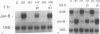Abstract
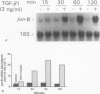
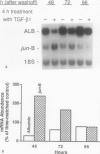
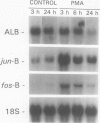
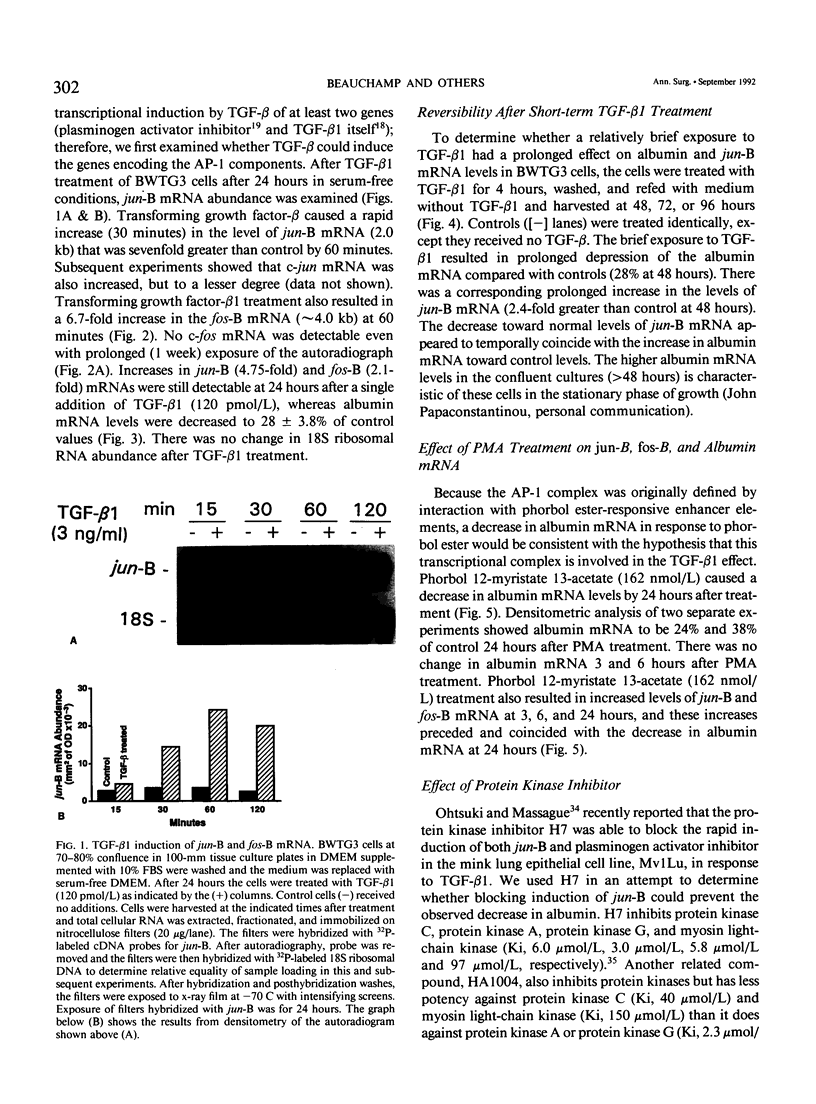
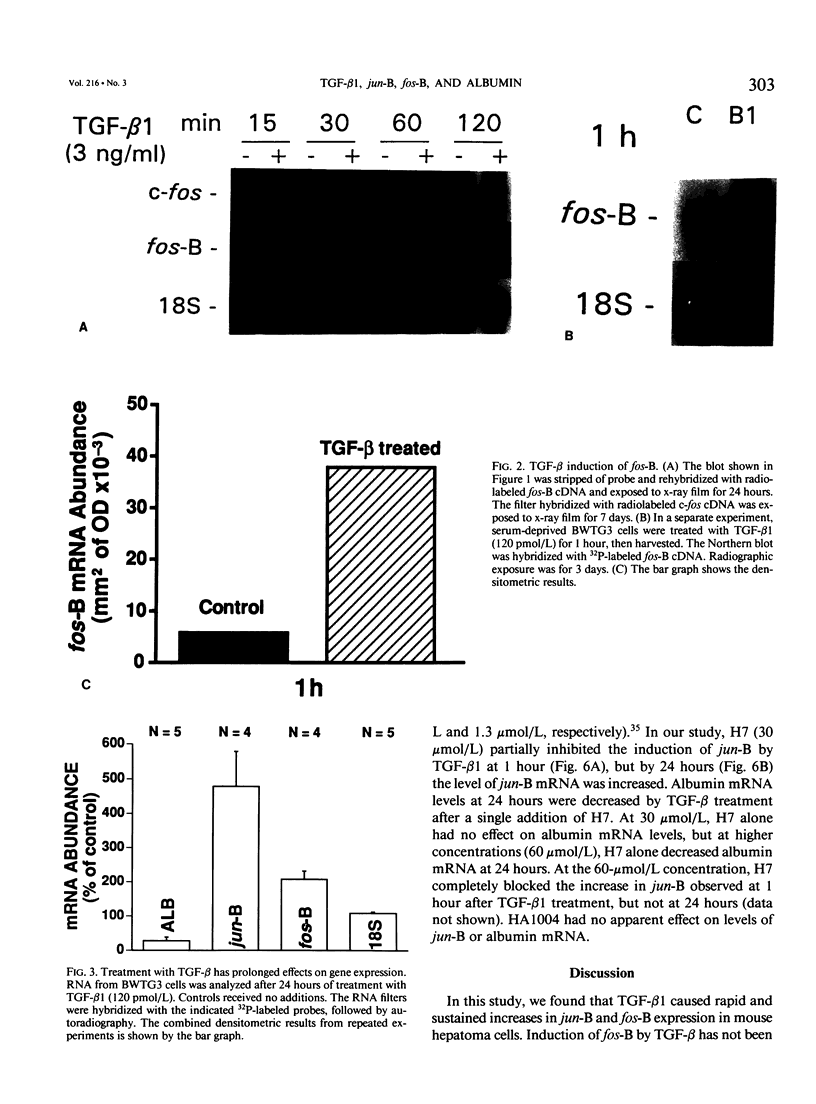
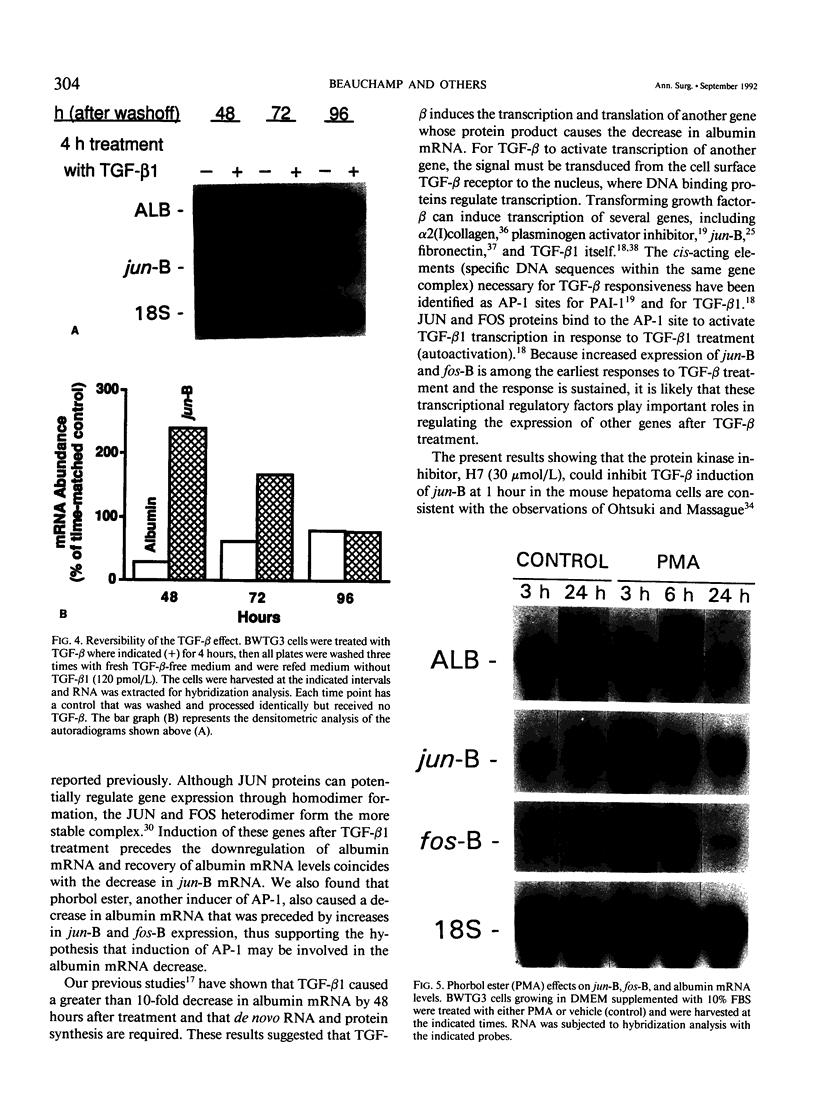
Transforming growth factor-beta (TGF-beta) modulates some components of the acute phase response in hepatic cells. The mechanisms for these actions of TGF-beta are largely unknown. The authors recently found that the decrease in albumin mRNA after TGF-beta 1 treatment required de novo RNA and protein synthesis, suggesting that TGF-beta acts through induction of another gene. The purpose of the current study was to determine whether TGF-beta 1 could regulate the expression of both the jun and fos genes that encode transcriptional regulatory proteins that constitute the AP-1 complex, and to determine whether expression of these genes may be coordinated with the decrease in albumin mRNA. Northern blot hybridization was used to determine levels of specific mRNAs. Transforming growth factor-beta 1 increased the levels of both jun-B and fos-B mRNA by 60 minutes after treatment of mouse hepatoma (BWTG3) cells. When TGF-beta 1 was removed from the media after 4 hours, there was a sustained effect of increased jun-B and decreased albumin mRNA (greater than 48 hours), and the subsequent decrease in jun-B levels coincided with the increase in albumin mRNA. The tumor-promoting phorbol ester (phorbol 12-myristate 13-acetate [PMA]), known to induce jun and fos gene expression, caused increases in jun-B and fos-B that preceded the decrease in albumin mRNA levels at 24 hours. These observations are consistent with our hypothesis that jun-B and fos-B induction may participate in downregulation of albumin synthesis as well as other hepatic responses to TGF-beta.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angel P., Imagawa M., Chiu R., Stein B., Imbra R. J., Rahmsdorf H. J., Jonat C., Herrlich P., Karin M. Phorbol ester-inducible genes contain a common cis element recognized by a TPA-modulated trans-acting factor. Cell. 1987 Jun 19;49(6):729–739. doi: 10.1016/0092-8674(87)90611-8. [DOI] [PubMed] [Google Scholar]
