Abstract
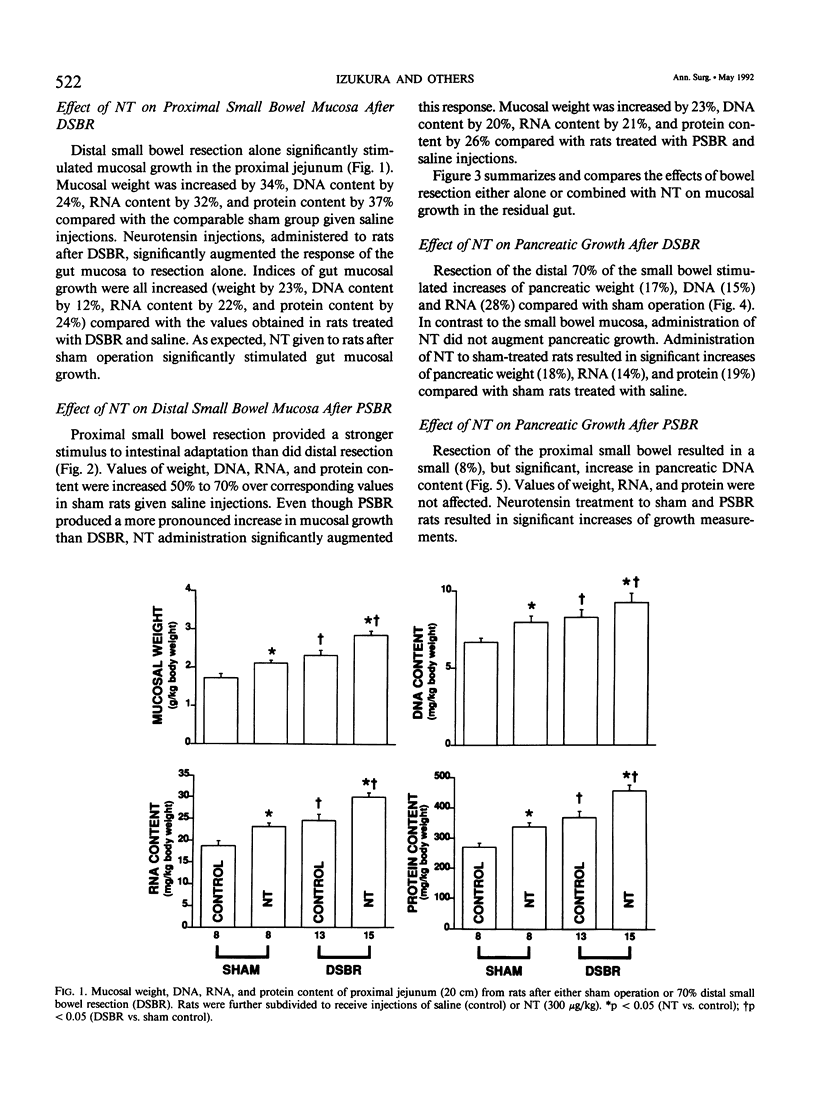
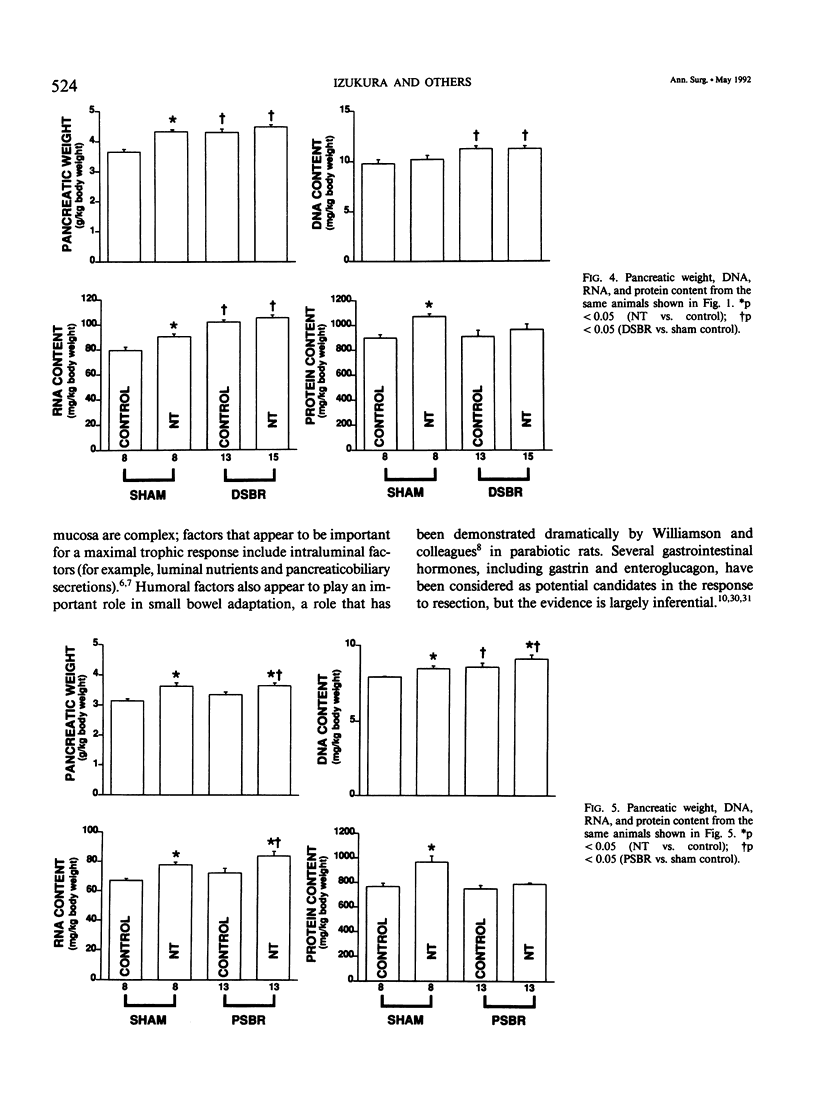
Massive small bowel resection (SBR) is characterized by increased proliferation of residual gut mucosa and pancreas. Neurotensin (NT), a gut tridecapeptide, stimulates growth of normal gut mucosa and pancreas. This study examined whether NT affected growth of the small intestine and the pancreas after either distal or proximal SBR. Male Fischer 344 rats were divided into four groups. Group 1 underwent ileal transection with reanastomosis (SHAM) and group 2 underwent 70% distal SBR. Group 3 underwent SHAM operation (jejunal transection), and group 4 underwent 70% proximal SBR. After operation, each group was further subdivided to receive either saline (control) or NT (300 micrograms/kg) subcutaneously in gelatin every 8 hours for 7 days. At death, the pancreas and proximal jejunum (from groups 1 and 2) or distal ileum (from groups 3 and 4) were removed, weighed, and analyzed for DNA, RNA, and protein content. Both proximal and distal SBR significantly increased mucosal growth in the remnant intestine; a more pronounced effect was noted with proximal SBR. Administration of NT significantly augmented the adaptive changes in both groups of rats by mechanisms involving increases in both cell size (hypertrophy) and cell number (hyperplasia). Pancreatic growth was stimulated by distal (but not proximal) SBR; NT did not augment this response. The authors conclude that NT augments intestinal growth after SBR by mechanisms involving an increase in overall mucosal cellularity. Administration of NT may be therapeutically useful to enhance mucosal regeneration during the early period of adaptive hyperplasia after SBR.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altmann G. G., Leblond C. P. Factors influencing villus size in the small intestine of adult rats as revealed by transposition of intestinal segments. Am J Anat. 1970 Jan;127(1):15–36. doi: 10.1002/aja.1001270104. [DOI] [PubMed] [Google Scholar]
- Andersson S., Rosell S., Hjelmquist U., Chang D., Folkers K. Inhibition of gastric and intestinal motor activity in dogs by (Gln4) neurotensin. Acta Physiol Scand. 1977 Jun;100(2):231–235. doi: 10.1111/j.1748-1716.1977.tb05941.x. [DOI] [PubMed] [Google Scholar]
