Fat2 can initiate rotation
The above results show that the follicle cells can start migrating from an unpolarized state and that rotation initiation likely occurs with rigid-body dynamics. We further hypothesize that Fat2 operates within a mechanochemical feedback loop to promote rotation, in which Fat2 acts at the trailing edge of each cell to align the cells’ protrusions, and the resulting tissue motion localizes Fat2 to the cells’ trailing edges. Here, we develop a minimal theoretical model to elucidate whether these conditions can recapitulate rotation initiation.
We modeled the egg chamber as an ellipsoidal rigid body (Fig. 5A). Crawling forces are generated by lamellipodial protrusions at the basal epithelial surface, which we model as point forces generated by each follicle cell at positions (Fig. 5B). Throughout, we denote vectors and matrices in bold and scalars in regular font. The BM provides the substrate for cell crawling but also generates resistance to motion. We model this resistance as a viscous drag force with viscous coefficients for motion tangential to the follicle cell-BM interface (Fig. 5B). The BM elastically resists motion perpendicular to the egg chamber-BM interface. Intuitively, the egg chamber can be thought of as a rigid ellipsoid elastically confined by a surrounding BM. Any motion distinct from rotation around the long axis generates restoring elastic forces/torques. Given estimates of the stiffness of the BM (≈ 50 kPa) [30, 44] and traction stress generated by other crawling cells in vivo (order-of-magnitude ≈ 10Pa [45]), we infer that this elastic response strongly constrains translational motion. Furthermore, for an ellipsoidal egg chamber (eccentricity ), elastic confinement restricts rotation to the long axis, which reflects what we observe with the delayed migration assay (See Sec. S1.1.1 for details). These observations allow us to reduce the full equation of motion for the egg chamber to a single equation describing rotation around its long axis. In the overdamped limit (negligible inertia), the non-dimensional equation for the rotation of the egg chamber along the long axis (Sec. S1.1) is
| (1) |
where the angular velocity vector is and is the unit vector pointing to the anterior pole along the AP axis (Fig. 5A).
Figure 5: Rigid-body dynamics and the mechanosensitive behavior of Fat2 can initiate rotation.
A) To model the delayed migration assay, the egg chamber is represented as an ellipsoidal rigid body with eccentricity . The dimensions of the egg chamber are rescaled with the radius of the equatorial cross section. B) The crawling force models protrusive activity at the basal epithelial surface and the viscous drag models the interaction between the epithelium and the BM for cell . C) The Fat2 distribution is modeled by where is the angular direction in the tangent space. D) Fat2 dynamics are mechanosensitive, whereby Fat2 is recruited to each cell’s trailing edge with respect to the migration direction . E) The model breaks rotational symmetry, generating either clockwise or counter-clockwise rotation about the AP axis. model trajectories starting from an isotropic initial condition (). F) Representative model trajectory with symmetry broken, resulting in sustained counterclockwise rotations and the corresponding evolution of the cell-averaged Fat2 distribution . The Fat2 distribution evolves from an isotropic configuration to a polar configuration centered around . The time evolution of the quantities in F is given in Movie 7.
Next, we modeled the interplay between the planar polarization of Fat2 and protrusions. We account for the Fat2 distribution as a concentration field , where is the angular coordinate in the tangent plane at (Fig. 5C). Although Fat2 acts in trans to orient protrusions, its function is restricted to the cell-cell interface where it resides [32]. Given this local effect, we simplify the model by representing Fat2’s function as being cell-autonomous. A minimal model of Fat2’s effect on follicle cell protrusions is
| (2) |
where is the ratio of the characteristic time-scale of rotation initiation to the time-scale over which the crawling force changes directions in response to a change in Fat2 distribution. The effect of the Fat2 distribution on crawling force dynamics is encoded through on the tangent plane at , where is a stochastic variable sampled from the distribution proportional to (Sec. S1.2). Eq. (2) models the alignment of the protrusions at the cell edges opposite to those with Fat2 enrichment (Fig. 5C,D).
Last, we modeled the mechanosensitive dynamics of Fat2, in which Fat2 localizes to cell edges opposite to the local cell migration direction (Fig. 5D and Sec. S1.2.1)
| (3) |
where is the characteristic concentration scale of Fat2, is a characteristic rate of Fat2 dynamics, sets the relative strength of the mechanosensitivity compared to saturation dynamics, is the radius of the equatorial cross-section of the egg chamber and is a unit tangent vector along the angular direction at (Sec. S1.2.1).
Consistent with observations of pre-rotation egg chambers in the delayed migration assay, we solve Eqns (1–3) with initially uniform (i.e. symmetric) Fat2 and crawling force distribution (See Sec. S1.5 for details). For parameter selection (ie, ) and sensitivity analysis see Sec. S1.3. Using these initial conditions and parameters, the model breaks symmetry and generates sustained rotations (Movie 7). These rotations occur about the AP axis, with equal probability of clockwise and counterclockwise directions, consistent with experiments (Fig. 5E). The Fat2 distribution and crawling force transition from an isotropic to a polar distribution centered around for clockwise and for counter-clockwise rotations. These observations reflect Fat2 being localized to the cells’ trailing edges and the progressive alignment of cell protrusive activity as rotation initiates. Fig. 5F shows one simulation iteration that undergoes counterclockwise rotational symmetry breaking and the cell-averaged Fat2 distribution , transitioning from an isotropic to a polar distribution centered around . Our results remain robust to large parameter changes (Sec. S1.3).
