Abstract
Recombinant adenoviral vectors are effective in transferring foreign genes to a variety of cells and tissue types, both in vitro and in vivo. However, during the gene transfer, they may alter the principal function and local environment of transfected cells. Increasing evidence exists for a selective adrenotropism of adenovirus during infections and gene transfer. Therefore, using bovine adrenocortical cells in primary culture, we analyzed the influence of different adenoviral deletion mutants on cell morphology and physiology. Transfection of cells with an E1/E3-deleted adenoviral vector, engineered to express a modified form of the Aequorea victoria green fluorescent protein, was highly efficient, as documented by fluorescent microscopy. Ultrastructural analysis, however, demonstrated nuclear fragmentation and mitochondrial alterations in addition to intranuclear viral particles. Basal secretion of 17-OH-progesterone, 11-deoxycortisol, and cortisol was significantly increased by E1/E3-deleted vectors; yet, the corticotropin-stimulated release of these steroids was decreased. Interestingly, neither purified viral capsids nor E3/E4-deleted adenoviral mutants altered basal and stimulated steroidogenesis of adrenocortical cells. An intact adrenal response is crucial for adaptation to stress and survival. Therefore, the implications of our findings need to be considered in patients with adenoviral infections and those undergoing clinical studies using adenoviral gene transfer. At the same time, the high level of transfection in adrenocortical cells might make appropriately modified adenoviral vectors suitable for gene therapy of adrenocortical carcinomas with poor prognosis.
Gene therapy, that is, the treatment or prevention of disease by gene transfer, has been considered a revolution in medicine. As a result, approximately 300 clinical protocols involving gene transfer have been approved in the U.S. and Europe over the last 20 years, with more than 3,500 patients treated (1). Yet, there is still no conclusive evidence for its efficacy, and recent concern has arisen about its safety. Although this outcome disappointed both the professional and lay communities, important information has been collected from preclinical and clinical studies, and with the recent advances in “genomics” and “proteomics,” gene therapy is likely to become a reality within the next decades. Together with retroviruses, adenoviruses are the most commonly used viral vectors in clinical trials. Recombinant adenoviral vectors (RAVs), genetically modified by deletions within the viral genome to allow insertion of foreign genes (transgenes), have been successfully used for gene transfer to different tissues (2). Their limited effectiveness has been proven in phase I clinical trials to treat cystic fibrosis (3, 4), as well as in the treatment of several tumors (5, 6), including endocrine neoplasias (7, 8). Recently, however, there have also been reports of significant toxicity associated with administration of RAVs to primates (9, 10) and humans (11).
There is evidence that the adrenal gland is a major target for adenovirus. Indeed, a selective adrenotropism of this virus has been demonstrated in mice (12, 13) and calves (14, 15) during experimentally induced infections. In the former, viral attack affected all three zones of adrenal cortex, with 80% or more of the cells exhibiting intranuclear inclusions (12). Electron microscopy revealed changes in nuclear morphology, with the appearance of nucleolar hypertrophy and angular crystals associated with intranuclear virion accumulation (13). In the latter, acute focal nonsuppurative necrosis was present in the zona glomerulosa and fasciculata and occasionally in the zona reticularis and medulla of the adrenal glands, with pyknotic nuclei and eosinophilic intranuclear inclusions (14, 15). Morphologic and histologic changes in the adrenal cortices, including edema of the capsule and stroma, loss of trabecular structure, and delipidization, have also been described in infants with generalized adenoviral infections (16).
More recently, animal studies using RAVs for fetal gene therapy have demonstrated an efficient and persistent transgene expression in the fetal adrenal gland (17–20). Our group has recently shown that a single intraadrenal injection of an adenoviral vector encoding the cytochrome P450 21-hydroxylase gene induces compensation of biochemical, endocrine, and histological alterations in 21-hydroxylase-deficient mice, suggesting that corrective gene therapy is a feasible option for the treatment of congenital adrenal hyperplasia (21). Furthermore, adenoviral-mediated transfer of a suicide gene in vitro has successfully been tested in human NCI-H295 and mouse Y-1 adrenocortical cancer cells (22).
Despite this evidence, the direct effect of RAVs on adrenocortical cell function and hormone release has not been explored thus far. Therefore, we transduced bovine adrenal cortical cells (BACCs) in primary culture with different adenoviral deletion mutants and studied their effect on cell morphology, ultrastructure, proliferation, and steroidogenesis under both basal and corticotropin (ACTH)-stimulated conditions.
Material and Methods
Reagents.
DMEM/F12, FBS, Hepes, antibiotics, fungizone, and trypsin were purchased from Life Technologies (Rockville, MD). All of the other reagents used, unless otherwise specified, were from Sigma.
