Abstract
Introduction
In Europe, surveillance of respiratory syncytial virus (RSV) has been recently incorporated into existing influenza monitoring platforms that are based on influenza-like illness (ILI) or acute respiratory infection (ARI) case definitions. This study aims to compare RSV rates captured by ARI versus ILI case definitions and to describe the clinical and economic trajectories of RSV in older adults.
Methods
The study was conducted in Italy during the 2023/2024 and 2024/2025 seasons. Thirty-eight general practitioners were randomized 1:1 to enroll individuals ≥ 50 years presenting for care and meeting the European criteria for ARI or ILI, respectively. Alternative definitions were also explored. All subjects were tested for respiratory pathogens. RSV-positive individuals were followed for up to one month.
Results
Of 1431 patients (ARI: 741; ILI: 690) included, 5.2% tested positive for RSV. Odds of RSV in the ARI group (5.8%) was 26% higher than in the ILI group (4.6%) [odds ratio (OR) 1.26; 95% CI 0.60–2.65]. Exclusion of GPs with unexpectedly low enrollment rates increased the OR to 1.67 (95% CI 0.80–3.42). Conversely, adults in the ILI group showed higher rates of influenza A (OR 0.83; 95% CI 0.47–1.44) and SARS-CoV-2 (OR 0.57; 95% CI 0.34–0.95). A proposed alternative case definition, denoted as ARI with wheezing and/or productive cough and/or rhonchi and/or dyspnea was sensitive at 92.0% and specific at 30.8%. Among 75 RSV-positive outpatients, the case-complication, case-hospitalization and case-fatality rates were 30.7%, 2.7%, and 1.3%, respectively. The mean costs per RSV case were € 168.71 from the payer perspective and up to € 899.51 from the societal perspective.
Conclusions
Compared to a highly sensitive ARI definition, ILI-based surveillance likely underestimates the incidence of RSV. Further qualifiers can enhance specificity of the ARI case definition. The study confirms a significant burden of RSV in older adults.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40121-025-01205-3.
Keywords: Respiratory syncytial virus, RSV, Acute respiratory infection (ARI), Influenza-like illness (ILI), Surveillance, Case definition, Burden of disease, Complications, Cost of illness, Older adults
Key Summary Points
| Why carry out this study? |
| Currently available studies on the epidemiology and burden of respiratory syncytial virus (RSV) in older adults underscore critical knowledge gaps, including the need for an accurate clinical case definition and prospectively collected data on the burden of disease. |
| Driven by these evidence gaps, the RESPIRA-50 study envisaged a direct comparison of acute respiratory infection (ARI) and influenza-like illness (ILI) case definitions for their ability to capture RSV in primary care and a prospective tracking of RSV-related clinical and economic consequences. |
| What was learned from the study? |
| Although the difference was not statistically significant, a highly sensitive ARI case definition identified about one-third more RSV cases than ILI-based surveillance platforms, which are more specific for influenza or SARS-CoV-2. Consequently, ARI-based definitions may better reflect the true burden of disease in older adults. |
| Prospectively collected and granular data highlighted a high rate of RSV-related complications, which are responsible for a significant economic impact. |
Introduction
Initially identified as a predominantly pediatric pathogen, respiratory syncytial virus (RSV) has been increasingly recognized as a major cause of respiratory illness in older adults [1]. Among older people living in high-income countries, the average RSV-related incidence, hospitalization, and case-fatality rates attributable to RSV are estimated at 1.62%, 0.15%, and 7.13%, respectively. With the corresponding population of 321.3 million, these rates translate into 5.2 million symptomatic cases, 470,000 hospitalizations, and 33,000 in-hospital deaths [1]. To reduce this burden of disease, three effective RSV vaccines have recently become available for older adults [2].
Comprehensive literature reviews [3–5] have indicated some critical evidence gaps in epidemiological and clinical features of RSV in older adults. First, there is not any universally accepted RSV clinical case definition that triggers collection of a biological sample for virological analyses. Previous surveillance studies used a variety of case definitions, most of which have been specifically developed for influenza [3]. Several European countries, including Italy, have recently incorporated surveillance of RSV into the existing influenza monitoring platforms, which are based on influenza-like illness (ILI) case definitions. Surveillance of respiratory viruses in other countries is based on a broader acute respiratory infection (ARI) case definition, while still other countries report both ILI and ARI incidence rates [6]. As a substantial proportion of RSV-positive older adults lacks fever as a symptom, it seems that fever-based case definitions underestimate the burden of RSV [7]. While more sensitive case definitions are useful for detecting the majority of RSV cases, more specific (and thus less sensitive) case definitions require fewer resources and still allow pursuit of common surveillance objectives [8].
Regarding RSV-related outcomes in older adults, most evidence is limited to retrospective, register-based studies conducted in hospital settings, while very few community-based prospective surveys have investigated the natural history of RSV in adult outpatients [4, 5]. For instance, the current Italian data are generally restricted to mere estimation of positivity prevalence, with little information on the complication rates, their spectrum, and associated resource consumption [5].
This study, named RESPIRA-50, aims to reproduce two parallel sentinel surveillance networks, which are based on ARI and ILI case definitions, in order to compare rates of RSV and other respiratory pathogens detected using these established syndromic case definitions. The study also aims to identify an alternative RSV-specific case definition and to scrutinize clinical and economic trajectories of RSV infection in community-dwelling adults aged ≥ 50 years. The chosen population target reflects the broadened age indication for an RSV vaccine, now encompassing individuals ≥ 50 years, as opposed to the prior ≥ 60-year threshold [9].
Methods
Study Design and Setting
The study was conducted in Genoa (northern Italy) during the 2023/2024 and 2024/2025 RSV seasons. Interim first-season results can be assessed elsewhere [10]. Each RSV season was defined as a period between week 42 (mid-October) and week 17 (end of April). General practitioners (GPs) who took part of a large cooperative were invited to participate in the study. To simulate two different sentinel surveillance networks, volunteer GPs were randomly (1:1) allocated to one of two groups to enroll individuals seeking care for ARI or ILI, respectively. Separate ARI and ILI training modules were administered to all GPs before the start of each season.
The European Centre for Disease Prevention and Control (ECDC) criteria [11] were used for both ARI [sudden onset of ≥ 1 respiratory symptom (cough, sore throat, shortness of breath, coryza) and GP’s judgment of an underlying infection] and ILI [sudden onset of ≥ 1 systemic (fever or feverishness, malaise, headache, myalgia) and ≥ 1 respiratory (cough, sore throat, shortness of breath) symptoms] case definitions (Table S1).
The Italian sentinel surveillance network, which is based on the ECDC ILI case definition, recommends covering ≥ 4% of the population [12]. For this study, we aimed to cover a more conservative threshold of ≥ 5% of the total Genoa population aged ≥ 50 years (n = 297,486). With an average of 1300 patients aged ≥ 14 years per GP, of which 59% were ≥ 50 years, 24 GPs would cover 6.2% of the population aged ≥ 50 years. By assuming that each GP would swab approximately 20–30 patients per season, we expected to enroll 960–1440 subjects. However, at the end of the first season, several GPs enrolled < 30 patients and, for the second season, we additionally randomized 12 GPs. In all, 38 GPs contributed to ≥ 1 season (n = 24 in the first season and n = 36 in the second season).
During the study period, adult RSV vaccination was not publicly funded in Italy; however, the authorized vaccines could be purchased out-of-pocket. By the end of the study, we checked RSV vaccination status in a local immunization registry, where the registration of all administered vaccines is mandatory. Of the 1443 subjects enrolled, only 16 (1%) had received a single dose of an adjuvanted RSV vaccine (between March and June 2024), and none of these individuals tested positive for RSV.
This study was conducted in accordance with the Helsinki Declaration of 1964 and its later amendments. The study protocol was approved by the Liguria Region Ethics Committee (#13354 of 18/09/2023). Written informed consent was obtained from all patients.
Eligibility Criteria
Inclusion criteria were formulated as follows: individuals aged ≥ 50 years, who were able to provide informed consent and met the ARI/ILI case definitions. Subjects were excluded if they did not meet the above-described ECDC criteria, were residents of long-term care facilities, had known positivity for RSV in the current season or developed symptoms > 7 days before the GP visit.
Study Procedures
On the day of enrollment, GPs obtained the informed consent and performed a baseline assessment (t0) by gathering demographic (sex, age, nationality) and medical history (presence of underlying health conditions) data and evaluated signs and symptoms associated with the current visit. The list of respiratory and systemic symptoms collected is reported later in the manuscript. The GPs then collected a nasopharyngeal swab specimen that was eluted in a transport medium (UTM, Copan Italia; Brescia, Italy), barcoded, stored at 2–8 °C for a maximum of 72 h and shipped in batch in an isothermal bag to the regional reference laboratory located at San Martino Research Hospital (Genoa, Italy).