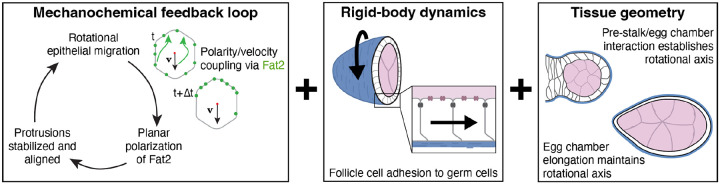
Here, egg chamber geometry sets the rotation axis and the rigid body dynamics provides the global synchronizing mechanical cue for follicle cells, while the mechanosensitive behavior of Fat2 transduces this cue to align cell polarity. The transduction of the global rotational cue into the Fat2 distribution further promotes crawling force in the direction of rotation, thereby forming positive feedback between rotation, Fat2 distribution, and cell crawling force (Fig. 7).
Figure 7: Proposed biophysical mechanism for egg chamber rotation.
Fat2 operates in a mechanochemical feedback loop, in which cell motion polarizes Fat2 to cells’ trailing edges, which in turn stabilizes and aligns individual cell crawling forces. Rigid-body dynamics of the egg chamber synchronize the individual movements of the follicle cells for collective migration. Tissue geometry provides a mechanical cue that ensures rotation around the AP axis. Synergy between the mechanosensitive behavior of Fat2, egg chamber rigid-body dynamics, and egg chamber geometry initiates and maintains the rotational migration of the follicle cells.
Mechanical constraints specify the rotational axis
Wild-type egg chambers always rotate around the AP axis [27]. Notably, this is also true when the onset of rotation is delayed using 109-30>fat2-RNAi. In the above model, elongation of the egg chamber along its AP axis (i.e., egg chamber geometry), combined with elastic confinement from the BM, ensures that rotation occurs around the AP axis. However, egg chambers are thought to be spherical during the earliest developmental stages when rotation normally begins. Simulating the above model with a sphere , the epithelium still breaks symmetry, but rotation is not restricted to any specific axis (See Sec. S1.4). We therefore asked how the rotational axis is specified under wild-type conditions.
Having shown that rotation begins at stage 1, we looked more closely at the shape of the egg chamber within the germarium. Although stage 1 egg chambers are mostly round, the follicle cells at the anterior pole form a flattened interface with the pre-stalk cells that will eventually separate individual egg chambers after they bud from the germarium (Fig. 6A, S2D) [46]. Live imaging of rotating stage 1 egg chambers showed that these anterior-most follicle cells rotate with the rest the tissue and slide past the pre-stalk cells, which remain stationary (Fig. 6B, S2E; Movie 8). This led us to hypothesize that a mechanical interaction between the egg chamber and the pre-stalk cells could constrain the direction of rotation.
Figure 6: Mechanical constraints specify the rotational axis.
A) Illustration of a transverse section through a rotating egg chamber at stage 1, highlighting the shape of the interface between a subset of pre-stalk cells (gold) and the anterior-most follicle cells (magenta) in the germarium. B) Movie stills of maximum intensity projections of nuclei generated from a 2.5-hour movie (Movie 8). Stationary pre-stalk cell nuclei are pseudocolored gold, migrating anterior follicle cell nuclei are pseudocolored magenta. The overlay shows the change in the cells positions at these time points. C) The polar angle quantifies the extent of contact between the pre-stalk cells and the egg chamber. The unit vector is along the pre-stalk axis, which aligns with the egg chamber’s AP axis. D) The motion of the egg chamber tangential (normal) to the prestalk/egg chamber interface generates viscous (elastic) resisting forces. Rotational motion of the egg chamber is described in the inertial (i.e. fixed) frame . The orientation of a vector is represented in spherical coordinates . E) The rotational symmetry-breaking dynamics of a stage 1 egg chamber is described using and average Fat2 distribution . F) The evolution of the orientation of the rotational axis and the AP axis is described using the spherical coordinates in the inertial frame . The time evolution of the quantities (F-G) is given in Movie 9.
To test this hypothesis, we extended our model to account for the mechanical interaction with pre-stalk cells. The areal extent of the egg chamber/pre-stalk interface is quantified by the polar angle (Fig. 6C). The interaction is assumed to be elastic for motion normal to this interface. For motion tangential to the interface, instead, we assume a viscous drag force (Fig. 6D). We use the same Fat2 and protrusion dynamics as in Eq. 2 and Eq. 3. By numerically simulating this extended model (See Sec. S2 for more details), the egg chamber breaks symmetry, generating sustained rotations about the AP axis (Fig. 6E,F; Movie 9). There are three relevant axes here. The stationary pre-stalk axis and two dynamic axes: the AP axis that comoves with the egg chamber, and the egg chamber rotational axis (Fig. 6D). The AP axis is defined by the axis normal to the flattened interface with the pre-stalk cells. The time taken to align the rotational axis with the pre-stalk axis depends on the strength of the elastic interaction with the pre-stalk cells. Stronger elastic interactions induce faster alignment.