Cell Culture.
Primary cultures of BACCs were prepared weekly from freshly slaughtered steers' adrenal glands transported to our laboratory on ice from a local slaughterhouse. Cells were isolated as described (23, 24). Briefly, adrenals were submerged in 70% ethanol for 10 sec and freed of fat and connective tissue. Glands were then cut into halves, the medulla was removed, and the cortex was scraped off the capsule and cut into small pieces. After being washed in DMEM/F12 medium (containing 200 units/ml of penicillin, 200 μg/ml of streptomycin, 50 μg/ml of gentamicin, 0.5 μg/ml of fungizone, 2.438 mg/ml of NaHCO3, and 1% of 1 M Hepes), cortical tissue was digested with 2.5% trypsin dissolved in the same medium. Cortical cells were then separated by centrifugation (800 × g) and filtration after erythrocyte lysis. Viability of isolated cells, tested by trypan blue exclusion, was greater than 90%.
Cortical cells were grown in DMEM/F12 medium adjusted to contain 10% FBS, 100 units/ml of penicillin, 100 μg/ml of streptomycin, 25 μg/ml of gentamicin, 0.25 μg/ml of fungizone, 2.438 mg/ml of NaHCO3, and 1% of 1 M Hepes at 37°C in a 5% CO2 atmosphere. Culture medium was replaced every 24 h. For the experiments, cells were seeded either on 24-well polystyrene plates (Corning) at a density of 2 × 105 cells per well or in 75-cm2 tissue culture flasks (Becton Dickinson) at a density of 4 × 106 cells per flask. After 2–3 days in culture, 50% confluent cells were transduced with the different adenoviral vectors.
Adenoviral Vectors and Empty Capsids.
The following human adenovirus (Ad) serotype 5 mutants were used in this study: (i) AdGFP [E1/E3-deleted, in which the cytomegalovirus (CMV) early/intermediate promoter/enhancer drives the cDNA for a modified form of the Aequorea victoria green fluorescent protein (GFP)] (25); (ii) AdLacZ (E1/E3-deleted, in which the CMV promoter/enhancer drives the Escherichia coli lacZ gene) (26); (iii) dl1011 (E3/E4-deleted, expressing none of the products of the early region 4) (27); and (iv) dl1014 (E3-deleted/E4 partially deleted, expressing none of the products of the early region 4 except for those of the ORF 4 domain) (27). All viral stocks were purified twice by cesium chloride density-gradient centrifugation (Beckman L-80 ultra-centrifuge) and plaque titered in triplicate by serial dilution and agar overlay on their respective transcomplementing cell lines according to standard protocols (27, 28). Viruses were amplified by standard techniques and analyzed by restriction digestion of proteinase K-digested viral DNA. Clinical-grade lipopolysaccharide- and endotoxin-free stocks of 1.5 × 1011 particles per ml were prepared and stored at −80°C. Viral titers of 4.5 × 105 viral particles per well (24-well plates) or 25 × 106 viral particles per flask (75-cm2 flasks) from these stocks were used for the experiments.
In addition to these vectors, purified viral capsids were also tested in some experiments. Viral capsids were isolated by double banding in CsCl gradients and dialyzed 6 times against 1,000 volumes of buffer containing 10 mM Tris (pH 7.4), 1 mM MgCl2, and 50% glycerol (29). The protein concentration of empty adenoviral capsids was determined by the Bradford method (Bio-Rad) and compared with protein concentrations of plaque-titered virus to determine capsid protein viral equivalents.
Optical and Fluorescent Microscopy.
Twenty-four hours after transduction by the AdGFP vector, cortical cells in 24-well plates were examined by an Olympus (New Hyde Park, NY) Inverted System Microscope IX70 with Inverted Reflected Light Fluorescence Observation Attachment. A specific GFP cube (U-MWIB/GFP) was selected for fluorescence. Cells were photographed with the integrated camera.
Electron Microscopy.
For electron microscopy, cells transduced by AdGFP for 24 h in 75-cm2 flasks were harvested with PBS/EDTA and pelleted at 800 × g for 10 min. Cellular pellets were fixed in 4% glutaraldehyde in 0.1 M phosphate buffer (pH 7.3) for 2 h, postfixed in 2% OsO4 in 0.1M cacodylate (pH 7.3) for 1 h, dehydrated in ethanol, and embedded in epoxy resin. Ultrathin sections (70 nm) were stained with uranyl acetate and lead citrate and examined at 80 kV under a Philips (Electronic Instruments, Mahwah, NJ) electron microscope 301.
Cell Proliferation.