- Appleton G. V., Bristol J. B., Williamson R. C. Proximal enterectomy provides a stronger systemic stimulus to intestinal adaptation than distal enterectomy. Gut. 1987;28 (Suppl):165–168. doi: 10.1136/gut.28.suppl.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOOTH C. C., EVANS K. T., MENZIES T., STREET D. F. Intestinal hypertrophy following partial resection of the small bowel in the rat. Br J Surg. 1959 Jan;46(198):403–410. doi: 10.1002/bjs.18004619821. [DOI] [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baca I., Feurle G. E., Schwab A., Mittmann U., Knauf W., Lehnert T. Effect of neurotensin on exocrine pancreatic secretion in dogs. Digestion. 1982;23(3):174–183. doi: 10.1159/000198725. [DOI] [PubMed] [Google Scholar]
- Buchan A. M., Griffiths C. J., Morris J. F., Polak J. M. Enteroglucagon cell hyperfunction in rat small intestine after gut resection. Gastroenterology. 1985 Jan;88(1 Pt 1):8–12. doi: 10.1016/s0016-5085(85)80125-6. [DOI] [PubMed] [Google Scholar]
- DISCHE Z. Qualitative and quantitative colorimetric determination of heptoses. J Biol Chem. 1953 Oct;204(2):983–997. [PubMed] [Google Scholar]
- Deltz E., Gebhardt J. H., Preissner C., Schroeder P., Hansmann M. L., Kaiserling E., Müller-Hermelink H. K., Thiede A. Distribution of gastrointestinal hormones in the adaptive response after small bowel transplantation. Gut. 1987;28 (Suppl):217–220. doi: 10.1136/gut.28.suppl.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowling R. H., Booth C. C. Structural and functional changes following small intestinal resection in the rat. Clin Sci. 1967 Feb;32(1):139–149. [PubMed] [Google Scholar]
- Evers B. M., Beauchamp R. D., Ishizuka J., Townsend C. M., Jr, Alam T., Papaconstantinou J., Thompson J. C. Posttranscriptional regulation of neurotensin in the gut. Surgery. 1991 Aug;110(2):247–252. [PubMed] [Google Scholar]
- Evers B. M., Izukura M., Townsend C. M., Jr, Uchida T., Thompson J. C. Differential effects of gut hormones on pancreatic and intestinal growth during administration of an elemental diet. Ann Surg. 1990 May;211(5):630–638. [PMC free article] [PubMed] [Google Scholar]
- Evers B. M., Izukura M., Townsend C. M., Jr, Uchida T., Thompson J. C. Neurotensin prevents intestinal mucosal hypoplasia in rats fed an elemental diet. Dig Dis Sci. 1992 Mar;37(3):426–431. doi: 10.1007/BF01307738. [DOI] [PubMed] [Google Scholar]