Therefore, the mechanochemical feedback loop that breaks symmetry, combined with mechanical interaction with the pre-stalk cells, can generate sustained rotation about the AP axis in stage 1 egg chambers. In this natural condition, the geometry of the pre-stalk/egg chamber interface sets the rotational axis as rotation initiates. At later stages, we envision that the elongated shape of the egg chamber ensures that the rotational axis remains stable over time.
Discussion
This work sheds light on two key aspects of egg chamber rotation in Drosophila - how rotation initiates and how the rotational axis is specified. We show that the follicle cells initiate rotational migration at stage 1, when they are still in the germarium, and that chiral symmetry breaking is a self-organized process. Our data and modeling further suggest that a Fat2-based mechanochemical feedback combined with the rigid-body motion of the egg chamber is sufficient to both initiate rotational migration and make it self-sustaining. Finally, our work suggests that rotation about the AP axis is specified by a tissue-geometry-mediated mechanical interaction between the anterior-most follicle cells and the pre-stalk cells at stage 1, and that the axis is then maintained by the progressive lengthening of the egg chamber over time (Fig. 7). The central features of our proposed mechanism for egg chamber rotation are detailed below.
We propose that Fat2 operates in a mechanochemical feedback loop to initiate and maintain the rotational migration of the follicle cells. By delaying the onset of Fat2 expression, we found that Fat2 can initiate rotation at multiple developmental stages and that initiation can occur ex vivo, showing that chiral symmetry breaking is a self-organized process. Moreover, because follicle cell number [47–49] and egg chamber volume [43] both increase roughly 10-fold over the stages at which we have seen rotation initiate, symmetry-breaking is robust to these factors. We also found that Fat2 becomes planar polarized concurrent with the onset of rotation. This observation, combined with prior data [32] and current modeling, suggests a positive feedback loop in which Fat2 aligns the crawling forces of individual cells and the resulting rotational motion polarizes Fat2 to the cells’ trailing edges. We envision that Fat2 aligns the crawling forces by acting in trans to create a stable domain of protrusive activity at the leading edge of the cell behind [32, 33]. However, Fat2 may also act on other components of the migration machinery [31, 34, 35, 37]. Elucidating how the resulting collective motion, in turn, leads to polarized localization of Fat2 is an important area for future research.
We further propose that the rigid-body dynamics of the egg chamber synchronizes individual follicle cell movements for collective migration. Unlike most epithelial cells, which have free apical surfaces, the apical surfaces of the follicle cells tightly adhere to the germ cells [27]. This adhesion rigidifies the tissue and limits neighbor exchange on short time scales. Consistent with this mechanical constraint, we found that cell motility is restricted to the basal epithelial surface before rotation begins and that this local motility depends on Fat2, likely through its effect on cell protrusive activity [33]. For rotation to initiate, the movements of the individual cells must become aligned in a global migration direction, which we also observed. Our model posits that rigid-body dynamics provides this global synchronizing cue. Indeed, combining the rigid-body assumption with the mechanosensitive behavior of Fat2 described above (polarity-velocity coupling) [11, 50] is sufficient to break symmetry and generate sustained egg chamber rotations. Exploiting rigid-body dynamics, our model yields closed-form parametric expressions that relate tissue geometry (e.g., eccentricity, pre-stalk interface size and shape) to mechanical outputs, offering insight into how geometry influences tissue dynamics. Overall, our biophysical mechanism extends prior models of symmetry breaking proposed for rotating epithelial spheroids, based on cell flocking behaviors through Viscek-like polarity alignment [17, 18] or cell substrate curvature [22]. Beyond the egg chamber, we anticipate that the same principles can drive chiral symmetry breaking even when the rigid-body assumption is relaxed to allow elastic deformations of the rotating tissue—a generalization we will pursue in future work.
Another major finding of this work is that tissue-geometry-mediated mechanical constraints may ensure that rotation occurs around the AP axis. It has been proposed that two pairs of specialized follicle cells (polar cells) define the rotational axis [51], as they are located at the anterior and posterior poles of the egg chamber. However, the polar cells have not yet differentiated when rotation initiates at stage 1 [52]. Instead, the anterior-most follicle cells directly contact the pre-stalk cells and can remodel their contacts along this flattened interface as rotation initiates. Our model suggests that an elastic interaction between these two cell types is sufficient to specify the rotational axis at this stage. Delaying the onset of migration revealed that rotation also occurs around the AP axis when it initiates at stages 4–6. Here, slight elongation of the egg chamber, combined with elastic confinement from the BM can specify the rotational axis (Sec. S1.1.1). At these stages, polar cells would be required because they control the early, rotation-independent phase of tissue elongation [53]. We speculate that progressive lengthening of the tissue maintains the rotational axis in wild-type egg chambers, ensuring its stability over roughly two days when this motion occurs in vivo.