- Assoian R. K., Sporn M. B. Type beta transforming growth factor in human platelets: release during platelet degranulation and action on vascular smooth muscle cells. J Cell Biol. 1986 Apr;102(4):1217–1223. doi: 10.1083/jcb.102.4.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnard J. A., Lyons R. M., Moses H. L. The cell biology of transforming growth factor beta. Biochim Biophys Acta. 1990 Jun 1;1032(1):79–87. doi: 10.1016/0304-419x(90)90013-q. [DOI] [PubMed] [Google Scholar]
- Bascom C. C., Wolfshohl J. R., Coffey R. J., Jr, Madisen L., Webb N. R., Purchio A. R., Derynck R., Moses H. L. Complex regulation of transforming growth factor beta 1, beta 2, and beta 3 mRNA expression in mouse fibroblasts and keratinocytes by transforming growth factors beta 1 and beta 2. Mol Cell Biol. 1989 Dec;9(12):5508–5515. doi: 10.1128/mcb.9.12.5508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchamp R. D., Barnard J. A., McCutchen C. M., Cherner J. A., Coffey R. J., Jr Localization of transforming growth factor alpha and its receptor in gastric mucosal cells. Implications for a regulatory role in acid secretion and mucosal renewal. J Clin Invest. 1989 Sep;84(3):1017–1023. doi: 10.1172/JCI114223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatti S. P., Foster D. N., Ranganathan G., Moses H. L., Getz M. J. Induction of fibronectin gene transcription and mRNA is a primary response to growth-factor stimulation of AKR-2B cells. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1119–1123. doi: 10.1073/pnas.85.4.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun L., Mead J. E., Panzica M., Mikumo R., Bell G. I., Fausto N. Transforming growth factor beta mRNA increases during liver regeneration: a possible paracrine mechanism of growth regulation. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1539–1543. doi: 10.1073/pnas.85.5.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busso N., Chesne C., Delers F., Morel F., Guillouzo A. Transforming growth-factor-beta (TGF-beta) inhibits albumin synthesis in normal human hepatocytes and in hepatoma HepG2 cells. Biochem Biophys Res Commun. 1990 Sep 14;171(2):647–654. doi: 10.1016/0006-291x(90)91195-x. [DOI] [PubMed] [Google Scholar]