Cell proliferation was assayed by [3H]thymidine incorporation as described (30). Briefly, 24 h after transduction by AdGFP in 24-well plates, cells were washed in serum-free medium and maintained for 3 h in fresh culture medium. After, cells were incubated with 10 μCi per well [3H]thymidine (Amersham Pharmacia Biotech, Piscataway, NJ) for 2, 4, 8, and 24 h. [3H]Thymidine uptake was stopped by chilling the plates on ice for 2 min, medium was aspirated, and wells were washed 2 times with ice-cold PBS + 0.5% BSA under 30 min of continual shaking. Then, 1 ml per well of ice-cold 5% trichloroacetic acid was added, and cells were left at 4°C for 30 min. After one more washing in PBS, cells were solubilized in 0.1 N NaOH at room temperature under gentle shaking. Aliquots of the cell solution were collected into scintillation vials, the Bio-Safe II counting mixture (Research Products International) was added, and radioactivity was counted with the LS 6000IC scintillation counter (Beckman Coulter). Twelve replicates for each time point were used.
Basal and Stimulated Steroid Secretion.
For these experiments, cortical cells were incubated with the above described adenoviral mutants or purified viral capsids in 24-well plates for 24 h. After this procedure, plates were gently washed with serum-free medium, fresh culture medium was added, and cells were cultivated for 3 more days. Cells were then incubated in serum-free medium containing 5 × 10−3 M ascorbic acid, 0.001% wt/vol transferrin, and 0.01% wt/vol bacitracin (23, 24) in the absence (basal steroidogenesis) or presence (stimulated steroidogenesis) of different concentrations of ACTH (from 10−10 M to 10−8 M). After 24 h, supernatants were collected and cortical cells were lysed in 0.1% Triton X-100. Both supernatants and cell lysates were stored at −80°C until thawed on ice for analysis. Experiments were performed in quadruplicate.
Steroid Measurements.
Steroids were measured in supernatants. Pregnenolone, 17-OH-pregnenolone, progesterone, 17-OH-progesterone, and 11-deoxycortisol were measured by RIA after extraction with hexane-ethyl acetate and chromatography (Esoterix Endocrinology, Calabasas Hills, CA). Cortisol was measured by RIA with a kit from DPC (Los Angeles) (for specifications of the assays, see Table 2, which is published as supporting information on the PNAS web site, www.pnas.org). Results were corrected for cell protein concentration, measured in the cell lysates by the Bradford method (Bio-Rad).
Data Analysis.
Results are presented as the mean ± SEM from a minimum of three experiments. All data were tested for normal distribution and equal variance. Statistical significance was determined by unpaired Student's t test or Mann–Whitney rank sum test with commercially available software. Differences were considered significant at P < 0.05.
Results
Efficacy of Adenoviral Transduction in BACCs.
Twenty-four hours after transduction by the AdGFP vector, inspection of the cells by phase-contrast microscopy revealed a higher confluence in transduced BACCs (80–90%) (Fig. 1b) vs. nontransduced cells (60–70%) (Fig. 1a). In contrast, the confluence of cells transduced by dl1011 and dl1014 was not different from that of nontransduced cells (not shown). When compared with background (Fig. 1c), fluorescent microscopy of AdGFP-transduced cells showed a level of transgene expression close to 100% (Fig. 1d). One week after transduction, GFP was still expressed at a high level (not shown).
Figure 1.
Phase-contrast (a and b) and fluorescent (c and d) microscopy (×4) of BACCs 24 h after transduction by AdGFP (b and d) vs. nontransduced cells (a and c). Confluence was higher in transduced cells (b) than in nontransduced cells (a), suggesting increased cell proliferation. The level of GFP expression, after subtraction for background (c), was close to 100% of transduced cells (d).
Effect of Adenoviral Transduction on BACCs Ultrastructure.
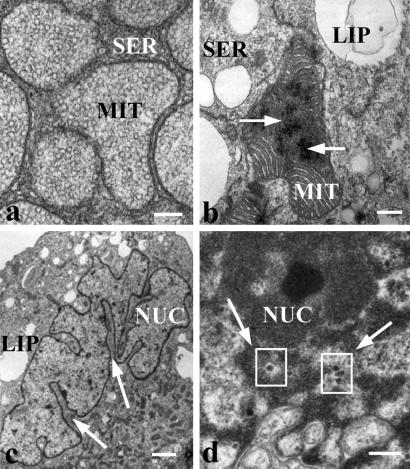
The ultrastructure of nontransduced BACCs was characterized by a cytoplasm typically filled with ample smooth endoplasmic reticulum and round or elongated mitochondria with tubulovesicular internal membranes (Fig. 2a). In contrast, mitochondria of AdGFP-transduced cells appeared pleiomorphic, exhibiting a reduced amount of tubular internal membranes. More specifically, the cristae had a lamellar morphology, characteristic of nonsteroidogenic cells or steroidogenic cells after deprivation of trophic hormones (31). In addition, the mitochondrial matrix frequently contained crystalline structures (Fig. 2b). Segmentation of the nucleus was also observed (Fig. 2c) and, viral particles of 60 nm in diameter were found in the nucleus of transduced cells (Fig. 2d).