Importantly, three features of our proposed mechanism for egg chamber rotation require interactions between the migrating follicle cells and other non-migratory cell types – the germ cells, the pre-stalk cells and the polar cells. This contrasts with many in vitro systems of rotational epithelial migration that focus on uniform monolayers of migrating cells [10, 14–17, 22]. One notable exception is human mammary organoids, which have rotating spherical acini attached to a central system of ducts [23]. Unlike isolated epithelial spheres that do not maintain a coherent rotational axis, these acini rotate persistently around the ducts in a manner that is remarkably like that of a stage 1 egg chamber rotating around the pre-stalk cells. Thus, interactions with non-migratory cell types may provide a general mechanism to constrain rotational epithelial migrations, even though rotational migration, itself, is a spontaneous and self-organized process.
Methods
Fly genetics, sources and care
Drosophila melanogaster were fed cornmeal molasses agar food and raised at 25°C. Experimental females were collected 0–3 days post-eclosion and then aged in the presence of males with yeast before dissection. In all RNAi experiments using 109-30-Gal4 or tj-Gal4, experimental and control females were kept at 29°C for 48 hours prior to dissection, otherwise experimental females were kept at 25°C. The genotypes used in each experiment are listed in Supplementary Table 2 and indexed by figure panel.
Sample preparation
Egg chamber dissection
Ovaries were dissected into live imaging media (Schneider’s Drosophila medium containing 1X Penicillin-Streptomycin + 15% fetal bovine serum + 200μg/mL insulin, [54]) in one well of a glass spot plate using forceps. Ovarioles were carefully removed from the muscle sheath to not damage the stages of egg chambers being analyzed. For standard live imaging, any egg chambers older than the most mature egg chamber to be analyzed were trimmed from the ovariole and discarded. This was done by slicing through the stalk connecting the adjacent egg chambers with a 27-gauge needle. For extended live imaging, any egg chambers older than stage 9 were removed in the same manner. For a detailed protocol and movies of the dissection process see Cetera et al., 2016 [55].
Sample preparation for standard live imaging
Following dissection and trimming of the ovarioles, the tissue was transferred to another well in the spot plate containing live imaging media with CellMask (1:500) for 8 minutes and then washed with fresh live imaging media to remove excess dye. Stained ovarioles were transferred in 16μl of media to a slide, and glass spacer beads were added to the drop of media to limit egg chamber compression by the coverslip. The size of the beads varied based on the egg chamber stage of interest (51/53μm for stage 6–8, 40μm for stage 5–6, 30μm for stage 4–5, 25μm for stage 3–4, 15μm for germaria-stage 2). A coverslip was then placed on top of the ovarioles and beads and sealed with petroleum jelly to prevent evaporation. Imaging was performed with this setup for up to 1 hour.
Sample preparation for extended/overnight live imaging
Here, we adapted a method based on Wilcockson and Ashe, 2021 [38] to perform extended or overnight live imaging of egg chamber rotation. Following dissection and trimming of the ovarioles, the tissue was transferred to another well of the spot plate containing live imaging media with 1x SpyDNA for 15 minutes. During this 15-minute incubation, 200μl of prewarmed (37°C) live imaging media without insulin was added to an Eppendorf tube containing 0.002 g of fibrinogen (final concentration 10 mg/ml), vortexed briefly, then placed back at 37°C for the remainder of the staining period. Of note, increasing the volume of fibrinogen/media mixture or over-vortexing often resulted in premature clotting. After staining, the fibrinogen mixture was transferred to an adjacent well in the spot plate. Ovarioles were transferred to the fibrinogen mixture, pipetting slowly up and down to ensure complete incorporation, then transferred in 10μl to an imaging dish (MatTek, 35mm petri dish, 14mm microwell). Thrombin (10U/μl) was added to the drop of fibrinogen in two 0.5μl increments (1.0μl total), and left for 10 minutes. If the clot had not formed after 10 minutes another 0.5μl of thrombin was added. The clot was determined to be sufficiently formed if an eyelash could not break the surface tension of the clot-droplet, or polymerization was visible. In some, but not all samples, a Millicell culture insert (Sigma, 12 mm diameter, 8μm membrane pore size) was placed on top of the clot to prevent Z-drift but this was not a necessary step. The clot was then covered in 0.3 ml of live imaging media with insulin, a wet Kimwipe was added to the side of the dish to maintain humidity and the lid was placed on the dish. The z-stack acquisition feature was used to capture at least half the volume of egg chambers imaged. Tissues were imaged overnight for up to 13 hours; however, egg chamber rotation often slowed after 8–9 hours of imaging.
F-actin staining and immunostaining of fixed tissues
All fixed tissues were stained for F-actin (phalloidin) to facilitate the staging of egg chambers and N cadherin (anti-DN cadherin) to facilitate the segmentation of cells in the follicular epithelium for quantitative analyses. Dissected ovarioles were fixed in a 4% EM-grade formaldehyde in phosphate buffered saline (PBS) plus 0.1% Triton X-100 (PBT) for 15 minutes and then washed 3×5 minutes in PBT. Egg chambers were incubated with primary antibodies (anti-DN cadherin, 1:200) in PBT either overnight at 4°C or for 2 hours at room temperature while rocking, then washed 3×5 minutes in PBT. Ovarioles were then incubated in secondary antibodies (1:200) with the addition of either TRITC phalloidin (1:200) or Alexa Fluor 647 phalloidin (1:100) overnight at 4°C or 2 hours at room temperature while rocking. Ovarioles were then washed 3×5 minutes in PBT and mounted in 30μL SlowFade Diamond antifade on a slide using a 22×50 mm # 1.5 coverslip, sealed with nail polish and stored at 4°C until imaged.