Individuals who tested positive for RSV were followed for up to 30 days, while those who tested negative for RSV ended the study at t0. The first follow-up call (t1) was performed 14 ± 1 days after the onset of symptoms and the following information deemed to be related to the current RSV episode was collected: (1) eventual development of complication(s); (2) further GP visits; (3) specialist visits (type and number); (4) medications used (brand, type, dosage, frequency and duration); (5) diagnostic tests and procedures (type and number); (6) emergency department (ED) referrals and/or hospitalizations; and (7) for working adults, number of full-time or part-time working days lost. Patients who fully recovered (self-judged as return to the premorbid state) by day 14 ended the study, while a second follow-up call (t2) was performed on day 30 ± 1 for not fully recovered individuals and the same data were collected. All follow-up calls were systematically performed by a trained physician.
All specimens were tested in two different real-time polymerase chain reaction (RT-PCR) assays [Allplex Respiratory Panels 1–4 (RSV is included in Panel 1) and Allplex SARS-CoV-2/FluA/FluB/RSV; Seegene, Seoul, Korea] and one rapid RSV antigen detection test (RADT; STANDARD Q RSV Ag; SD Biosensor, Suwon-si, Korea). To ensure the same testing conditions and avoid a double swab collection, which may reduce patient’s compliance, both RT-PCR and RADT were performed at the laboratory using the same specimen. Laboratory staff was blinded to the sample origin. Considering their high sensitivity and specificity for RSV [13], the Allplex Respiratory Panel 1, which also differentiates between RSV subtypes A and B, was considered the reference standard. In summary, all swabs were tested for 25 viral [RSV A and B; influenza A(H1N1)pdm09, A(H3N2) and B; SARS-CoV-2; adenovirus; rhinovirus; enterovirus; metapneumovirus; parainfluenza viruses 1, 2, 3 and 4; bocaviruses 1–4; coronaviruses 229E, NL63 and OC43] and bacterial [Hemophilus (H.) influenzae; Streptococcus (S.) pneumoniae; Bordetella pertussis; Bordetella parapertussis; Mycoplasma pneumoniae; Legionella pneumophila, and Chlamydophila pneumoniae] pathogens. Finally, 64 (85.3%) RSV-positive samples with a sufficient viral load were sequenced using either whole-genome next generation or Sanger G gene sequencing. All sequences generated were uploaded to the GISAID (Global Initiative on Sharing All Influenza Data) database (https://gisaid.org/). Descriptions of all the laboratory methods are provided in Table S2.
Study Endpoints
The study has two co-primary endpoints. The first endpoint was the RSV detection rate in both ARI and ILI groups defined as a proportion of swabs tested positive for RSV to the total of swabs processed. The second endpoint was prevalence of complications (including hospitalizations) among subjects testing positive for RSV to the total of RSV-positive subjects.
The study had several secondary and exploratory endpoints. First, RSV detections were described by month and season, age-class (50–64, 65–74, and ≥ 75 years), subtype, clade, and co-detection patterns (single detections or co-detections). Second, the association between signs/symptoms and RSV status was tested to develop an alternative RSV-specific case definition, and the performance of the latter was assessed by computing sensitivity, specificity, positive (PPV), and negative (NPV) predictive values and the area under the curve (AUC). For comparison, all patients were also re-classified according to the World Health Organization’s (WHO)-like (ARI with measured fever ≥ 38 °C and cough) [14] and the United States (US) Centers for Disease Control and Prevention (CDC) (fever ≥ 37.8 °C with cough and/or sore throat) [15] ILI case definitions (Table S1). Third, ARI and ILI groups were compared in terms of the detection rates of other respiratory pathogens. Fourth, diagnostic accuracy [positive (PPA) and negative (NPA) percent agreements, PPV and NPV] of the alternative RT-PCR kit and RADT, compared to the reference RT-PCR kit, was evaluated. Finally, resource consumption in terms of GP and specialist visits, diagnostic tests and procedures, medicines, ED referrals and hospitalizations, and relative costs incurred by RSV-positive individuals were quantified.
Analysis of Costs
The bottom-up costing approach was implemented. All analyses were conducted using costs expressed in Euros (€) as of 2024. Cost calculation, performed by the study staff, specifically included only resources associated with the RSV episode, thereby excluding other healthcare expenditures, such as pre-existing medications or clearly unrelated visits. Direct costs included costs related to medicines, GP and specialist visits, diagnostic procedures, ED referrals, and hospitalizations associated with the current RSV episode (time horizon 30 days). Ex-factory prices were considered for reimbursable medicines [16], while list prices were used for non-reimbursable products. The cost of only effectively consumed doses was considered. The costs of GP visits performed at either GP’s office or patient’s home derived from an Italian costing study [17] and were actualized to 2024 [18]. Analogously, the costs related to ED visits were taken from a cost study conducted in Genoa [19]. Official tariffs [20, 21] were used for specialist visits, diagnostic tests, and hospitalizations. Costs related to RT-PCR and RADT were not included. For indirect costs, we considered productivity losses that were applied to working adults only [22]. Perspective of the Italian National Health Service (NHS) considered all direct costs except for non-reimbursable medicines. Societal perspective included all direct and indirect costs. Costs were summarized as both means with standard deviations (SDs) and medians with ranges. A summary of all cost items, assumptions, and data sources is provided in Table S3.
Statistical Analysis
RSV detection and complication rates were expressed as proportions with Clopper–Pearson’s exact 95% confidence intervals (CIs). Characteristics of patients enrolled in the ARI and ILI groups, as well as those tested positive and negative for RSV, were compared by means of the Fisher’s exact or t tests. Notably, the statistical significance (p < 0.05) of these tests should be interpreted cautiously, as the clustered nature of the data was ignored.
Considering the clustered nature of the data, generalized estimating equations (GEE) logistic regression with an exchangeable working correlation structure was used to study the association between RSV positivity and surveillance group, as well as between RSV positivity and reported signs/symptoms. The effect size was expressed as odds ratios (ORs) with 95% CIs. The estimands of participant-average effect and the participant-level estimator were therefore of interest. The dependent variable was either any RSV detection or RSV mono-detection. The base-case GEE models were controlled for the effect of varying cluster sizes (i.e., the number of swabs collected by single GPs) by including the logarithm of the cluster size as an offset. Several sensitivity analyses were then conducted. First, the offset of cluster size was excluded from the model specification. Second, we added potential confounders of season, sex, age, nationality, smoking status, and presence of co-morbidities. Third, as informative cluster size cannot be ruled out, we changed the GEE working correlation structure to independent [23]. Finally, to reduce noise due to low-enrolling GPs, we limited the dataset to clusters with ≥ 30 swabs. In all models, the sandwich variance estimator was used to calculate robust standard errors. Data were analyzed in R packages, v. 4.5.0 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Characteristics of the Study Population
Across two seasons, 38 GPs (18 in the ARI and 20 in the ILI groups) enrolled 1443 subjects (2023/2024: n = 524; 2024/2025: n = 919). Twelve (0.8%) subjects were excluded for the following reasons: (1) five were < 50 years; (2) four were swabbed outside the allowed time window; (3) two did not meet the definition of ARI/ILI; and (4) one swab had no cotton flock inside the transport tube. A total of 1431 adults were therefore included in the analysis.
The mean age of participants was 67.9 (SD 11.3) years and females prevailed (62.4%). Two-thirds (61.7%) of patients had ≥ 1 co-morbidity, of which cardiovascular (42.9%) and respiratory (17.7%) diseases were the most prevalent (Table 1). Of the study population, 51.8% (n = 741) and 48.2% (n = 690) of subjects were enrolled by GPs randomized to the ARI and ILI groups, respectively. The number of adults enrolled by single GPs varied from 2 to 97. As shown in Table 1, ARI and ILI patients were comparable for most variables, but there were some imbalances in terms of mean age (Δ 1.6 years), nationality, prevalence of cancer, and smoking status.
Table 1.