Figure 2.
Electron microscopy of BACCs. (a) Round and elongated mitochondria with ample tubulovesicular internal membrane filled the cytosol of nontransduced cells (White bar = 200 nm). (b) Crystalline structures (arrows) within the mitochondrial matrix of AdGFP-transduced cells. (White bar = 200 nm.) (c) Nuclear membrane segmentation (arrows) in AdGFP-transduced cells. (White bar = 1 μm.) (d) Viral particles of 60 nm in diameter (arrows and squares) in the cell nucleus after transduction by AdGFP. (White bar = 200 nm.) NUC, nucleus; MIT, mitochondrion; SER, smooth endoplasmic reticulum; LIP, liposome.
Effect of Adenoviral Transduction on BACCs Proliferation.
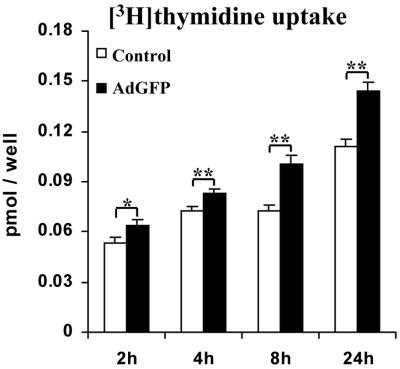
A significant time-dependent increase in [3H]thymidine uptake was found in BACCs transduced by AdGFP vs. nontransduced cells (0.064 ± 0.003 vs. 0.053 ± 0.004 pmol per well at 2h, P = 0.022; 0.083 ± 0.003 vs. 0.073 ± 0.002 at 4 h, P = 0.009; 0.1 ± 0.006 vs. 0.073 ± 0.003 at 8 h, P = 0.002; and 0.14 ± 0.006 vs. 0.11 ± 0.004 at 24 h, P = 0.002) (Fig. 3), consistent with their higher confluence (Fig. 1b).
Figure 3.
Proliferation assay. A significant time-dependent increase in [3H]thymidine incorporation was found in BACCs transduced by AdGFP. Results are the mean ± SEM of dodecaduplicates from at least three experiments. *, P < 0.05; **, P < 0.01.
Effect of Adenoviral Vectors on BACCs Cortisol Secretion.
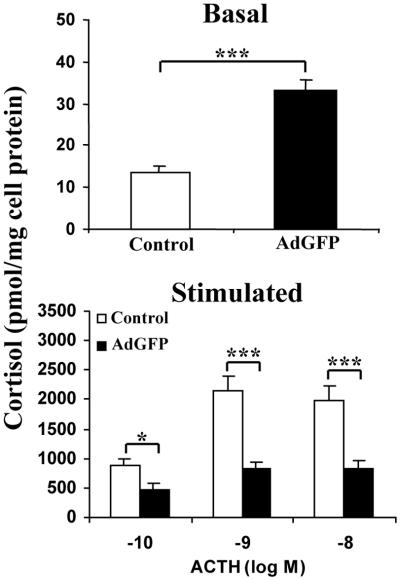
When compared with controls, BACCs transduced by the E1/E3-deleted AdGFP vector showed a significantly increased basal cortisol secretion (32.04 ± 3.48 vs. 13.73 ± 1.53 pmol/mg of cell protein, P < 0.001) (Fig. 4 Upper). On the contrary, the stimulated cortisol secretion, which in both non- and AdGFP-transduced cells reached a peak at 10−9 M ACTH, was significantly decreased in the latter vs. the former (468.8 ± 99 vs. 868.6 ± 126.5 pmol/mg cell protein at 10−10 M ACTH, P = 0.018; 832.1 ± 92.7 vs. 2159.7 ± 225.3 at 10−9 M, P < 0.001; 838.4 ± 129.4 vs. 1978.6 ± 245.4 at 10−8 M, P < 0.001) (Fig. 4 Lower). To confirm that this effect was related to the adenoviral vector itself, rather than to the GFP expression, we transduced BACCs with a second E1/E3-deleted vector (AdLacZ) carrying a different transgene (lacZ). In addition, we tested purified viral capsids (Cap) and two E3/E4-deleted mutants (dl1011 and dl1014) in our model (Fig. 5). When compared with that of nontransduced cells (5.41 ± 0.92), the basal cortisol secretion was significantly increased by AdLacZ (16.06 ± 1.68, P = 0.002) but not by Cap (5.12 ± 0.85, P = 1.0), dl1011 (6.92 ± 0.92, P = 0.081) or dl1014 (6.24 ± 0.99, P = 0.296) (Fig. 5 Upper). Similarly, when compared with the stimulated cortisol secretion in nontransduced BACCs (853 ± 140.64 at 10−10 M; 2198.38 ± 238.67 at 10−9 M; 1929.93 ± 232.19 at 10−8 M), cortisol response to ACTH was significantly decreased by AdLacZ (346.48 ± 54.7 at 10−10 M, P = 0.002; 894.75 ± 175.4 at 10−9 M, P < 0.001; 759.6 ± 148.47 at 10−8 M, P < 0.001), but it was not affected by Cap (769.69 ± 99.7, P = 0.626; 1896.02 ± 226.65, P = 0.364; 1870.32 ± 225.70, P = 0.717), dl1011 (703.23 ± 95.65, P = 0.373; 1839.99 ± 271.03, P = 0.159; 1860.07 ± 301.42, P = 0.202), or dl1014 (843.77 ± 120.4, P = 0.961; 2003.96 ± 263.9, P = 0.370; 1918.41 ± 238.4, P = 0.728) (Fig. 5 Lower).