Microscopy
All live and fixed imaging was performed at room temperature using laser scanning confocal microscopy. Extended live imaging was performed using a Zeiss LSM 880 inverted microscope running Zen Black software with a 40x Plan-APOCHROMAT 1.3NA oil immersion objective (Fig. 1, 2E, 6A,B). All other images and movies were collected using a Zeiss LSM 800 upright microscope running Zen Blue software with a 63x Plan-APOCHROMAT 1.4NA oil immersion objective. All images were collected such that the egg chambers’ AP axes aligned with the horizontal (x) image axis, except in cases where this alignment occurred post-imaging in Fiji as described below.
Analysis of live imaging data
Kymograph generation
To produce the kymographs shown in Fig. 1I, 2E, and S1C movies were aligned in Fiji (imageJ) so that the egg chambers’ AP axis corresponds with the horizontal (x) image axis. A maximum-intensity projection was generated to capture the height of follicular epithelium for the whole duration of the timelapse. In Fiji, the straight line tool (1 pixel) was used to draw a vertical line and the KymoResliceWide plugin was used to create the final kymograph.
Line-scans of Col IV-GFP
Timelapse movies of a central optical plane through stage 1 egg chambers were rotated in Fiji so that the AP axes corresponded with the horizontal (x) image axis. In Fiji, straight lines of 0.28μm (1 pixel) thickness were drawn vertically through the anterior-most pole of the egg chamber where the anterior BM will eventually form as determined from the movie. The average intensities of Col IV-GFP were measured at the beginning of the timelapse using the Plot Profile function in Fiji.
Cell segmentation and centroid tracking of live tissues
In brief, timelapse movies of CellMask stained epithelia were segmented in each frame using the pretrained ‘cytoplasm’ model in Cellpose [56] and tracked by linking regions of high overlap in consecutive frames. Napari tools were used to manually correct any cell segmentation and tracking errors. To account for XY drift, Python scikit-image and scipy libraries were used to generate a mask of the tissue. Tissue masks were centered in each frame based on their centroid position, then movies were aligned to centered tissue masks. Scikit-image regionprop was then used to determine the individual cell centroid positions at each frame of the movie. See Williams et al., 2022 [33] and Williams and Horne-Badovinac, 2023 [36] for more detailed protocols. See also ‘Code’ section below.
Migration rate data collection and analysis
For quantification of follicle cell migration rates as shown in Fig. 2D and S1F, ovarioles were stained with Orange or Deep Red CellMask (1:500) and mounted for standard live imaging. Timelapse movies were collected at a single Z plane just below the apical surface of the epithelium every 30 seconds for 10 minutes. The multiple position acquisition feature was used to image many ovarioles in a single imaging session. Cells were segmented and tracked, and tissues were aligned to center, as described above. For stage 6–7 egg chambers, migration rate was calculated based on cell speeds at the central region of the egg chamber between the two poles, to account for variation across the AP axis. For stage 5 and under, all cells were used in the analysis. For younger egg chambers (stage 2–3), only egg chambers in which at least 3 cells could be tracked were included in the analysis. Migration rates were determined from centroid displacement [36]. See also ‘Code’ section below. To quantify migration rates at stage 1 (Fig. 1), we generated kymographs from timelapse movies by drawing single lines through the epithelium in the direction of migration. The migration rate was determined by measuring the slope of 3 kymograph lines and averaging the values. See Barlan et al., 2017 [32] for in-depth methods.
Analysis of relative cell movement at basal versus apical surfaces
For each egg chamber, a 30-minute timelapse movie (15 second intervals) was acquired at the basal surface of the epithelium. After 30 minutes, the z-plane was shifted apically and another 30-minute timelapse movie was collected. Cells were segmented and tracked, and centroid positions were obtained as described above. For analysis, cells present for less than 10 consecutive frames were discarded. First, we determined the difference between the first and last position of each cell centroid in all tissues (Fig. S2B). To compare cell movement at basal versus apical surfaces in one egg chamber, we calculated the average cell displacement by finding the total of all cell displacements at each z-plane divided by the number of cells analyzed in that plane (Fig. 4B, S2B).
Quantifying polar order from cell centroid tracks
For each egg chamber, a 30-minute timelapse movie was acquired at the basal surface of the epithelium at 15 second intervals. Cells were segmented and tracked, and centroid positions were obtained as described above. Cells present for less than 10 consecutive frames and cells at the boundary of the tissue were not analyzed. The position of cell centroids were defined as for each cell at time , where is computed every minute for 30 minutes. We quantify the centroid movement for cell at time as . To quantify the coordination between the centroid movements of all cells in the field of view (FOV) over a span of 30 minutes, we compute a polar order parameter for an egg chamber. We calculate from by
| (4) |
where is the total number of time points and is the total number of follicle cell centroids in the FOV at both and . For perfectly ordered centroid movements where all the cells align in a single direction . For random isotropic centroid movements . See also the ‘Code Availability’ section below.