Characteristics of the study population; Genoa (Italy), 2023/2024 and 2024/2025 seasons
| Characteristic | Level | n (%) | p value (ARI vs. ILI) | ||
|---|---|---|---|---|---|
| Total (n = 1431) | ARI (n = 741) | ILI (n = 690) | |||
| Sex | Female | 893 (62.4) | 465 (62.8) | 428 (62.0) | 0.79 |
| Male | 538 (37.6) | 276 (37.2) | 262 (38.0) | ||
| Age, years | Mean (SD) | 67.9 (11.3) | 68.7 (11.3) | 67.1 (11.1) | 0.009 |
| 50–64 | 622 (43.5) | 300 (40.5) | 322 (46.7) | 0.058 | |
| 65–74 | 379 (26.5) | 204 (27.5) | 175 (25.4) | ||
| ≥ 75 | 430 (30.0) | 237 (32.0) | 193 (28.0) | ||
| Nationality | Italian | 1340 (93.6) | 711 (96.0) | 629 (91.2) | < 0.001 |
| Foreign-born | 91 (6.4) | 30 (4.0) | 61 (8.8) | ||
| Season | 2023/2024 | 517 (36.1) | 250 (33.7) | 267 (38.7) | 0.054 |
| 2024/2025 | 914 (63.9) | 491 (66.3) | 423 (61.3) | ||
| Underlying health conditions | ≥ 1 | 883 (61.7) | 446 (60.2) | 437 (63.3) | 0.23 |
| Cardiovascular | 614 (42.9) | 319 (43.0) | 295 (42.8) | 0.92 | |
| Respiratory | 254 (17.7) | 129 (17.4) | 125 (18.1) | 0.73 | |
| Diabetes | 121 (8.5) | 70 (9.4) | 51 (7.4) | 0.18 | |
| Hepatic | 34 (2.4) | 14 (1.9) | 20 (2.9) | 0.23 | |
| Renal | 63 (4.4) | 35 (4.7) | 28 (4.1) | 0.61 | |
| Anemia | 56 (3.9) | 29 (3.9) | 27 (3.9) | 0.99 | |
| Obesity | 142 (9.9) | 74 (10.0) | 68 (9.9) | 0.99 | |
| Dementia | 33 (2.3) | 15 (2.0) | 18 (2.6) | 0.49 | |
| Cancer | 149 (10.4) | 64 (8.6) | 85 (12.3) | 0.024 | |
| Immunosuppression | 52 (3.6) | 24 (3.2) | 28 (4.1) | 0.48 | |
| Current smoker | No | 808 (56.5) | 641 (86.5) | 568 (82.3) | 0.034 |
| Yes | 222 (15.5) | 100 (13.5) | 122 (17.7) | ||
ARI acute respiratory infection, ILI influenza-like illness, SD standard deviation
All patients in the ILI group satisfied the ECDC ARI criteria, while 92.6% (686/741) of patients in the ARI group also satisfied the ECDC ILI criteria.
Epidemiology of RSV and its Characteristics
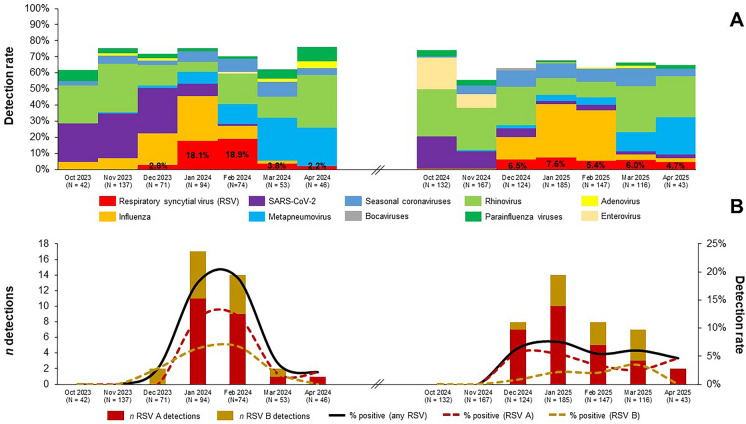
RSV was detected in 5.2% (75/1431; 95% CI 4.1–6.5%) of participants and its detection rate was higher in the 2023/2024 season than in the 2024/2025 season (7.0% vs. 4.3%). RSV accounted for 7.8% (75/965) of all viral detections and of 5.7% (75/1315) of all viral/bacterial detections. During the first season, RSV clearly peaked between January and February 2024 with a monthly peak positivity rate of up to 18.9%. Conversely, in the 2024/2025 season, the observed detection rates plateaued at a comparatively low level (Fig. 1A).
Fig. 1.
Detection rates of respiratory syncytial virus (RSV) and other respiratory viruses overall (A) and by RSV subtype (B); Genoa (Italy), 2023/2024 and 2024/2025 seasons
Although both RSV subtypes co-circulated, RSV A was predominant in both seasons (2023/2024: 61.1%; 2024/2025: 69.2%) (Fig. 1B). The population of molecularly characterized RSV A strains (n = 41) was heterogeneous and represented by subclades adjoining A.D.1* (26.8%), A.D.3* (51.2%), and A.D.5* (22.0%). Sequences of RSV B strains (n = 23) belonged to four different (sub)clades, of which B.D.E.1 (69.6%) was the most frequent (Table S4).
Of 75 RSV cases, 47 (62.7%) were single RSV detections, 10 (13.3%) samples were co-detected with other respiratory viruses, of which human rhinovirus was the most common, 11 (14.7%) were co-detected with H. influenzae and/or S. pneumoniae, while the remaining seven (9.3%) specimens showed a mixed viral/bacterial co-detection pattern (Table S5).
Compared with RT-PCR, RADT had low PPA (22.7%) and perfect NPA (100%). Indeed, if only RADT was used, the overall RSV prevalence would drop from 5.2% to 1.2%. RSV RADT performed comparatively well (PPA 63.2%) only for high viral load samples (cycle threshold < 25) (Table S6). Analogously, diagnostic performance of the two RT-PCR assays differed: compared with the Allplex Respiratory Panel 1, PPA and NPA of the Allplex SARS-CoV-2/FluA/FluB/RSV were 85.3% and 100%, respectively. The lower sensitivity of the latter kit was driven by low load samples (cycle threshold > 30) (Table S7).
Compared to RSV-negative subjects, those who tested positive for RSV exhibited a higher proportion of females (77.3% vs. 61.6%), and had ≥ 1 chronic condition (72.0% vs. 61.1%), particularly renal disease (9.3% vs. 4.1%) and obesity (18.7% vs. 9.4%). Other characteristics were comparable (Table S8).
Detection Rates of RSV and Other Respiratory Pathogens by Surveillance Group
Overall, RSV detection rate in the ARI group (5.8%; 43/741) was higher than in the ILI group (4.6%; 32/690). When only RSV mono-detections were considered, the difference was similar (ARI: 3.9%; ILI: 2.6%). This pattern was consistently observed in both 2023/2024 (ARI: 8.0%; ILI: 6.0%) and 2024/2025 (ARI: 4.7%; ILI: 3.8%) seasons and for both RSV A (ARI: 3.8%; ILI: 3.0%) and RSV B (ARI: 2.0%; ILI: 1.6%) subtypes. Of 75 RSV cases, 100% (75/75), 98.7% (74/75), 28.4% (21/74) and 33.8% (25/74) of individuals satisfied the ECDC ARI, ECDC ILI, CDC ILI, and WHO ILI criteria, respectively. RSV detection rates were slightly higher in individuals aged ≥ 75 years (5.6%; 24/430) than in younger adults (65–74 years: 4.7%, 18/379; 50–64 years: 5.3%, 33/622).
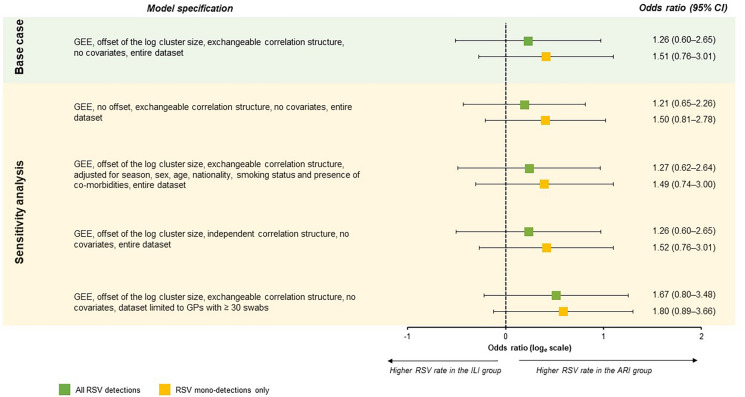
As shown in Fig. 2, in the base-case GEE model, the odds of any RSV and RSV mono-detections were 26% and 51% higher for subjects in the ARI than in the ILI groups, respectively. Alternative model specifications produced concordant results and the ORs were always higher for RSV mono-detections. Notably, when only GPs with ≥ 30 swabs were considered, the ORs of any and single RSV detections were 1.67 and 1.80, respectively. However, all 95% CIs included the null.
