A microbe becomes a pathogen by evading—to a greater or lesser extent—the immune defenses of its host. The mechanisms that have evolved for so doing are legion in number and striking in their diversity and ingenuity. They involve mechanisms to evade recognition by—and mechanisms to subvert the effector mechanisms of—both the innate and the adaptive immune response (see Table 1). All classes of infectious agents from the smallest viruses to helminth worms use these techniques albeit in somewhat different ways. An almost ubiquitous target for these subversion mechanisms is the complement system and it is with the subversion of complement by smallpox virus that the report in this issue of PNAS by Rosengard et al. (1) is concerned.
Table 1.
Mechanisms for microbial subversion of the immune response
| • To avoid recognition: |
| Antigenic variation: Flu; HIV; trypanosomes; plasmodia |
| Acquiring a host coat: Worms & retroviruses |
| • To avoid the effector mechanisms: |
| Subvert CTLs: Produce HLA molecule homologues |
| Subvert Fc-function: Produce Fc-R homologues |
| Subvert complement: Produce complement control protein homologues |
| Subvert apoptosis |
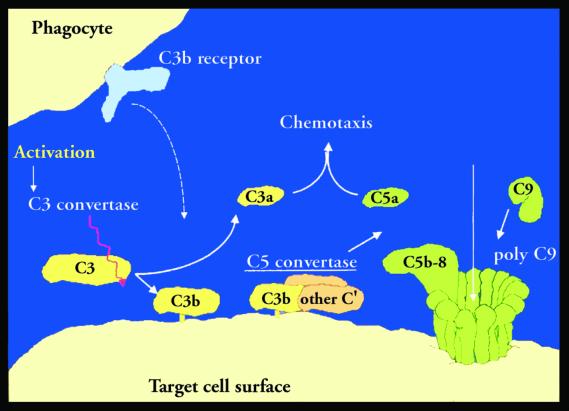
An extremely simplified view of the complement system is shown in Fig. 1. There are two principal biologically important steps. The first is brought about by the activation (and subsequent fixation) of C3 by C3-converting enzymes. This step generates the bound C3 fragments (C3b and iC3b) that interact with complement receptors on inflammatory cells and the activation fragments C3a and C5a, which also activate inflammatory cells. The second important step is the formation of the membrane attack complex, which inserts a channel into cell membranes.
Figure 1.
Simplified view of the complement system.
Regulation of the C3 activation step is achieved by a series of proteins of highly homologous structure that are composed of oligomers of the so-called “short consensus repeat” (SCR) protein domain and are coded in the genome at a single large locus on chromosome 1q. They are known as complement control proteins (CCPs). These include secreted plasma proteins (Factor H and C4-binding protein) and membrane-bound proteins [Membrane cofactor protein (CD46), the complement receptors 1(CD35) and 2(CD21), and decay accelerating factor (CD55)], the last named having a glycolipid anchor. They have two principal activities. They act as cofactors for the proteolytic cleavage by Factor I of C3b to iC3b and (for CR1) from iC3b to C3c and C3dg; and they have “decay accelerating” activity on the C3-converting enzymes of both the classical and the alternative complement pathways. Some bacteria can bind Factor H and C4-binding protein to their surfaces where they act as a form of membrane-bound CCP. Viruses have at their disposal the protein-synthesizing apparatus of the host and can make use of this to synthesize homologues of the host CCPs to protect themselves from host complement.
Vaccinia virus has a CCP composed of four SCRs, which is known as VCP, and has been extensively studied particularly by Kotwal and Moss and their colleagues (2, 3). Rosengard et al. (1) have now performed the ingenious experiment of synthesizing the corresponding protein of variola—the smallpox virus—and investigating how its properties differ from that of the vaccinia protein. The synthesis was achieved by introducing into the vaccinia protein the 11 amino acid substitutions that are all that distinguish the vaccinia and the variola complement control proteins (which show amino acid sequence homology of around 95%). The variola complement control protein—called SPICE by the authors—shows important differences in its properties from its vaccinia counterpart. It shows a substantially greater degree of specificity for human complement and is 100-fold more active in inactivating human C3b.
These findings are of considerable interest in at least two separate contexts. They will help in determining the structural basis of the specificity that the CCPs have for different mammalian C3b. The 11 amino acid differences are distributed through 3 of the 4 SCRs, which suggests that the binding may be quite complex.
The findings are also of interest in considering the pathogenesis of small pox and the high specificity that small pox infection has for humans. It is an attractive hypothesis to believe that, as the authors do, the high efficiency of inactivation of human complement may be responsible for the restriction of variola host range to humans. For a variety of reasons this hypothesis cannot be tested experimentally. There is (it is to be hoped) no smallpox virus available for experimentation to see whether replacing SPICE with VCP would confer on variola the power to infect other species. Nor can one envisage introducing SPICE into vaccinia to see whether this increases its pathogenicity to humans.
Indeed, the idea that bioterrorists might be tempted to attempt such an experiment has been suggested as a reason for considering it imprudent to publish observations of this nature. This line of argument should, however, be resisted except in all but exceptional circumstances. In the present sad state of the world it is clearly undesirable to publish “cook books” on how to manufacture novel weapons, whether nuclear or chemical or biological. This is, however, not the same as restricting the publication of valuable scientific information, especially when this is information that can be exploited for beneficial ends, merely because it might give a potential terrorist ideas. In the present case, it is very unlikely that vaccinia virus carrying SPICE in place of VCP would approach the pathogenicity of either genuine variola or, indeed, of some other poxviruses that can infect humans. Furthermore, the information on SPICE could plausibly be used to enhance immunity to smallpox by immunization with SPICE. Although this possibility cannot now be tested with smallpox in humans, analogous experiments could be done with other poxviruses to see whether immunization with a viral CCP with good specificity for a particular host complement affects virulence and host range.
It is, however, quite unlikely that interference with complement alone will be sufficient to determine the pathogenicity of a poxvirus. It is well known from studies of spontaneous mutations in humans that it is not complement deficiencies but defects in cellular immunity that give rise to exaggerated reactions to vaccinia. The cellular immune response dominates the effect of the host response on the course of these viral infections. Recruitment of the complement system can, however, influence the cell-mediated response (4) and it is likely that this is the pathway with which the viral complement control proteins can interfere.
The experiments of Rosengard et al. (1) are an illustration of how the exploitation of microbial genomics can allow studies of the biology of viruses that cannot themselves be studied safely—or at all. The work is far more likely to stimulate advances in vaccinology or viral therapy than it is to threaten biosecurity.
Footnotes
See companion article on page 8808.
References
- 1.Rosengard A M, Liu Y, Nie Z, Jimenez R. Proc Natl Acad Sci USA. 2002;99:8808–8813. doi: 10.1073/pnas.112220499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kotwal G J, Moss B. Nature (London) 1988;335:176–178. doi: 10.1038/335176a0. [DOI] [PubMed] [Google Scholar]
- 3.Murthy K H, Smith S A, Ganesh V K, Judge K W, Mullin N, Barlow P N, Ogata C M, Kotwal G J. Cell. 2001;104:301–311. doi: 10.1016/s0092-8674(01)00214-8. [DOI] [PubMed] [Google Scholar]
- 4.Tsuji R F, Kawikova I, Ramabhadran R, Akahira-Azuma M, Taub D, Hugli T E, Gerard C, Askenase P W. J Immunol. 2000;165:1588–1598. doi: 10.4049/jimmunol.165.3.1588. [DOI] [PubMed] [Google Scholar]