Analysis of fixed tissue data
Fat2-3xGFP fluorescence intensity quantification
To measure Fat2 brightness at the basal cell edges (Fig. 2B), a single optical section was captured and the Fiji oval selection tool was used to measure the mean intensity of Fat2-3xGFP at the whole basal surface and three cell interiors at the basal surface of each sample. To control for variation between samples, the average of the basal surface cell interiors intensity measurement was subtracted from the total basal surface intensity measurement.
Fat2 enrichment at leading-trailing cell edges
As a measure of Fat2 planar polarity at the basal surface, we determined the ratio of Fat2-3xGFP fluorescence intensity at horizontal (migratory leading/trailing) to vertical (lateral) cell edges. To do this, cells were segmented based on a cell membrane marker (anti-DN cadherin) using the pretrained ‘cytoplasm’ model in Cellpose [56]. Next, we segmented the cell-cell edges which are the cellular interfaces between each pair of neighboring cells. Then we measured the angle of each cell-cell edge relative to the egg chamber’s AP axis and Fat2 fluorescence intensity along those edges. Finally, we calculated the average fluorescence intensities of edges with angles between 0° and 10° (migratory leading/trailing) and between 80° and 90° (lateral) and determined the ratio between these values. A detained description of cell-cell edge segmentation can be found in Williams et al., 2022 [33]. See also ‘Code’ section below.
Reproducibility and statistical analysis
Egg chambers damaged during sample preparation were excluded from analysis. All experiments were performed at least twice. Each replicate included egg chambers pooled from multiple females. Experiments were not randomized, nor was the data analysis performed blind. No statistical method was used to determine sample size. The number of biological replicates (n), statistical tests, and significance can be found in figures or figure legends. All statistical tests were performed in GraphPad Prism10. All data in which statistical tests were performed was tested for normality, and found to follow an approximate normal distribution. Paired t-tests were used for comparison of relative cell movements at basal versus apical surfaces within epithelia (Fig. 4B). A two-way ANOVA with Tukey’s multiple comparisons test was performed on datasets measuring migration rates or Fat2 planar polarity at multiple stages for multiple conditions (Fig. 2B,D, Fig. 3B, S1F). An ordinary one-way ANOVA was performed when two or more conditions were compared within one stage (Fig. S2B).
Movie generation
Fiji was used to add labels and annotations and export timelapse videos as .avi, which were then converted to .mp4 using HandBrake. Simulations were generated using MATLAB R2023a.
Supplementary Material
Acknowledgements
We are grateful to the members of the Horne-Badovinac and Serra labs and to Noah Mitchell for helpful discussions and comments on the manuscript, and to the Bloomington Drosophila Stock Center and FlyBase for the essential reagents and resources they provided for this work. M.S. and S.H.B. further thank The Company of Biologists and the organizers of the 2022 workshop ‘From Physics to Function’ (Buxted Park, UK), where this project was initiated.
Funding
This work was supported by NIH T32HD055164 (Schwabach and Williams), NIH T32 GM007183 (Cetera) – NSF PHY-2413073, NSF CAREER PHY-2443851 and NIGMS/NIH R35GM156889 (Serra) and NIH R01GM126047 and NIH R35GM148485 (Horne-Badovinac).
Funding Statement
This work was supported by NIH T32HD055164 (Schwabach and Williams), NIH T32 GM007183 (Cetera) – NSF PHY-2413073, NSF CAREER PHY-2443851 and NIGMS/NIH R35GM156889 (Serra) and NIH R01GM126047 and NIH R35GM148485 (Horne-Badovinac).
Footnotes
Competing Interests
The authors declare no competing interests.
Code Availability
The code used for the experimental analysis and model simulation is available in https://github.com/SreejithSanthosh/egg-chamber-rotation.