Fig. 2.
Association between positivity for respiratory syncytial virus (RSV) and surveillance group; Genoa (Italy), 2023/2024 and 2024/2025 seasons. ARI acute respiratory infection, CI confidence interval, GP general practitioner, GEE generalized estimating equation, ILI influenza-like illness
In an exploratory analysis of all respiratory pathogens tested (Table S9), positivity to ≥ 1 pathogen was very similar (OR 0.96; 95% CI 0.60–1.53) between the ARI (74.9%) and ILI (73.2%) groups. With regard to single pathogens detected, patients in the ARI group more frequently tested positive for adenovirus (ARI: 1.1% vs. ILI: 0.3%; OR 3.75, 95% CI 0.85–16.60), enterovirus (ARI: 3.2% vs. ILI: 2.5%; OR 1.32, 95% CI 0.54–3.23), metapneumovirus (ARI: 6.5% vs. ILI: 5.2%; OR 1.25, 95% CI 0.71–2.22), and bacteria (ARI: 25.9% vs. ILI: 18.4%; OR 1.52, 95% CI 0.84–2.77). Conversely, SARS-CoV-2 (ARI: 7.4% vs. ILI: 12.2%; OR 0.57, 95% CI 0.34–0.95), influenza A(H1N1)pdm09 (ARI: 6.1% vs. ILI: 8.0%; OR 0.74, 95% CI 0.46–1.19), and parainfluenza viruses (ARI: 1.9% vs. ILI: 3.0%; OR 0.61, 95% CI 0.30–1.26) were more common findings in the ILI group.
Symptomatic Profile of Older Adults Who Tested Positive for RSV
Among RSV-positive adults, malaise (92.0%) was the most common systemic symptom, while cough (97.3%) and coryza (90.7%) were the most frequently reported respiratory symptoms (Table 2). Fever ≥ 38 °C was reported by only 31.1% of subjects. Compared to RSV-negative individuals, those positive for RSV had a higher prevalence of several respiratory symptoms (wheezing, productive cough, dyspnea, rhonchi, coryza, altered smell, and desaturation), while no systemic symptoms were predictive of RSV. An analysis based on RSV mono-detections showed generally comparable results apart from the altered smell that was no longer associated with RSV (Table S10). Considering both a low prevalence of desaturation in the entire cohort (5.1%) and a high prevalence of coryza among subjects tested positive for rhinovirus (88.4%), we selected the symptoms of wheezing, productive cough, rhonchi, and dyspnea for further evaluation.
Table 2.
Association between positivity for respiratory syncytial virus (RSV) and signs and symptoms reported by adults aged ≥ 50 years; Genoa (Italy), 2023/2024 and 2024/2025 seasons
| Symptom | RSV-positive (n = 75)a | RSV-negative (n = 1356) | OR (95% CI) | aOR (95% CI)b |
|---|---|---|---|---|
| Any fever or feverishness | 49 (65.3) | 908 (67.0) | 1.00 (0.62–1.60) | 1.14 (0.69–1.90) |
| Fever ≥ 38 °Cc | 23 (31.1) | 499 (37.5) | 0.79 (0.53–1.18) | 0.86 (0.55–1.32) |
| Shivering | 45 (60.0) | 766 (56.5) | 1.29 (0.84–1.98) | 1.35 (0.90–2.04) |
| Headache | 39 (52.0) | 802 (59.1) | 0.81 (0.52–1.27) | 0.81 (0.48–1.34) |
| Myalgia | 40 (53.3) | 865 (63.8) | 0.69 (0.43–1.11) | 0.69 (0.44–1.08) |
| Arthralgia | 37 (49.3) | 830 (61.2) | 0.66 (0.41–1.07) | 0.66 (0.40–1.08) |
| Malaise | 69 (92.0) | 1156 (85.3) | 2.17 (0.81–5.84) | 2.13 (0.83–5.47) |
| Decreased appetite | 39 (52.0) | 607 (44.8) | 1.44 (0.87–2.38) | 1.40 (0.84–2.35) |
| Nausea | 17 (22.7) | 267 (19.7) | 1.29 (0.71–2.37) | 1.23 (0.65–2.32) |
| Diarrhea | 11 (14.7) | 149 (11.0) | 1.52 (0.74–3.12) | 1.63 (0.77–3.43) |
| Any cough | 73 (97.3) | 1275 (94.0) | 2.54 (0.54–11.96) | 2.54 (0.50–12.76) |
| Productive cough | 56 (74.7) | 798 (58.8) | 2.35 (1.31–4.20) | 2.52 (1.34–4.75) |
| Dyspnea | 24 (32.0) | 260 (19.2) | 2.15 (1.24–3.72) | 2.12 (1.19–3.78) |
| Tachypnea | 2 (2.7) | 34 (2.5) | 1.14 (0.28–4.68) | 1.20 (0.30–4.81) |
| Rhonchi | 29 (38.7) | 316 (23.3) | 2.28 (1.37–3.81) | 2.45 (1.41–4.24) |
| Wheezing | 23 (30.7) | 181 (13.3) | 3.15 (1.76–5.63) | 3.12 (1.61–6.04) |
| Need for supplemental O2 | 0 (0) | 13 (1.0) | – | – |
| Low/decreased SaO2d | 7 (9.3) | 64 (4.8) | 2.20 (0.86–5.68) | 2.60 (0.99–6.81) |
| Sore throat | 38 (50.7) | 790 (58.3) | 0.78 (0.47–1.31) | 0.77 (0.47–1.26) |
| Coryza | 68 (90.7) | 1112 (82.0) | 2.81 (1.02–7.79) | 2.88 (1.09–7.58) |
| Altered smell | 16 (21.3) | 216 (15.9) | 1.57 (0.99–2.48) | 1.71 (1.07–2.75) |
| Altered taste | 15 (20.0) | 222 (16.4) | 1.39 (0.81–2.38) | 1.48 (0.87–2.53) |
aOR adjusted odds ratio, CI confidence interval, OR odds ratio
aIncludes also subjects with co-detections
bAdjusted for surveillance group, age, sex, smoking status, and presence of co-morbidities
cData on measured body temperature were available for 48 and 884 febrile subjects testing positive and negative for RSV, respectively
dData on SaO2 were available for 75 and 1329 subjects testing positive and negative for RSV, respectively
All available ILI criteria (ECDC, CDC, and WHO) performed poorly for RSV with AUC values ≤ 0.51 (Table S11). Specificity was maximized (64.9%; 95% CI 62.3–67.5%) with the WHO case definition, but its sensitivity was only 28.4% (95% CI 19.4–39.5%). The newly proposed alternative RSV-specific case definition of ARI (RSV-ARI) with wheezing and/or productive cough and/or rhonchi and/or dyspnea performed better, the corresponding AUC, sensitivity and specificity parameters being 0.61 (95% CI 0.58–0.65), 92.0% (95% CI 83.6–96.3%), and 30.8% (95% CI 28.4–33.3%), respectively. Eventual use of only “wheezing” qualifier would increase specificity up to 86.7%, but the sensitivity would drop to 30.7% (Table S11). When applied to influenza, the alternative RSV-ARI case definition performed worse (AUC: 0.52; sensitivity: 74.2%; specificity: 30.1%), while the WHO (AUC: 0.71; sensitivity: 71.1%; specificity: 71.0%) and CDC (AUC: 0.71; sensitivity: 76.8%; specificity: 64.6%) ILI criteria performed the best.
Clinical Consequences of RSV Infection
All participants completed the follow-up period. Of 75 RSV infections, 66.7% (50/75) did not fully recover by day 14 and the average duration of RSV episode was 20.1 (SD 7.8) days. Twenty-three (30.7%; 95% CI 20.5–42.4%) patients developed ≥ 1 complication that involved mostly (73.9%; 17/23) lower respiratory tract (Table 3). Two pneumonia cases, one of which was with pleural effusion, required hospitalization (case-hospitalization rate 2.7%; 95% CI 0.3–9.3%) and involved 71- and 96-year-old women. The sample of the former was positive for RSV B and H. influenzae, while the latter was positive for RSV A only. The 96-year-old woman developed acute respiratory failure and died 16 days after the initial GP visit (case-fatality rate 1.3%; 95% CI 0.0–7.2%). All hospitalizations and the death occurred in the ARI group.
Table 3.