- Castilla A., Prieto J., Fausto N. Transforming growth factors beta 1 and alpha in chronic liver disease. Effects of interferon alfa therapy. N Engl J Med. 1991 Apr 4;324(14):933–940. doi: 10.1056/NEJM199104043241401. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Chiu R., Boyle W. J., Meek J., Smeal T., Hunter T., Karin M. The c-Fos protein interacts with c-Jun/AP-1 to stimulate transcription of AP-1 responsive genes. Cell. 1988 Aug 12;54(4):541–552. doi: 10.1016/0092-8674(88)90076-1. [DOI] [PubMed] [Google Scholar]
- Cohen D. R., Curran T. fra-1: a serum-inducible, cellular immediate-early gene that encodes a fos-related antigen. Mol Cell Biol. 1988 May;8(5):2063–2069. doi: 10.1128/mcb.8.5.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czaja M. J., Weiner F. R., Flanders K. C., Giambrone M. A., Wind R., Biempica L., Zern M. A. In vitro and in vivo association of transforming growth factor-beta 1 with hepatic fibrosis. J Cell Biol. 1989 Jun;108(6):2477–2482. doi: 10.1083/jcb.108.6.2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fey G. H., Fuller G. M. Regulation of acute phase gene expression by inflammatory mediators. Mol Biol Med. 1987 Dec;4(6):323–338. [PubMed] [Google Scholar]
- Greenberg M. E., Ziff E. B. Stimulation of 3T3 cells induces transcription of the c-fos proto-oncogene. Nature. 1984 Oct 4;311(5985):433–438. doi: 10.1038/311433a0. [DOI] [PubMed] [Google Scholar]
- Hidaka H., Inagaki M., Kawamoto S., Sasaki Y. Isoquinolinesulfonamides, novel and potent inhibitors of cyclic nucleotide dependent protein kinase and protein kinase C. Biochemistry. 1984 Oct 9;23(21):5036–5041. doi: 10.1021/bi00316a032. [DOI] [PubMed] [Google Scholar]
- Ito Y., Hayashi H., Taira M., Tatibana M., Tabata Y., Isono K. Depression of liver-specific gene expression in regenerating rat liver: a putative cause for liver dysfunction after hepatectomy. J Surg Res. 1991 Aug;51(2):143–147. doi: 10.1016/0022-4804(91)90085-z. [DOI] [PubMed] [Google Scholar]
- Kehrl J. H., Wakefield L. M., Roberts A. B., Jakowlew S., Alvarez-Mon M., Derynck R., Sporn M. B., Fauci A. S. Production of transforming growth factor beta by human T lymphocytes and its potential role in the regulation of T cell growth. J Exp Med. 1986 May 1;163(5):1037–1050. doi: 10.1084/jem.163.5.