Figure 4.
Effect of AdGFP on the basal (Upper) and ACTH-stimulated (Lower) cortisol secretion of transduced BACCs. Results are presented as mean ± SEM from at least 5 experiments performed in quadruplicate. *, P < 0.05; ***, P < 0.001.
Figure 5.
BACCs basal (Upper) and stimulated (Lower) secretion of cortisol after incubation with purified viral capsids (Cap) or transduction by the AdLacZ, dl1011, and dl1014 vectors. Results are the mean ± SEM from at least 5 experiments performed in quadruplicate. **, P < 0.01; ***, P < 0.001.
Effect of Adenoviral Vectors on BACCs Secretion of Other Steroids.
The effect of AdLacZ, dl1011, and dl1014 on the release of other steroids from BACCs was also studied and compared with that of nontransduced (control) cells. Results from these experiments are summarized in Table 1. Progesterone secretion from cells transduced either by AdLacZ or dl1011 or dl1014 was not different from that of control cells. However, AdLacZ affected the secretion of both 17-OH-progesterone and 11-deoxycortisol with the same pattern observed for cortisol (increased basal secretion/decreased stimulated secretion). On the contrary, and similarly to what had been observed with cortisol, there was no effect of dl1011 or dl1014 on the secretion of these two steroids. Pregnenolone and 17-OH-pregnenolone were also measured but their levels were below the detection limit of the assays in both transduced and nontransduced cells.
Table 1.
Effect of different adenoviral vectors on the basal and stimulated (10−9 M ACTH) secretion of cortisol precursors by BACCs
| Progesterone, pmol/mg cell protein
|
17-OH-progesterone, pmol/mg cell protein
|
11-Deoxycortisol, pmol/mg cell protein
|
||||
|---|---|---|---|---|---|---|
| Basal | Stimulated | Basal | Stimulated | Basal | Stimulated | |
| Control | 1.91 ± 0.17 | 2.97 ± 0.65 | 1.38 ± 0.16 | 11.32 ± 1.87 | 2.92 ± 0.90 | 50.42 ± 5.50 |
| AdLacZ | 1.47 ± 0.03 | 3.24 ± 0.63 | 4.55 ± 1.22* | 5.96 ± 1.24† | 13.73 ± 0.89‡ | 31.57 ± 6.12§ |
| dl1011 | 1.40 ± 0.13 | 2.84 ± 0.71 | 1.33 ± 0.36 | 12.24 ± 1.6 | 2.16 ± 0.30 | 56.23 ± 6.10 |
| dl1014 | 1.41 ± 0.12 | 2.42 ± 0.47 | 1.34 ± 0.70 | 9.78 ± 1.45 | 1.34 ± 0.41 | 47.18 ± 7.56 |
Results are presented as mean ± SEM from at least 5 experiments performed in quadruplicate.
, P = 0.03 vs. control.
, P = 0.038 vs. control.
, P = 0.001 vs. control.
, P = 0.024 vs. control.
Discussion
Currently, there are two main categories of gene therapy vehicles, nonviral and viral. The former is generally less toxic and immunogenic than the latter, but also suffers from inefficient gene transfer and low gene expression, especially in the in vivo condition. On the other hand, viral vectors have often been the first choice in gene-transfer studies because of the inherent capacity of certain viruses to enter the cell, translocate into the nucleus, and efficiently express their genes, properties that were acquired through millions of years of evolution and selective pressures. However, there is now growing concern about the safety of viral vectors (9–11).