- Feldman E. J., Dowling R. H., McNaughton J., Peters T. J. Effects of oral versus intravenous nutrition on intestinal adaptation after small bowel resection in the dog. Gastroenterology. 1976 May;70(5 PT1):712–719. [PubMed] [Google Scholar]
- Feurle G. E., Müller B., Rix E. Neurotensin induces hyperplasia of the pancreas and growth of the gastric antrum in rats. Gut. 1987;28 (Suppl):19–23. doi: 10.1136/gut.28.suppl.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregor M., Menge H., Stössel R., Riecken E. O. Effect of monoclonal antibodies to enteroglucagon on ileal adaptation after proximal small bowel resection. Gut. 1987;28 (Suppl):9–14. doi: 10.1136/gut.28.suppl.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haegel P., Stock C., Marescaux J., Petit B., Grenier J. F. Hyperplasia of the exocrine pancreas after small bowel resection in the rat. Gut. 1981 Mar;22(3):207–212. doi: 10.1136/gut.22.3.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson W. R. Proliferative and morphological adaptation of the intestine to experimental resection. Scand J Gastroenterol Suppl. 1982;74:11–20. [PubMed] [Google Scholar]
- Hanson W. R., Rijke R. P., Plaisier H. M., Van Ewijk W., Osborne J. W. The effect of intestinal resection on Thiry-Vella fistulae of jejunal and ileal origin in the rat: evidence for a systemic control mechanism of cell renewal. Cell Tissue Kinet. 1977 Nov;10(6):543–555. doi: 10.1111/j.1365-2184.1977.tb00311.x. [DOI] [PubMed] [Google Scholar]
- Helmstaedter V., Feurle G. E., Forssmann W. G. Ultrastructural identification of a new cell type--the N-cell as the source of neurotensin in the gut mucosa. Cell Tissue Res. 1977 Nov 23;184(4):445–452. doi: 10.1007/BF00220968. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Obertop H., Nundy S., Malamud D., Malt R. A. Onset of cell proliferation in the shortened gut. Rapid hyperplasia after jejunal resection. Gastroenterology. 1977 Feb;72(2):267–270. [PubMed] [Google Scholar]
- Olsen P. S., Pedersen J. H., Poulsen S. S., Yamashita Y., Kirkegaard P. Neurotensin-like immunoreactivity after intestinal resection in the rat. Gut. 1987 Sep;28(9):1107–1111. doi: 10.1136/gut.28.9.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PORUS R. L. EPITHELIAL HYPERPLASIA FOLLOWING MASSIVE SMALL BOWEL RESECTION IN MAN. Gastroenterology. 1965 Jun;48:753–757. [PubMed] [Google Scholar]