Clinical outcomes in adults aged ≥ 50 years who tested positive for respiratory syncytial virus (RSV); Genoa (Italy), 2023/2024 and 2024/2025 seasons (n = 75)
| Outcome | n (%)a | 95% CI |
|---|---|---|
| Any complication | 23 (30.7) | 20.5–42.4 |
| Any upper respiratory tract complication | 8 (10.7) | 4.7–19.9 |
| Otitis media | 4 (5.3) | 1.5–13.1 |
| Sinusitis | 4 (5.3) | 1.5–13.1 |
| Any lower respiratory tract complication | 17 (22.7) | 13.8–33.8 |
| Bronchitis | 10 (13.3) | 6.6–23.2 |
| Pneumonia | 7 (9.3) | 3.8–18.3 |
| Hospitalization | 2 (2.7) | 0.3–9.3 |
| Death | 1 (1.3) | 0.0–7.2 |
CI confidence interval
aTwo subjects developed both sinusitis and bronchitis
Resource Consumption and Costs
The average number of GP visits associated with the RSV episode was 1.7 (SD 0.9), while 48.0% (36/75) had ≥ 1 follow-up visit. All but one (98.7%) patient took ≥ 1 medicine, of which antibiotics (57.3%), mucolytics (46.7%), and inhaled corticosteroids (41.3%) were the most prevalent. Additionally, 21.3% of patients performed ≥ 1 diagnostic test (mostly chest X-ray), while 6.7% had a specialist visit (Table S12).
The mean outpatient costs incurred by RSV-positive adults were € 74.40. The average hospitalization costs across all RSV-positive subjects were € 107.49. The mean hospitalization costs of the two hospitalized subjects were instead € 4031.00 (€ 3802.00 and € 4260.00, respectively). From the Italian NHS perspective, the mean cost of an RSV episode was € 168.71. Direct costs were generally skewed to the right with median costs being significantly lower than mean costs. Working adults (n = 28) lost on average 5.5 (SD 5.1) 8-h working days with the associated productivity loss of € 840.61. From the societal perspective, the mean cost of an RSV episode was € 899.51 for working adults and € 255.17 for non-working adults (Table 4).
Table 4.
Direct and indirect episode costs incurred by adults aged ≥ 50 years who tested positive for respiratory syncytial virus (RSV) in the first 30 days after the onset of symptoms; Genoa (Italy), 2023/2024 and 2024/2025 seasons (n = 75)
| Cost item | Mean (SD), € | Median [Min–Max], € |
|---|---|---|
| Reimbursable medicines | 14.29 (12.51) | 12.96 [0.00–64.99] |
| Non-reimbursable medicines | 13.18 (13.26) | 12.50 [0.00–53.68] |
| General practitioner visits | 33.58 (18.30) | 18.39 [18.39–120.18] |
| Specialist visits | 1.80 (6.83) | 0.00 [0.00–33.60] |
| Diagnostic procedures | 7.31 (22.07) | 0.00 [0.00–136.95] |
| Emergency department visits | 4.25 (20.94) | 0.00 [0.00–106.15] |
| Hospitalizations | 107.49 (654.88) | 0.00 [0.00–4,260.00] |
| Productivity lossa | 840.61 (782.02) | 687.24 [0.00–3,054.40] |
| Total outpatient costsb | 74.40 (47.99) | 63.00 [18.39–291.65] |
| Total costs (Italian National Health Service perspective)c | 168.71 (680.93) | 49.11 [18.39–4,533.79] |
| Total costs (societal perspective, non-working adults)d | 255.17 (855.04) | 74.83 [18.39–4,551.65] |
| Total costs (societal perspective, working adults)e | 899.51 (802.08) | 738.42 (24.02–3,155.03) |
SD standard deviation
aOnly working adults (n = 28) were considered
bIncludes costs of reimbursable and non-reimbursable medicines, general practitioner, specialist and emergency department visits, and diagnostic procedures
cIncludes costs of reimbursable medicines, general practitioner, specialist and emergency department visits, diagnostic procedures, and hospitalizations
dIncludes costs of reimbursable and non-reimbursable medicines, general practitioner, specialist and emergency department visits, diagnostic procedures, and hospitalizations
eIncludes costs of reimbursable and non-reimbursable medicines, general practitioner, specialist and emergency department visits, diagnostic procedures, hospitalizations, and productivity losses
In an analysis stratified by RSV co-detection pattern, subjects with single RSV detections generally showed lower costs than subjects with co-detections (Table S13). For instance, among RSV mono-detections, the mean outpatient costs and total costs from the Italian NHS perspective were € 70.35 and € 138.51, respectively. The corresponding costs across subjects with co-detections were € 81.20 and € 219.42. However, owing to a smaller number of co-detections, the total mean costs from the Italian NHS perspective were influenced by a single hospitalized case. Indeed, median costs were more balanced between subjects with mono-detections (€ 49.11) and those with co-detections (€ 54.92) (Table S13).
Discussion
The RESPIRA-50 study was conceived as an attempt to compare available case definitions for RSV in a randomized fashion and to prospectively collect data on the natural history of the community-acquired RSV infection in adults aged ≥ 50 years. According to the WHO [24], interpretation of the global RSV surveillance data is challenged by the absence of a standard case definition. Current data on the burden of RSV in older adults also have various limitations, were often collected retrospectively [3], and therefore heavily depended on the accuracy and completeness of the primary data sources.
In the main analysis of the first primary endpoint, the odds of RSV detection in the ARI versus ILI groups was 26% greater, while in the sensitivity analyses the difference increased by up to 80%. This trend was consistently observed in both RSV seasons and for both RSV subtypes. While the magnitude of effect sizes is undoubtedly of public health relevance, the 95% CIs of all point estimates were comparatively wide and included the null. We believe that this imprecision was mainly driven by two concurrent factors. First, there was a large variability in terms of GP enrollment rates: several GPs enrolled an unexpectedly low number of patients. An increase in cluster size variability may lead to loss of efficiency and decrease in power [25]. Ideally, an unbiased estimate of RSV detection rate could be achieved by tracking a random sample of the general population. In this study, as well as in actual sentinel surveillance networks, the random assumption is likely missed because the sample of symptomatic individuals is self-selected by the GPs [26]. When we excluded the GPs with low swabbing rates, the OR of the ARI versus the ILI group increased from 1.26 to 1.67. It can be inferred that the testing behavior of “poorly engaged” GPs systematically differed from their “well engaged” counterparts. For example, the former group could experience waning initial enthusiasm, have busier practices, and thus enroll only the most severe cases. This bias is difficult to control since all participating GPs are volunteers (rather than carefully chosen) and are implicitly assumed to be willing to participate in surveillance activities [27]. The second reason for the failure to reject the null hypothesis is a comparatively low circulation of RSV, especially in the second season. In this regard, the number of SARS-CoV-2 cases was about twice (139 vs. 75) that of RSV and the detection rate of the former virus was significantly higher in the ILI group.