Intact adrenal function is a prerequisite for adaptation to stress and survival in all mammalian species (32). In the present study, we tested the possibility that in vitro transfection with RAVs commonly used in gene therapy may cause functional and morphological alterations of the adrenal cortex.
Transgene expression mediated by an E1/E3-deleted vector was highly efficient in our model of isolated BACCs in primary culture, which reinforces the evidence for a natural adrenotropism of adenoviruses, reported in animal and human studies (12–22). Adenoviral transduction was followed by alteration of the ultrastructure of adrenocortical cells. The most significant changes involved mitochondria, in which a significant part of steroidogenesis takes place. Under physiologic conditions, the structure of adrenal mitochondria reflects the functional status of the gland, and reduction of internal membrane is usually accompanied by a decreased steroidogenic capacity (33). The mitochondrial matrix of transduced BACCs frequently contained crystalline structures. Similar structures have been observed in the nuclei of mouse adrenocortical cells during adenoviral infection, and it was suggested that they may represent aggregates of viral particles (13). In addition to mitochondrial alterations, we could demonstrate fragmentation of the cell nucleus, which was correlated to the number of intranuclear viral particles. Interestingly, fragmented nuclei have also been described in adrenal cells after infection by herpes simplex virus type I (34). Adenoviral transduction can cause cell apoptosis (35) and nuclear fragmentation may be a sign of this process. However, we did not detect other signs of programmed cell death, such as increased number of condensed nuclei or apoptotic bodies. On the other hand, cell proliferation was increased after transduction.
Adrenocortical response to ACTH was significantly suppressed by E1/E3-deleted vectors. The marked decrease in the stimulated secretion of 17-OH-progesterone, 11-deoxycortisol, and cortisol may suggest an interference of these vectors with the activities of 17α-, 21-, and/or 11β-hydroxylases. On the other hand, the increased basal steroidogenesis may be related to the increased cell proliferation. Similar modifications in patterns of steroidogenesis have been reported in Y-1 mouse adrenal tumor cells after transformation by a simian adenovirus (36, 37). Neither purified empty capsids nor E3/E4-deleted vectors affected adrenocortical cells secretion of cortisol and cortisol precursors. These observations indicate that the changes in steroidogenesis after adenoviral transduction are secondary to internalization of the vector into the cells, which is in line with our ultrastructural findings. The altered steroid secretion may be related to transcription and/or translation within the adenovirus E4 region, because E4 deletion annulled it. However, the lack of effect on adrenal steroidogenesis from the dl1014 mutant, deleted in the entire E4 region except for the ORF 4 domain, suggests that this domain is not implicated in the steroid changes.
In summary, our data show that adenoviral-mediated gene transfer can interfere with the normal function of the adrenal cortex. As the “end organ” of the human stress system, the adrenal gland has the capacity to maintain through appropriate morphologic, physiologic, and molecular changes the steroid balance necessary to cope with the altered demands of stressful states (32). Therefore, its integrity must be preserved in clinical studies using adenoviral vectors for gene transfer. From this point of view, our findings suggest that E4 deletion should be taken into consideration when designing RAVs for clinical gene therapy.
More generally, impairment of adrenal steroidogenesis may become relevant not only during adenovirus-mediated gene transfer, but also during adenoviral infection. Recently, evidence is accumulating for life-threatening adenoviral infections not only in immunocompromised patients, but also in immunocompetent individuals (38–40). Interestingly, substantial corticosteroid elevations have been described during the course of acute, severe, natural adenovirus 4 respiratory illness in young men (41). An impaired adrenal glucocorticoid release during adenoviral infection may lead to a feedback increase in central corticotropin releasing hormone (CRH) levels, which, considering the well documented role of CRH and glucocorticoids in mood, anxiety, and depression (42), might explain some of the milder symptoms seen in patients with adenoviral infections, such as malaise, disrupted sleep, and negative emotional reactions. Finally, adrenal insufficiency has been implicated in the mortality of patients with generalized adenoviral infections (16). Therefore, based on these considerations and on our results, we propose testing of the hypothalamo–pituitary–adrenal axis function in patients with severe adenoviral infections.
In conclusion, this study demonstrates a direct effect of adenoviral vectors on adrenal cell function and steroidogenesis. Considering the wide use of adenovirus-mediated gene transfer, these findings need to be considered in experimental and clinical studies applying such vectors. In addition, the high efficacy of adrenocortical transfection can make adenoviral vectors promising for cancer gene therapy of adrenocortical carcinomas with poor prognosis.