References
- 1.Cheung K. J. & Horne-Badovinac S. Collective migration modes in development, tissue repair and cancer. Nature Reviews Molecular Cell Biology, 1–18 (2025). [Google Scholar]
- 2.Theveneau E. & Mayor R. Collective cell migration of epithelial and mesenchymal cells. Cellular and Molecular Life Sciences 70, 3481–3492 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shaw T. J. & Martin P. Wound repair: a showcase for cell plasticity and migration. Current Opinion in Cell Biology 42, 29–37 (2016). [DOI] [PubMed] [Google Scholar]
- 4.Krndija D. et al. Active cell migration is critical for steady-state epithelial turnover in the gut. Science 365, 705–710 (2019). [DOI] [PubMed] [Google Scholar]
- 5.Mayor R. & Etienne-Manneville S. The front and rear of collective cell migration. Nature Reviews Molecular Cell Biology 17, 97–109 (2016). [DOI] [PubMed] [Google Scholar]
- 6.Poujade M. et al. Collective migration of an epithelial monolayer in response to a model wound. Proceedings of the National Academy of Sciences 104, 15988–15993 (2007). [Google Scholar]
- 7.Jain S., Ladoux B. & M`ege R.-M. Mechanical plasticity in collective cell migration. Current Opinion in Cell Biology 72, 54–62 (2021). [DOI] [PubMed] [Google Scholar]
- 8.SenGupta S., Parent C. A. & Bear J. E. The principles of directed cell migration. Nature Reviews Molecular Cell Biology 22, 529–547 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang S., Brangwynne C., Parker K. & Ingber D. Symmetry-breaking in mammalian cell cohort migration during tissue pattern formation: Role of random-walk persistence. Cell Motility 61, 201–213 (2005). [Google Scholar]
- 10.Doxzen K. et al. Guidance of collective cell migration by substrate geometry. Integrative Biology 5, 1026–1035 (2013). [DOI] [PubMed] [Google Scholar]
- 11.Jain S. et al. The role of single-cell mechanical behaviour and polarity in driving collective cell migration. Nature Physics 16, 802–809 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lo Vecchio S., Pertz O., Szopos M., Navoret L. & Riveline D. Spontaneous rotations in epithelia as an interplay between cell polarity and boundaries. Nature Physics 20, 322–331 (2024). [Google Scholar]
- 13.Segerer F. J., Thüroff F., Piera Alberola A., Frey E. & Rädler J. O. Emergence and Persistence of Collective Cell Migration on Small Circular Micropatterns. Physical Review Letters 114, 228102 (2015). [DOI] [PubMed] [Google Scholar]
- 14.Tanner K., Mori H., Mroue R., Bruni-Cardoso A. & Bissell M. J. Coherent angular motion in the establishment of multicellular architecture of glandular tissues. Proceedings of the National Academy of Sciences 109, 1973–1978 (2012). [Google Scholar]
- 15.Wang H., Lacoche S., Huang L., Xue B. & Muthuswamy S. K. Rotational motion during three-dimensional morphogenesis of mammary epithelial acini relates to laminin matrix assembly. Proceedings of the National Academy of Sciences 110, 163–168 (2013). [Google Scholar]
- 16.Chin A. S. et al. Epithelial Cell Chirality Revealed by Three-Dimensional Spontaneous Rotation. Proceedings of the National Academy of Sciences 115, 12188–12193 (2018). [Google Scholar]
- 17.Tan T. H. et al. Emergent chirality in active solid rotation of pancreas spheres. PRX Life 2, 033006 (2024). [Google Scholar]
- 18.Brandstätter T. et al. Curvature induces active velocity waves in rotating spherical tissues. Nature Communications 14, 1643 (2023). [Google Scholar]
- 19.Xie G., Wang Y. J., Thirumalai D., Li X. & Irianto J. Rotational migration in human pancreatic ductal organoids depends on actin and myosin activity. [Preprint] bioRxiv 2024.10.01.616188 (2024). [Google Scholar]
- 20.Ender P. et al. Spatiotemporal control of ERK pulse frequency coordinates fate decisions during mammary acinar morphogenesis. Developmental Cell 57, 2153–2167.e6 (2022). [DOI] [PubMed] [Google Scholar]
- 21.Hirata E., Ichikawa T., Horike S. i. & Kiyokawa E. Active K-RAS induces the coherent rotation of epithelial cells: A model for collective cell invasion in vitro. Cancer Science 109, 4045–4055 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Glentis A. et al. The emergence of spontaneous coordinated epithelial rotation on cylindrical curved surfaces. Science Advances 8, eabn5406 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fernández P. A. et al. Surface-tension-induced budding drives alveologenesis in human mammary gland organoids. Nature Physics 17, 1130–1136 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rørth P. Fellow travellers: emergent properties of collective cell migration. EMBO Reports 13, 984–991 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Horne-Badovinac S. & Bilder D. Mass transit: epithelial morphogenesis in the Drosophila egg chamber. Developmental Dynamics 232, 559–574 (2005). [DOI] [PubMed] [Google Scholar]
- 26.Berg C., Sieber M. & Sun J. Finishing the egg. Genetics 226, iyad183 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haigo S. L. & Bilder D. Global tissue revolutions in a morphogenetic movement controlling elongation. Science 331, 1071–1074 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cetera M. et al. Epithelial rotation promotes the global alignment of contractile actin bundles during Drosophila egg chamber elongation. Nature Communications 5, 5511 (2014). [Google Scholar]