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S. J., Angel P., Lafyatis R., Hattori K., Kim K. Y., Sporn M. B., Karin M., Roberts A. B. Autoinduction of transforming growth factor beta 1 is mediated by the AP-1 complex. Mol Cell Biol. 1990 Apr;10(4):1492–1497. doi: 10.1128/mcb.10.4.1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S. J., Jeang K. T., Glick A. B., Sporn M. B., Roberts A. B. Promoter sequences of the human transforming growth factor-beta 1 gene responsive to transforming growth factor-beta 1 autoinduction. J Biol Chem. 1989 Apr 25;264(12):7041–7045. [PubMed] [Google Scholar]
- Kushner I., Ganapathi M., Schultz D. The acute phase response is mediated by heterogeneous mechanisms. Ann N Y Acad Sci. 1989;557:19–30. doi: 10.1111/j.1749-6632.1989.tb23996.x. [DOI] [PubMed] [Google Scholar]
- Lee W., Mitchell P., Tjian R. Purified transcription factor AP-1 interacts with TPA-inducible enhancer elements. Cell. 1987 Jun 19;49(6):741–752. doi: 10.1016/0092-8674(87)90612-x. [DOI] [PubMed] [Google Scholar]
- Li L., Hu J. S., Olson E. N. Different members of the jun proto-oncogene family exhibit distinct patterns of expression in response to type beta transforming growth factor. J Biol Chem. 1990 Jan 25;265(3):1556–1562. [PubMed] [Google Scholar]
- Lin H. Y., Wang X. F., Ng-Eaton E., Weinberg R. A., Lodish H. F. Expression cloning of the TGF-beta type II receptor, a functional transmembrane serine/threonine kinase. Cell. 1992 Feb 21;68(4):775–785. doi: 10.1016/0092-8674(92)90152-3. [DOI] [PubMed] [Google Scholar]
- Mackiewicz A., Ganapathi M. K., Schultz D., Brabenec A., Weinstein J., Kelley M. F., Kushner I. Transforming growth factor beta 1 regulates production of acute-phase proteins. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1491–1495. doi: 10.1073/pnas.87.4.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackiewicz A., Kushner I. Transforming growth factor beta 1 influences glycosylation of alpha 1-protease inhibitor in human hepatoma cell lines. Inflammation. 1990 Oct;14(5):485–497. doi: 10.1007/BF00914270. [DOI] [PubMed] [Google Scholar]
- Miller-Graziano C. L., Szabo G., Griffey K., Mehta B., Kodys K., Catalano D. Role of elevated monocyte transforming growth factor beta (TGF beta) production in posttrauma immunosuppression. J Clin Immunol. 1991 Mar;11(2):95–102. doi: 10.1007/BF00917745. [DOI] [PubMed] [Google Scholar]