Supplementary Material
Acknowledgments
We are indebted to Professor Gary Ketner (Johns Hopkins University) for kindly providing us with the dl1011 and dl1014 adenoviral mutants used in this study and for offering generous advice on their use. This work, which is part of a Ph.D. program from the University of Messina, School of Medicine (Messina, Italy), in collaboration with the Pediatric and Reproductive Endocrinology Branch at the National Institute of Child Health and Human Development, National Institutes of Health, has been recently awarded a National Institutes of Health FARE 2002 award.
Abbreviations
- BACCs
bovine adrenal cortical cells
- RAVs
recombinant adenoviral vectors
- Ad
adenovirus
- ACTH
corticotropin
- GFP
green fluorescent protein
References
- 1.Mountain A. Trends Biotechnol. 2000;18:119–128. doi: 10.1016/s0167-7799(99)01416-x. [DOI] [PubMed] [Google Scholar]
- 2.Ali M, Lemoine N R, Ring C J. Gene Ther. 1994;1:367–384. [PubMed] [Google Scholar]
- 3.Zuckerman J B, Robinson C B, McCoy K S, Shell R, Sferra T J, Chirmule N, Magosin S A, Propert K J, Brown-Parr E C, Hughes J V, et al. Hum Gene Ther. 1999;10:2973–2985. doi: 10.1089/10430349950016384. [DOI] [PubMed] [Google Scholar]
- 4.Harvey B G, Leopold P L, Hackett N R, Grasso T M, Williams P M, Tucker A L, Kaner R J, Ferris B, Gonda I, Sweeney T D, et al. J Clin Invest. 1999;104:1245–1255. doi: 10.1172/JCI7935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anklesaria P. Curr Opin Mol Ther. 2000;2:426–432. [PubMed] [Google Scholar]
- 6.Wasil T, Buchbinder A. Cancer Invest. 2000;18:740–746. doi: 10.3109/07357900009012206. [DOI] [PubMed] [Google Scholar]
- 7.Barzon L, Bonaguro R, Palu G, Boscaro M. Eur J Endocrinol. 2000;143:447–466. doi: 10.1530/eje.0.1430447. [DOI] [PubMed] [Google Scholar]
- 8.Stone D, David A, Bolognani F, Lowenstein P R, Castro M G. J Endocrinol. 2000;164:103–118. doi: 10.1677/joe.0.1640103. [DOI] [PubMed] [Google Scholar]
- 9.Lozier J N, Metzger M E, Donahue R E, Morgan R A. Blood. 1999;94:3968–3975. [PubMed] [Google Scholar]
- 10.Nunes F A, Furth E E, Wilson J M, Raper S E. Hum Gene Ther. 1999;10:2515–2526. doi: 10.1089/10430349950016852. [DOI] [PubMed] [Google Scholar]
- 11.Marshall E. Science. 1999;286:2244–2245. doi: 10.1126/science.286.5448.2244. [DOI] [PubMed] [Google Scholar]
- 12.Margolis G, Kilham L, Hoenig E M. Am J Pathol. 1974;75:363–374. [PMC free article] [PubMed] [Google Scholar]
- 13.Hoenig E M, Margolis G, Kilham L. Am J Pathol. 1974;75:375–394. [PMC free article] [PubMed] [Google Scholar]
- 14.Stauber E, Card C. Can J Comp Med. 1978;42:466–472. [PMC free article] [PubMed] [Google Scholar]
- 15.Cutlip R C, McClurkin A W. Am J Vet Res. 1975;36:1095–1098. [PubMed] [Google Scholar]
- 16.Medvedev N I, Shastina G V. Arch Pathol. 1978;40:32–36. [PubMed] [Google Scholar]
- 17.Schachtner S, Buck C, Bergelson J, Baldwin H. Gene Ther. 1999;6:1249–1257. doi: 10.1038/sj.gt.3300939. [DOI] [PubMed] [Google Scholar]
- 18.Senoo M, Matsubara Y, Fujii K, Nagasaki Y, Hiratsuka M, Kure S, Uehara S, Okamura K, Yajima A, Narisawa K. Mol Genet Metab. 2000;69:269–276. doi: 10.1006/mgme.2000.2984. [DOI] [PubMed] [Google Scholar]
- 19.Coutelle C, Douar A M, Colledge W H, Froster U. Nat Med. 1995;1:864–866. doi: 10.1038/nm0995-864. [DOI] [PubMed] [Google Scholar]
- 20.Yang E Y, Cass D L, Sylvester K G, Wilson J M, Adzick N S. J Pediatr Surg. 1999;34:235–241. doi: 10.1016/s0022-3468(99)90181-1. [DOI] [PubMed] [Google Scholar]