In the WHO’s pilot surveillance program [24], compared to ILI (defined as measured fever ≥ 38 °C plus cough), the use of an extended ARI case definition (respiratory infection with shortness of breath or cough or sore throat or coryza) increased the number of RSV detections. Korsten et al. [7] showed that only 2 of 36 (6%) RSV-positive had fever ≥ 38 °C and the sensitivity of the WHO ILI definition for RSV was as low as 11%. Our findings are in line with these observations, although the sensitivity of the WHO ILI case definition in our study was higher (28.4%). On the other hand, the ECDC ILI criteria are much less stringent than the WHO ones and are therefore more sensitive to capturing RSV cases. For instance, among other systemic signs and symptoms, the ECDC ILI case definition includes malaise, which is highly non-specific. In this study, malaise was the most common systemic symptom reported by 92% of adults. This fact implies that the ECDC ILI case definition for RSV is much more sensitive than the WHO ILI criteria and approaches the ARI case definition; in other words, the ECDC ILI case definition is a subset of the ECDC ARI case definition. Indeed, in our study, all adults in the ILI group met the ECDC ARI criteria, and most (92.6%) patients in the ARI group also met the ECDC ILI criteria. A retrospective Portuguese study [28] found that the sensitivity of ECDC ILI and ARI-like (cough or sore throat or shortness of breath, while data on coryza were unavailable) case definitions for RSV yielded almost identical estimates of 81.1% and 82.1%, respectively. However, as sensitivity and specificity are inversely related, highly sensitive case definitions would be associated with a modest specificity. In the above-mentioned Portuguese study [28], in fact, the specificity of ECDC ILI and ARI-like case definitions for RSV were only 21.1% and 20.8%, respectively, while the AUC was 0.51 for both, suggesting no discrimination. While highly sensitive definitions, such as ARI, are useful for surveillance purposes as they can capture most symptomatic cases, their low specificity may be disadvantageous in other circumstances. For instance, in future cohort or test-negative case–control studies on RSV vaccine effectiveness, poorly specific case definitions would lead to an increase in the required sample size (especially during seasons characterized by a low RSV circulation), and consequently increased workload and costs. There is also some evidence [29] that RSV vaccination attenuates the severity of symptoms in breakthrough infections. In this latter case, the use of more sensitive case definitions would capture more vaccinated RSV cases with mild symptoms, which would potentially result in lower vaccine effectiveness [30]. Based on the clinical presentation of RSV episodes, we proposed the RSV-ARI case definition denoted as “ARI with wheezing and/or productive cough and/or rhonchi and/or dyspnea”, which performed better than the established alternatives and showed a balanced accuracy of 61.4%. This proposal is partially aligned with the alternative RSV-specific criteria identified in a prospective European study [7]. They found that infectious productive cough (i.e., respiratory infection with cough and production of sputum/phlegm) was a discriminating symptom among 36 RSV-positive community-dwelling older adults and was present in 94% of cases. Sensitivity, specificity, and AUC of the productive cough alone were 94%, 30%, and 0.62, respectively. Yet, a similar performance was observed for influenza (89%, 31%, and 0.60, respectively). To differentiate between RSV and influenza, the authors added a criterion of “no fever” (i.e., infectious productive cough without measured fever of feeling feverish); this determined an increase in specificity (55%) but also a substantial decrease in sensitivity (61%) [7]. In our study, which was based on a larger number of RSV cases, the prevalence of productive cough with sputum was lower (74.7% vs. 94%) and adding other qualifiers (wheezing, rhonchi, and dyspnea) improved the discriminating power. We also believe that excluding fever or feverishness, which were present in two-thirds of RSV cases, from the case definition would skew the surveyed population to mild infections.
RSV detection in this study was based solely on nasopharyngeal swabs, the "gold standard" and highest-yield sample type for respiratory viruses [31]. While some research [32] suggests that adding sputum and saliva samples significantly increases RSV detection in hospitalized patients compared to nasopharyngeal swabs alone, utilizing these alternative specimen types presents greater challenges in primary care than in a hospital setting.
Our second main finding confirms that RSV is responsible for a measurable burden in older adults. Being prospective, it contributes to existing literature, as most available studies are retrospective or model-based. The overall complication rate was 30.7%, while 9.3% of adults with RSV developed pneumonia and the case-hospitalization rate was 2.7%. Available research on the consequences of community-acquired RSV in older adults has yielded widely varying estimates. In a retrospective cohort of US adults aged ≥ 60 years [33], 9.5% of RSV-positive individuals developed pneumonia, while 11.9% were hospitalized within 4 weeks. The hospitalization rate in outpatients aged ≥ 50 years was lower (8.3%) [34]. Another US cohort among Medicare-insured US adults (≥ 60 years) [35] found a much higher complication rate of 47.9%, of which pneumonia (24.0%), chronic respiratory disease (23.6%), and hypoxia/dyspnea (22.0%) were the most common. A German claim-based retrospective study reported [36] that 86% of adults aged ≥ 60 years and 79% of those aged 50–59 years developed ≥ 1 complication following a confirmed RSV episode. Using three different US databases, Landi et al. [37] assessed the 28-day all-cause hospitalization rate following an outpatient diagnosis of RSV; these rates were 4.5–6.2% among adults aged ≥ 18 years and 7.9–10.3% among older adults ≥ 65 years. On the other hand, in a prospective European cohort of community-dwelling adults aged ≥ 50 years [38], the overall complication rate was 17.4% and no RSV-related hospitalizations or deaths occurred. In a small (n = 152, of which 33 were RSV-positive) prospective Italian study [39], the overall complication rate was 21% and one (3.0%) patient required hospitalization. In a nutshell, while severe RSV outcomes in older adults are undeniable, complication and hospitalization estimates are typically higher in retrospective compared to prospective studies, possibly because retrospective cohorts are skewed to more severe cases.
In this study, 57% of RSV-positive outpatients were prescribed antibiotics, which is very close to a previous Italian estimate of 52% [39]. The available literature, however, is highly heterogeneous and reports prescription rates between 6% [40] and 77% [33]. While regional variability in antibiotic prescription is well known [41], prescribing practices may be driven by the availability of microbiological diagnosis to prescribers. In our study, as well as in the earlier Italian study reporting a comparable rate [39], the RT-PCR output was shared with all GPs. However, there was a gap of 1–3 days between the swab collection and RT-PCR diagnosis sent to GPs, and several RSV-positive adults were prescribed an antibiotic before the test results were known. One solution to reduce the inappropriate prescription of antibiotics could be the use of RADTs, but in this study the latter was associated with a high false negative rate performing relatively well only for swabs with high viral loads. This finding is in line with previous observations [42] indicating that most adults have a comparatively low RSV load and consequently RADTs are of limited value. The limited sensitivity of RADTs can be circumvented by point-of-care multiplex RT-PCR kits able to provide results in 1 h or less, but these assays are much more expensive and there is no compelling evidence of their effectiveness in reducing antibiotic prescriptions [43]. Moreover, even RT-PCR assays produced by the same manufacturer may have different limits of detection. Therefore, when comparing (or combining) results of RSV surveillance studies, attention should be paid to the diagnostic accuracy of the tests used in those studies.
Most available RSV cost-of-illness studies comes from the US [44], whose healthcare system is different from Italy and other European countries. One recent US study showed [45] that 4% of hospitalized RSV cases determined 94% of all direct medical costs incurred by older adults. In our study, two hospitalized cases contributed about 60% of all direct medical costs. Few available European data [44] approach our cost estimates. In particular, a prospective study from the United Kingdom, Netherlands, and Belgium reported [46] that the mean (median) direct cost (Euros 2020) of a medically attended RSV episode in primary care was € 75.22 (€ 65.31). This appears to be very close to the outpatient costs of € 74.40 (€ 63.00) estimated in this study. Regarding Italy, available economic and resource use data [47] are confined to hospitalized older adults followed for 12 months post-hospitalization and therefore no comparison with our findings can be made.
This study suffers from some important limitations. As we discussed earlier, unequal cluster sizes and comparatively low circulation of RSV reduced statistical power. Furthermore, we were not able to consider the design effect due to a largely uncertain intra-class correlation, which is essential for sample size determination in cluster randomized studies. Second, owing to a relatively small number of GP clusters [48], there was some covariance imbalance between patients in the ARI and ILI groups. It is, however, unlikely that this imbalance had a significant impact on the primary endpoint, as after adjustment the estimated ORs changed by no more than 1.5%. Third, the proposed RSV-ARI case definition derived from a comparatively low number of RSV cases was not validated externally, and it is unclear whether it performs well in individuals aged < 50 years or in settings not specifically using the ECDC ARI/ILI criteria for surveillance. Fourth, the estimated costs, especially from the societal perspective, are likely underestimated since direct non-medical costs incurred by patients and their families (e.g., transportation, caregiving expenses) were not considered. As the follow-up period was limited to 30 days, long-term consequences and the associated increase in healthcare resource use (e.g., arising from deterioration of underlying conditions) were not captured. Furthermore, as laboratory testing for respiratory pathogens is not currently a routine diagnostics procedure, the costs of RT-PCR and RADT were not included. If they were, the total costs would increase significantly (e.g., the current tariff of the multiplex RT-PCR for respiratory viruses is € 142.20). Finally, the study was conducted in a limited geographic area and during seasons characterized by low-to-moderate circulation of RSV. It therefore remains unclear whether our findings are generalizable to other realities and high-incidence periods. In our opinion, however, Liguria is a good model for studying the epidemiology of respiratory viruses and the effectiveness of preventive measures, as it is currently the “oldest” European region with a mean population age of 52.3 years [49].