- 29.Isabella A. J. & Horne-Badovinac S. Rab10-Mediated Secretion Synergizes with Tissue Movement to Build a Polarized Basement Membrane Architecture for Organ Morphogenesis. Developmental Cell 38, 47–60 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Crest J., Diz-Muñoz A., Chen D.-Y., Fletcher D. A. & Bilder D. Organ sculpting by patterned extracellular matrix stiffness. eLife 6, e24958 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Squarr A. J. et al. Fat2 acts through the WAVE regulatory complex to drive collective cell migration during tissue rotation. Journal of Cell Biology 212, 591–603 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barlan K., Cetera M. & Horne-Badovinac S. Fat2 and Lar Define a Basally Localized Planar Signaling System Controlling Collective Cell Migration. Developmental Cell 40, 467–477.e5 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Williams A. M., Donoughe S., Munro E. & Horne-Badovinac S. Fat2 polarizes the WAVE complex in trans to align cell protrusions for collective migration. eLife 11, e78343 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Viktorinová I. & Dahmann C. Microtubule polarity predicts direction of egg chamber rotation in Drosophila. Current Biology 23, 1472–1477 (2013). [DOI] [PubMed] [Google Scholar]
- 35.Viktorinová I., Henry I. & Tomancak P. Epithelial rotation is preceded by planar symmetry breaking of actomyosin and protects epithelial tissue from cell deformations. PLoS Genetics 13, e1007107 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Williams A. M. & Horne-Badovinac S. Fat2 polarizes Lar and Sema5c to coordinate the motility of collectively migrating epithelial cells. Journal of Cell Science 137, jcs261173 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen D.-Y., Lipari K. R., Dehghan Y., Streichan S. J. & Bilder D. Symmetry Breaking in an Edgeless Epithelium by Fat2-Regulated Microtubule Polarity. Cell Reports 15, 1125–1133 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wilcockson S. G. & Ashe H. L. Live imaging of the Drosophila ovarian germline stem cell niche. STAR Protocols 2, 100371. [Google Scholar]
- 39.Buszczak M. et al. The Carnegie Protein Trap Library: A Versatile Tool for Drosophila Developmental Studies. Genetics 175, 1505–1531 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stedden C. G. et al. Planar-Polarized Semaphorin-5c and Plexin A Promote the Collective Migration of Epithelial Cells in Drosophila. Current Biology 29, 908–920.e6 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li M. A., Alls J. D., Avancini R. M., Koo K. & Godt D. The large Maf factor Traffic Jam controls gonad morphogenesis in Drosophila. Nature Cell Biology 5, 994–1000 (2003). [DOI] [PubMed] [Google Scholar]
- 42.Hartman T. R. et al. Drosophila Boi limits Hedgehog levels to suppress follicle stem cell proliferation. Journal of Cell Biology 191, 943–952 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Spradling A. C. in The Development of Drosophila melanogaster (eds Bate M. & Martinez Arias A.) Volume I, Chapter 1 (Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, 1993). [Google Scholar]
- 44.Töpfer U., Guerra Santillán K. Y., Fischer-Friedrich E. & Dahmann C. Distinct contributions of ECM proteins to basement membrane mechanical properties in Drosophila. Development 149, dev200456 (2022). [DOI] [PubMed] [Google Scholar]
- 45.Yamaguchi N. et al. Rear traction forces drive adherent tissue migration in vivo. Nature Cell Biology 24, 194–204 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Assa-Kunik E., Torres I. L., Schejter E. D., Johnston D. S. & Shilo B.-Z. Drosophila follicle cells are patterned by multiple levels of Notch signaling and antagonism between the Notch and JAK/STAT pathways. Development 134, 1161–1169 (2007). [DOI] [PubMed] [Google Scholar]
- 47.Fasano L. & Kerridge S. Monitoring positional information during oogenesis in adult Drosophila. Development 104, 245–253 (1988). [DOI] [PubMed] [Google Scholar]
- 48.Morris L. X. & Spradling A. C. Long-term live imaging provides new insight into stem cell regulation and germline-soma coordination in the Drosophila ovary. Development 138, 2207–2215 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen D.-Y., Crest J., Streichan S. J. & Bilder D. Extracellular matrix stiffness cues junctional remodeling for 3D tissue elongation. Nature Communications 10, 3339 (2019). [Google Scholar]
- 50.Alert R. & Trepat X. Physical models of collective cell migration. Annual Review of Condensed Matter Physics 11, 77–101 (2020). [Google Scholar]
- 51.Díaz de la Loza M. C. et al. Laminin Levels Regulate Tissue Migration and Anterior-Posterior Polarity during Egg Morphogenesis in Drosophila. Cell Reports 20, 211–223 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Besse F. & Pret A.-M. Apoptosis-mediated cell death within the ovarian polar cell lineage of Drosophila melanogaster. Development 130, 1017–1027 (2003). [DOI] [PubMed] [Google Scholar]
- 53.Alégot H., Pouchin P., Bardot O. & Mirouse V. Jak-Stat pathway induces Drosophila follicle elongation by a gradient of apical contractility. eLife 7, e32943 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Prasad M., Jang A. C.-C., Starz-Gaiano M., Melani M. & Montell D. J. A protocol for culturing Drosophila melanogaster stage 9 egg chambers for live imaging. Nature Protocols 2, 2467–2473 (2007). [DOI] [PubMed] [Google Scholar]
- 55.Cetera M., Lewellyn L. & Horne-Badovinac S. Cultivation and Live Imaging of Drosophila Ovaries. Methods in Molecular Biology 1478, 215–226 (2016). [DOI] [PubMed] [Google Scholar]
- 56.Stringer C., Wang T., Michaelos M. & Pachitariu M. Cellpose: a generalist algorithm for cellular segmentation. Nature Methods 18, 100–106 (2021). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The code used for the experimental analysis and model simulation is available in https://github.com/SreejithSanthosh/egg-chamber-rotation.