- Sagor G. R., Ghatei M. A., Al-Mukhtar M. Y., Wright N. A., Bloom S. R. Evidence for a humoral mechanism after small intestinal resection. Exclusion of gastrin but not enteroglucagon. Gastroenterology. 1983 May;84(5 Pt 1):902–906. [PubMed] [Google Scholar]
- Schraut W. H. Current status of small-bowel transplantation. Gastroenterology. 1988 Feb;94(2):525–538. doi: 10.1016/0016-5085(88)90449-0. [DOI] [PubMed] [Google Scholar]
- Thompson J. S., Bragg L. E., Saxena S. K. The effect of intestinal resection and urogastrone on intestinal regeneration. Arch Surg. 1990 Dec;125(12):1617–1621. doi: 10.1001/archsurg.1990.01410240099020. [DOI] [PubMed] [Google Scholar]
- Thompson J. S., Rikkers L. F. Surgical alternatives for the short bowel syndrome. Am J Gastroenterol. 1987 Feb;82(2):97–106. [PubMed] [Google Scholar]
- Tilson M. D., Livstone E. M. Early proliferative activity: its occurrence in the crypts of small bowel and colon after partial small-bowel resection. Arch Surg. 1980 Dec;115(12):1481–1485. doi: 10.1001/archsurg.1980.01380120049012. [DOI] [PubMed] [Google Scholar]
- Vanderhoof J. A., Euler A. R., Park J. H., Grandjean C. J. Augmentation of mucosal adaptation following massive small-bowel resection by 16,16-dimethyl-prostaglandin E2 in the rat. Digestion. 1987;36(4):213–219. doi: 10.1159/000199421. [DOI] [PubMed] [Google Scholar]
- Wiklund B., Rökaeus A., Hallberg D. Neurotensin-like immunoreactivity in plasma after fat intake in normal and obese subjects and after jejunoileal bypass. Acta Chir Scand. 1985;151(4):361–365. [PubMed] [Google Scholar]
- Williamson R. C., Buchholtz T. W., Malt R. A. Humoral stimulation of cell proliferation in small bowel after transection and resection in rats. Gastroenterology. 1978 Aug;75(2):249–254. [PubMed] [Google Scholar]
- Wood J. G., Hoang H. D., Bussjaeger L. J., Solomon T. E. Effect of neurotensin on pancreatic and gastric secretion and growth in rats. Pancreas. 1988;3(3):332–339. doi: 10.1097/00006676-198805000-00015. [DOI] [PubMed] [Google Scholar]
- Wood J. G., Hoang H. D., Bussjaeger L. J., Solomon T. E. Neurotensin stimulates growth of small intestine in rats. Am J Physiol. 1988 Dec;255(6 Pt 1):G813–G817. doi: 10.1152/ajpgi.1988.255.6.G813. [DOI] [PubMed] [Google Scholar]