Conclusions
This cluster randomized study showed that ARI-based case definitions may capture more RSV cases than ILI-based criteria, and therefore better quantify the true burden of RSV in older adults. Among the available fever-based ILI criteria historically developed for influenza surveillance, the ECDC ILI case definition was associated with the lowest RSV underestimation because the presence of fever or feverishness is not the only qualifying systemic symptom. While being highly sensitive, ARI case definitions have poor specificity, which may be improved by adding further qualifiers. The proposed RSV-ARI case definition significantly improved specificity without causing heavy loss in sensitivity, but warrants further independent evaluation. Our granular evaluation of the natural history of community-acquired RSV adds to the body of evidence, confirming a substantial clinical and socioeconomic burden of RSV in older adults. We believe that the data generated will be useful for the upcoming health technology assessment of different preventive and therapeutic strategies.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank the participants of the study. The authors are also grateful to Medicoop Liguria and all participating general practitioners (Francesca Adami, Fabio Amato, Susanna Baffi, Dino Bacca, Gemma Baldari, Pierfrancesco Barettoni, Federico Bianchi, Martina Boatti, Sara Bolloli, Elisa Cannavino, Marco Cardini, Stefano Caroggio, Andrea Carraro, Emmanuela Corradino, Simona Delucchi, Antonio Farese, Marco Fighetti, Ruggero Filimbaia, Giorgio Franzone, Elena Gaggero, Elisa Gaggero, Marco Grosso, Valeria Longhini, Alessandro Lombardi, Valeria Messina, Rossana Migliorini, Beatrice Musolino, Gianluca Nattero, Roberta Occhipinti, Davide Orlandi, Paola Orsi, Marco Polese, Dario Ponzoletti, Luca Properzi, Lorenza Quaranta, Federica Roncallo, Tommaso Vaccaro).
Author Contributions
Conceptualization: Alexander Domnich, Francesco Lapi, Alen Marijam, and Giancarlo Icardi. Methodology: Alexander Domnich, Francesco Lapi, Andrea Orsi, Marta Vicentini, and Anna Puggina. Software: Piero Luigi Lai. Validation: Carlo-Simone Trombetta. Formal analysis: Alexander Domnich and Alessio Signori. Investigation: Luca Pestarino, Pier Claudio Brasesco, Carlo-Simone Trombetta, Giada Garzillo, Federica Stefanelli, Valentina Ricucci, and Bianca Bruzzone. Resources: Andrea Orsi, Donatella Panatto, and Giancarlo Icardi. Data curation: Piero Luigi Lai and Carlo-Simone Trombetta. Writing – original draft preparation: Alexander Domnich. Writing – review and editing: Francesco Lapi, Andrea Orsi, Marta Vicentini, Anna Puggina, Alen Marijam, Maria João Fonseca, Elisa Turriani, Donatella Panatto and Giancarlo Icardi. Supervision: Giancarlo Icardi. Project administration: Alexander Domnich and Donatella Panatto. Funding acquisition: Alexander Domnich and Giancarlo Icardi. All listed authors met the criteria for authorship set forth by the International Committee for Medical Journal Editors (ICMJE).
Funding
The study (ID 221526) was funded by GlaxoSmithKline Biologicals SA, which was involved in all study aspects except for enrollment of subjects, laboratory and data analyses. GlaxoSmithKline Biologicals SA also took charge of all costs associated with the development and publication of this manuscript, including the journal’s Rapid Service Fee.
Data Availability
Raw data generated during the current study are not publicly available due to CIRI-IT internal policy and local ethical restrictions. Subject to certain criteria, conditions, and exceptions, and upon a reasonable request from qualified researchers, CIRI-IT may provide access to the data.
Declarations
Conflict of Interest
Alexander Domnich provided consultancies and/or received speaker fees from CSL Seqirus, GSK, Sanofi, and SD Biosensor. Francesco Lapi provided consultancies in protocol preparation for epidemiological studies and data analyses for CSL Seqirus, Moderna, AstraZeneca, Pfizer, and Viatris. Marta Vicentini, Anna Puggina, Alen Marijam, Maria João Fonseca and Elisa Turriani are employees of the GSK group of companies and may hold shares in the GSK group of companies as part of their employee remuneration. Andrea Orsi provided consultancies and/or received speaker fees from CSL Seqirus, Moderna, Novavax, and SD Biosensor. Donatella Panatto provided consultancies for Pfizer and CSL Seqirus and received grants for conducting observational studies from Sanofi, Pfizer, GSK, and Viatris. Giancarlo Icardi provided consultancies and/or received grants for conducting experimental and/or observational studies from GSK, Sanofi, MSD, CSL Seqirus, and Pfizer. Other authors declare no conflicts of interest.
Ethical Approval
This study was conducted in accordance with the Helsinki Declaration of 1964 and its later amendments. The study protocol was approved by the Liguria Region Ethics Committee (#13354 of 18 September 2023). Written informed consent was obtained from all patients.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Savic M, Penders Y, Shi T, Branche A, Pirçon JY. Respiratory syncytial virus disease burden in adults aged 60 years and older in high-income countries: a systematic literature review and meta-analysis. Influenza Other Respir Viruses. 2023;17(1): e13031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Britton A, Roper LE, Kotton CN, Hutton DW, Fleming-Dutra KE, Godfrey M, et al. Use of respiratory syncytial virus vaccines in adults aged ≥60 years: updated recommendations of the Advisory Committee on Immunization Practices—United States, 2024. MMWR Morb Mortal Wkly Rep. 2024;73(32):696–702. [DOI] [PubMed] [Google Scholar]
- 3.Rozenbaum MH, Begier E, Kurosky SK, Whelan J, Bem D, Pouwels KB, et al. Incidence of respiratory syncytial virus infection in older adults: limitations of current data. Infect Dis Ther. 2023;12(6):1487–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kenmoe S, Nair H. The disease burden of respiratory syncytial virus in older adults. Curr Opin Infect Dis. 2024;37(2):129–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Domnich A, Calabrò GE. Epidemiology and burden of respiratory syncytial virus in Italian adults: a systematic review and meta-analysis. PLoS ONE. 2024;19(3): e0297608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.European Centre for Disease Prevention and Control (ECDC). European respiratory virus surveillance summary. Available from: https://erviss.org/. Accessed 22 Apr 2025.
- 7.Korsten K, Adriaenssens N, Coenen S, Butler CC, Verheij TJM, Bont LJ, et al. World Health Organization influenza-like illness underestimates the burden of respiratory syncytial virus infection in community-dwelling older adults. J Infect Dis. 2022;226(Suppl 1):S71–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davis W, Duque J, Huang QS, Olson N, Grant CC, Newbern EC, et al. Sensitivity and specificity of surveillance case definitions in detection of influenza and respiratory syncytial virus among hospitalized patients, New Zealand, 2012–2016. J Infect. 2022;84(2):216–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.European Medicines Agency (EMA). Arexvy. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/arexvy. Accessed 27 June 2025.
- 10.Domnich A, Lapi F, Orsi A, Lai PL, Pestarino L, Brasesco PC, et al. Exploring case definitions and the natural history of respiratory syncytial virus in adult outpatients: first-season results of the RESPIRA-50 study. J Infect Public Health. 2025;18(3): 102653. [DOI] [PubMed] [Google Scholar]
- 11.World Health Organization and the European Centre for Disease Prevention and Control. Operational considerations for respiratory virus surveillance in Europe; 2022. Available from: https://www.ecdc.europa.eu/sites/default/files/documents/Operational-considerations-respiratory-virus-surveillance-euro-2022.pdf. Accessed 22 Apr 2025.
- 12.Bella A, Giombini E, Urdiales AM, Caraglia A, Maraglino F, Facchini M, et al. La sorveglianza integrata dei virus respiratori RespiVirNet in Italia: i risultati della stagione 2023–2024. Boll Epidemiol Naz. 2024;5(4):19–27. [Google Scholar]
- 13.Gimferrer L, Andrés C, Rando A, Piñana M, Codina MG, Martin MDC, et al. Evaluation of Seegene Allplex Respiratory Panel 1 kit for the detection of influenza virus and human respiratory syncytial virus. J Clin Virol. 2018;105:31–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.World Health Organization (WHO). WHO surveillance case definitions for ILI and SARI; 2014. Available from: https://www.who.int/teams/global-influenza-programme/surveillance-and-monitoring/case-definitions-for-ili-and-sari. Accessed 22 Apr 2025.
- 15.Centers for Disease Control and Prevention (CDC). U.S. influenza surveillance: Purpose and methods. Available from: https://www.cdc.gov/fluview/overview/index.html. Accessed 22 Apr 2025.
- 16.Italian Medicines Agency. Lists of Class A and Class H medicinal products. Available from: https://www.aifa.gov.it/en/liste-farmaci-a-h. Accessed 22 Apr 2025.
- 17.Garattini L, Castelnuovo E, Lanzeni D, Viscarra C. Gruppo di studio DYSCO VISITE DV. Durata e costo delle visite in medicina generale: il progetto DYSCO. Farmeconomia Health Econ Ther Pathways. 2003;4:109. [Google Scholar]
- 18.Italian Institute of Statistics. Rivaluta. Available from: https://rivaluta.istat.it/. Accessed 22 Apr 2025.