- Morrone G., Cortese R., Sorrentino V. Post-transcriptional control of negative acute phase genes by transforming growth factor beta. EMBO J. 1989 Dec 1;8(12):3767–3771. doi: 10.1002/j.1460-2075.1989.tb08553.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T., Tomita Y., Hirai R., Yamaoka K., Kaji K., Ichihara A. Inhibitory effect of transforming growth factor-beta on DNA synthesis of adult rat hepatocytes in primary culture. Biochem Biophys Res Commun. 1985 Dec 31;133(3):1042–1050. doi: 10.1016/0006-291x(85)91241-0. [DOI] [PubMed] [Google Scholar]
- Nishina H., Sato H., Suzuki T., Sato M., Iba H. Isolation and characterization of fra-2, an additional member of the fos gene family. Proc Natl Acad Sci U S A. 1990 May;87(9):3619–3623. doi: 10.1073/pnas.87.9.3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtsuki M., Massagué J. Evidence for the involvement of protein kinase activity in transforming growth factor-beta signal transduction. Mol Cell Biol. 1992 Jan;12(1):261–265. doi: 10.1128/mcb.12.1.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelton R. W., Dickinson M. E., Moses H. L., Hogan B. L. In situ hybridization analysis of TGF beta 3 RNA expression during mouse development: comparative studies with TGF beta 1 and beta 2. Development. 1990 Oct;110(2):609–620. doi: 10.1242/dev.110.2.609. [DOI] [PubMed] [Google Scholar]
- Rappolee D. A., Mark D., Banda M. J., Werb Z. Wound macrophages express TGF-alpha and other growth factors in vivo: analysis by mRNA phenotyping. Science. 1988 Aug 5;241(4866):708–712. doi: 10.1126/science.3041594. [DOI] [PubMed] [Google Scholar]
- Rossi P., Karsenty G., Roberts A. B., Roche N. S., Sporn M. B., de Crombrugghe B. A nuclear factor 1 binding site mediates the transcriptional activation of a type I collagen promoter by transforming growth factor-beta. Cell. 1988 Feb 12;52(3):405–414. doi: 10.1016/s0092-8674(88)80033-3. [DOI] [PubMed] [Google Scholar]
- Russell W. E., Coffey R. J., Jr, Ouellette A. J., Moses H. L. Type beta transforming growth factor reversibly inhibits the early proliferative response to partial hepatectomy in the rat. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5126–5130. doi: 10.1073/pnas.85.14.5126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryseck R. P., Bravo R. c-JUN, JUN B, and JUN D differ in their binding affinities to AP-1 and CRE consensus sequences: effect of FOS proteins. Oncogene. 1991 Apr;6(4):533–542. [PubMed] [Google Scholar]