- 21.Tajima T, Okada T, Ma X M, Ramsey W, Bornstein S, Aguilera G. Gene Ther. 1999;6:1898–1903. doi: 10.1038/sj.gt.3301018. [DOI] [PubMed] [Google Scholar]
- 22.Chuman Y, Zhan Z, Fojo T. J Clin Endocrinol Metab. 2000;85:253–262. doi: 10.1210/jcem.85.1.6244. [DOI] [PubMed] [Google Scholar]
- 23.Hornsby P J, McAllister J M. Methods Enzymol. 1991;206:371–380. doi: 10.1016/0076-6879(91)06107-e. [DOI] [PubMed] [Google Scholar]
- 24.Haidan A, Bornstein S R, Glasow A, Uhlmann K, Lubke C, Ehrhart-Bornstein M. Endocrinology. 1998;139:772–780. doi: 10.1210/endo.139.2.5740. [DOI] [PubMed] [Google Scholar]
- 25.Zolotukhin S, Potter M, Hauswirth W W, Guy J, Muzyczka N. J Virol. 1996;70:4646–4654. doi: 10.1128/jvi.70.7.4646-4654.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Berkner K L. BioTechniques. 1988;6:616–629. [PubMed] [Google Scholar]
- 27.Bridge E, Ketner G. J Virol. 1989;63:631–638. doi: 10.1128/jvi.63.2.631-638.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Graham F L, Smiley J, Russell W C, Nairn R. J Gen Virol. 1977;36:59–74. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- 29.Graham F L, Prevec L. In: Methods in Molecular Biology. Murray E J, editor. Clifton, NJ: Humana; 1991. pp. 109–128. [DOI] [PubMed] [Google Scholar]
- 30.Coward P, Wada H G, Falk M S, Chan S D, Meng F, Akil H, Conklin B R. Proc Natl Acad Sci USA. 1998;95:352–357. doi: 10.1073/pnas.95.1.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Purvis J L, Canick J A, Mason J I, Estabrook R W, McCarthy J L. Ann NY Acad Sci. 1973;212:319–343. doi: 10.1111/j.1749-6632.1973.tb47605.x. [DOI] [PubMed] [Google Scholar]
- 32.Chrousos G P, Gold P W. J Am Med Assoc. 1992;267:1244–1252. [PubMed] [Google Scholar]
- 33.Bornstein S R, Ehrhart-Bornstein M, Guse-Behling H, Scherbaum W A. Anat Rec. 1992;234:255–262. doi: 10.1002/ar.1092340212. [DOI] [PubMed] [Google Scholar]
- 34.Aita K, Irie H, Koyama A H, Fukuda A, Yoshida T, Shiga J. Arch Virol. 2001;146:2009–2020. doi: 10.1007/s007050170048. [DOI] [PubMed] [Google Scholar]
- 35.Teramoto S, Matsuse T, Matsui H, Ohga E, Ishii T, Ouchi Y. Eur Respir J. 1999;13:1125–1132. doi: 10.1034/j.1399-3003.1999.13e31.x. [DOI] [PubMed] [Google Scholar]
- 36.Tournier P, Faucon-Biguet N, Mathieu D, Champsaur H, Boullier D. C R Acad Sci Hebd Seances Acad Sci D. 1978;286:1033–1036. [PubMed] [Google Scholar]
- 37.Lefevre A, Faucon-Biguet N, Mathieu D, Tournier P, Saez J M. Steroids. 1981;31:315–325. doi: 10.1016/s0039-128x(81)90294-4. [DOI] [PubMed] [Google Scholar]
- 38.Munoz F M, Piedra P A, Demmler G J. Clin Infect Dis. 1998;27:1194–1200. doi: 10.1086/514978. [DOI] [PubMed] [Google Scholar]
- 39.Klinger J R, Sanchez M P, Curtin L A, Durkin M, Matyas B. Am J Respir Crit Care Med. 1998;157:645–649. doi: 10.1164/ajrccm.157.2.9608057. [DOI] [PubMed] [Google Scholar]
- 40.Price N O, Hacker J K, Silvers J H, Crawford-Miksza L, Hendry R M, Flood J, Hajjeh R A, Reingold A L, Passaro D J. Clin Infect Dis. 2001;33:260–262. doi: 10.1086/321831. [DOI] [PubMed] [Google Scholar]
- 41.Mason J W, Buescher E L, Belfer M L, Artenstein M S, Mougey E H. J Hum Stress. 1979;5:18–28. doi: 10.1080/0097840X.1979.9934524. [DOI] [PubMed] [Google Scholar]
- 42.Habib K E, Gold P W, Chrousos G P. Endocrinol Metab Clin North Am. 2001;30:695–728. doi: 10.1016/s0889-8529(05)70208-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.