- 19.Astengo M, Paganino C, Amicizia D, Trucchi C, Tassinari F, Sticchi C, et al. Economic burden of pneumococcal disease in individuals aged 15 years and older in the Liguria region of Italy. Vaccines. 2021;9(12): 1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ligurian Regional Health Service. Regional catalog of outpatient healthcare services. Available from: https://www.alisa.liguria.it/components/com_publiccompetitions/includes/download.php?id=9196:delibera-343-anno-2024.pdf. Accessed 22 Apr 2025.
- 21.Ligurian Regional Health Service. Liguria Region tariffs for inpatient services. Available from: https://www.alisa.liguria.it/component/publiccompetitions/document/4147:tariffe-delle-prestazioni-di-assistenza-ospedaliera-per-acuti-per-tipo-di-ricovero-4147.html?Itemid=101. Accessed 22 Apr 2025.
- 22.Italian Institute of Statistics. Structure of wages in Italy, year 2022. Available from: https://www.istat.it/wp-content/uploads/2025/01/REPORT_STRUTTURA_RETRIBUZIONI_2022.pdf. Accessed 22 Apr 2025.
- 23.Kahan BC, Li F, Copas AJ, Harhay MO. Estimands in cluster-randomized trials: choosing analyses that answer the right question. Int J Epidemiol. 2023;52(1):107–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.World Health Organization (WHO). RSV surveillance case definitions. Available from: https://www.who.int/teams/global-influenza-programme/global-respiratory-syncytial-virus-surveillance/case-definitions. Accessed 22 Apr 2025.
- 25.Lauer SA, Kleinman KP, Reich NG. The effect of cluster size variability on statistical power in cluster-randomized trials. PLoS ONE. 2015;10(4): e0119074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Souty C, Turbelin C, Blanchon T, Hanslik T, Le Strat Y, Boëlle PY. Improving disease incidence estimates in primary care surveillance systems. Popul Health Metr. 2014;12:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Souty C, Boëlle PY. Improving incidence estimation in practice-based sentinel surveillance networks using spatial variation in general practitioner density. BMC Med Res Methodol. 2016;16(1):156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sáez-López E, Pechirra P, Costa I, Cristóvão P, Conde P, Machado A, et al. Performance of surveillance case definitions for respiratory syncytial virus infections through the sentinel influenza surveillance system, Portugal, 2010 to 2018. Euro Surveill. 2019;24(45): 1900140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Curran D, Matthews S, Cabrera ES, Pérez SN, Breva LP, Rämet M, et al. The respiratory syncytial virus prefusion F protein vaccine attenuates the severity of respiratory syncytial virus-associated disease in breakthrough infections in adults ≥60 years of age. Influenza Other Respir Viruses. 2024;18(2): e13236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maurel M, Mazagatos C, Goerlitz L, Oroszi B, Hooiveld M, Machado A, et al. Exploring the effect of clinical case definitions on influenza vaccine effectiveness estimation at primary care level: results from the end-of-season 2022–23 VEBIS multicentre study in Europe. Vaccine. 2024;42(16):3547–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee RA, Herigon JC, Benedetti A, Pollock NR, Denkinger CM. Performance of saliva, oropharyngeal swabs, and nasal swabs for SARS-CoV-2 molecular detection: a systematic review and meta-analysis. J Clin Microbiol. 2021;59(5):e02881-e2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ramirez J, Carrico R, Wilde A, Junkins A, Furmanek S, Chandler T, et al. Diagnosis of respiratory syncytial virus in adults substantially increases when adding sputum, saliva, and serology testing to nasopharyngeal swab RT-PCR. Infect Dis Ther. 2023;12(6):1593–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Belongia EA, King JP, Kieke BA, Pluta J, Al-Hilli A, Meece JK, et al. Clinical features, severity, and incidence of RSV illness during 12 consecutive seasons in a community cohort of adults ≥60 years old. Open Forum Infect Dis. 2018;5(12):ofy316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sundaram ME, Meece JK, Sifakis F, Gasser RA Jr, Belongia EA. Medically attended respiratory syncytial virus infections in adults aged ≥ 50 years: clinical characteristics and outcomes. Clin Infect Dis. 2014;58(3):342–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.DeMartino JK, Lafeuille MH, Emond B, Rossi C, Wang J, Liu S, et al. Respiratory syncytial virus-related complications and healthcare costs among a Medicare-insured population in the United States. Open Forum Infect Dis. 2023;10(5):ofad203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marijic P, Kliemt R, Krammer M, Kolb N, Last T, Ambrosch A, et al. Costs and complications of respiratory syncytial virus and acute respiratory infections in the adult population: analysis of a German claims database. Pharmacoecon Open. 2025;9(3):445–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Landi SN, Garofalo DC, Reimbaeva M, Scott AM, Jiang L, Cappell K, et al. Hospitalization following outpatient diagnosis of respiratory syncytial virus in adults. JAMA Netw Open. 2024;7(11): e2446010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Narejos Pérez S, Ramón Torrell JM, Põder A, Leroux-Roels I, Pérez-Breva L, Steenackers K, et al. Respiratory syncytial virus disease burden in community-dwelling and long-term care facility older adults in Europe and the United States: a prospective study. Open Forum Infect Dis. 2023;10(4):ofad111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bracaloni S, Esposito E, Scarpaci M, Cosci T, Casini B, Chiovelli F, et al. RSV disease burden in older adults: an Italian multiregion pilot study of acute respiratory infections in primary care setting, winter season 2022–2023. Influenza Other Respir Viruses. 2024;18(12): e70049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Korsten K, Adriaenssens N, Coenen S, Butler C, Ravanfar B, Rutter H, et al. Burden of respiratory syncytial virus infection in community-dwelling older adults in Europe (RESCEU): an international prospective cohort study. Eur Respir J. 2021;57(4):2002688. [DOI] [PubMed] [Google Scholar]
- 41.Mölstad S, Lundborg CS, Karlsson AK, Cars O. Antibiotic prescription rates vary markedly between 13 European countries. Scand J Infect Dis. 2002;34(5):366–71. [DOI] [PubMed] [Google Scholar]
- 42.Nuwer R. Better awareness of RSV in older adults is needed to fight a growing burden. Nature. 2023;621(7980):S58–9. [DOI] [PubMed] [Google Scholar]
- 43.Domnich A, Massaro E, Icardi G, Orsi A. Multiplex molecular assays for the laboratory-based and point-of-care diagnosis of infections caused by seasonal influenza, COVID-19, and RSV. Expert Rev Mol Diagn. 2024;24(11):997–1008. [DOI] [PubMed] [Google Scholar]
- 44.Grace M, Colosia A, Wolowacz S, Panozzo C, Ghaswalla P. Economic burden of respiratory syncytial virus infection in adults: a systematic literature review. J Med Econ. 2023;26(1):742–59. [DOI] [PubMed] [Google Scholar]
- 45.Carrico J, Hicks KA, Wilson E, Panozzo CA, Ghaswalla P. The annual economic burden of respiratory syncytial virus in adults in the United States. J Infect Dis. 2024;230(2):e342–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mao Z, Li X, Korsten K, Bont L, Butler C, Wildenbeest J, et al. Economic burden and health-related quality of life of respiratory syncytial virus and influenza infection in European community-dwelling older adults. J Infect Dis. 2022;226(Suppl 1):S87-94. [DOI] [PubMed] [Google Scholar]
- 47.Puggina A, Dovizio M, Domnich A, Marijam A, Veronesi C, Rizzo C, et al. Healthcare resource utilization and economic outcomes of RSV-hospitalized patients aged ≥ 60 years: a retrospective cohort study. Diseases. 2025;13(3):68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moerbeek M, van Schie S. How large are the consequences of covariate imbalance in cluster randomized trials: a simulation study with a continuous outcome and a binary covariate at the cluster level. BMC Med Res Methodol. 2016;16:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Eurostat. Population structure indicators by NUTS 2 region. Population structure indicators by NUTS 2 region. Available from: https://ec.europa.eu/eurostat/databrowser/view/DEMO_R_PJANIND2/default/table?lang=en. Accessed 22 Apr 2025.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Raw data generated during the current study are not publicly available due to CIRI-IT internal policy and local ethical restrictions. Subject to certain criteria, conditions, and exceptions, and upon a reasonable request from qualified researchers, CIRI-IT may provide access to the data.