- Schuermann M., Neuberg M., Hunter J. B., Jenuwein T., Ryseck R. P., Bravo R., Müller R. The leucine repeat motif in Fos protein mediates complex formation with Jun/AP-1 and is required for transformation. Cell. 1989 Feb 10;56(3):507–516. doi: 10.1016/0092-8674(89)90253-5. [DOI] [PubMed] [Google Scholar]
- Sporn M. B., Roberts A. B. TGF-beta: problems and prospects. Cell Regul. 1990 Nov;1(12):875–882. doi: 10.1091/mbc.1.12.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szpirer C., Szpirer J. A mouse hepatoma cell line which secretes several serum proteins including albumin and alpha-foetoprotein. Differentiation. 1975 Oct 16;4(2):85–91. doi: 10.1111/j.1432-0436.1975.tb01446.x. [DOI] [PubMed] [Google Scholar]
- Vogt P. K., Bos T. J., Doolittle R. F. Homology between the DNA-binding domain of the GCN4 regulatory protein of yeast and the carboxyl-terminal region of a protein coded for by the oncogene jun. Proc Natl Acad Sci U S A. 1987 May;84(10):3316–3319. doi: 10.1073/pnas.84.10.3316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakefield L. M., Winokur T. S., Hollands R. S., Christopherson K., Levinson A. D., Sporn M. B. Recombinant latent transforming growth factor beta 1 has a longer plasma half-life in rats than active transforming growth factor beta 1, and a different tissue distribution. J Clin Invest. 1990 Dec;86(6):1976–1984. doi: 10.1172/JCI114932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerhausen D. R., Jr, Hopkins W. E., Billadello J. J. Multiple transforming growth factor-beta-inducible elements regulate expression of the plasminogen activator inhibitor type-1 gene in Hep G2 cells. J Biol Chem. 1991 Jan 15;266(2):1092–1100. [PubMed] [Google Scholar]
- Zerial M., Toschi L., Ryseck R. P., Schuermann M., Müller R., Bravo R. The product of a novel growth factor activated gene, fos B, interacts with JUN proteins enhancing their DNA binding activity. EMBO J. 1989 Mar;8(3):805–813. doi: 10.1002/j.1460-2075.1989.tb03441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]