Abstract
Disruption of spermatogenesis found in azoospermia and oligozoospermia is thought to be of primarily genetic origin. Sl/Sld mutant mice offer a model system in which lack of transmembrane type c-kit ligand (KL2) expression on the somatic Sertoli cell surface results in disruption of spermatogenesis. We investigated the ability of adeno-, adeno-associated-, retro-, and lentiviral vectors to transduce Sertoli cells and found that transduction with either adeno- or lentiviral vectors led to reporter gene expression for more than 2 mo after testicular tubule injection. Because adenoviral vectors showed toxicity, lentiviral vectors were used to express the c-kit ligand in Sl/Sld Sertoli cells. Restoration of spermatogenesis was observed in all recipient testes. Furthermore, the sperm collected from recipient testes were able to generate normal pups after intracytoplasmic sperm injection. None of the offspring carried the transgene, suggesting the inability of lentiviral vectors to infect spermatogenic cells in vivo. We propose that lentiviral vectors can be used for gene therapy of male infertility without the risk of germ-line transmission.
Worldwide, up to 20% of couples are infertile. Approximately 30–50% of human infertility is attributable to male infertility, 70–90% of which arises from disrupted or impaired spermatogenesis with a clinical outcome of azoo- or oligospermia (1, 2). Spermatogenesis takes place within the testicular seminiferous tubules that are composed of germ cells, Sertoli cells, and peritubular cells lining the tubule. This process involves a mitotic germ cell proliferation, meiosis, and morphological changes of haploid germ cells to mature spermatozoa. Successful spermatogenesis, however, requires participation outside tubules, interstitial cells such as Lydig cells that produce androgen, macrophages, mast cells, and lymphoid cells. This intricate network of interactions is regulated by many growth factors and hormones and depends on intimate contact between germ cells and somatic Sertoli cells (3). Sertoli cells play a seminal role in normal spermatogenesis by providing not only structural support but also a variety of growth factors required for differentiation and proliferation of germ cells. In addition, inter-Sertoli cellular tight junctions confer the “blood–testis barrier” and partition the testis into an intratubular compartment (4, 5). Therefore, Sertoli cell dysfunction will impair spermatogenesis and result in male infertility. Currently there are no effective modalities to correct such genetic defects in animals or humans (6, 7).
To investigate whether viral-vector mediated gene transfer can be used for correcting Sertoli cell dysfunction, we used Sl/Sld mutant male mice that have Sertoli cell dysfunction and are consequently infertile. The Sl locus (steel) encodes both the soluble and membrane-bound forms of c-kit ligand (KL) that bind to the c-kit tyrosine kinase receptor synthesized by the W locus (dominant white spotting). In the testis, the c-kit receptor is expressed on the germ cells from spermatogonia, whereas the c-kit ligand is produced by the somatic Sertoli cells, and interaction between these two factors is essential for spermatogonial cell proliferation. The Sl mutation deletes the entire Sl gene, whereas the Sld mutation deletes the transmembrane and intracellular domain, thereby generating only soluble forms of the c-kit ligand (KL1) and leading to azoospermia. Additionally, seminiferous tubules of these mice are virtually devoid of germ cells, a clinical condition known as Sertoli cell only syndrome (8). We therefore asked whether spermatogenesis can be restored in Sl/Sld mice by transducing Sertoli cells with lentiviral vectors generating functional the c-kit ligand, KL2. We report that not only is spermatogenesis restored in all recipient testes, but also spermatozoa collected from transduced testes were able to generate normal pups by microinsemination.
Materials and Methods
Preparation of Viral Vector Plasmids and Viral Vector Production.
The AdV-CMV-nlslacZ (nls, nuclear localization signal; AdV, adenoviral vector; CMV, cytomegalovirus) vector and the AAV-CMV-lacZ (AAV, adeno-associated virus type 2) vector were prepared as described (9, 10). In this study, we constructed the pRV-CMV-nlslacZ plasmid in the murine leukemia virus-based retroviral vector, pCLNCX (11) by replacing a fragment containing the neo resistant cassette and the CMV promoter with CMV-nlslacZ-WPRE (WPRE, woodchuck hepatitis virus posttranscriptional regulatory element). We constructed the pLV-CMV-nlslacZ and pLV-CMV-lacZ (LV, lentiviral vector) plasmids in the HIV-based self-inactivating lentiviral vector, pRRLsin-hPGK-EGFP (EGFP, enhanced green fluorescent protein) by replacing the hPGK-EGFP fragment with the CMV-nlslacZ or CMV-lacZ fragment, respectively (12). To construct pLV-CMV-KL2, we replaced the nlslacZ fragment of pLV-CMV-nlslacZ with the KL2 fragment amplified by PCR from the testicular cDNA library with primers (KL2for: 5′-ctggatccgccaccatgaagaagacacaaacttgg-3′) and (KL2rev: 5′-ctgtcgactattacacctcttgaaattctctc-3′). Vesicular stomatitis virus G envelope protein-pseudotyped retroviral and lentiviral vectors were generated as described (12).
Recipient Mice and Viral Vector Injection Procedure.
In the first experiment, we injected recombinant adenoviral, AAV, retroviral, and lentiviral vectors into male C57BL/6 × DBA2 F1 hybrid mice (B6D2F1) at 6 wk of age. Approximately 10 μl of viral vector solution containing 0.04% trypan blue was injected into seminiferous tubules via the efferent ductules. Viral vector was injected into the right testis and the left testis remained as control. In the second experiment, B6D2F1 male mice were injected with the LV-CMV-nlslacZ vector into both testes at 6 wk of age and housed with 3 B6D2F1 females for 3 mo. In the third experiment, ≈3 μl of LV-CMV-KL2 vector was injected into Sl/Sld male mice at 3–6 wk of age. In all experiments, recipient mice were anesthetized by i.p. injection of Avertin before the operation. The seminiferous tubule injection was performed according to the method described (13), and more than 70% of seminiferous tubules were filled with solution as determined by trypan blue.
5-Bromo-4-chloro-3-indolyl β-d-galactoside (X-Gal) Staining and Histological Analysis.
Testes were removed from the recipient male mice and fixed in PBS containing 4% paraformaldehyde (PFA) for 2 h before X-Gal staining. Whole testes or frozen sections (20 μm) were washed three times with PBS and stained in X-Gal staining solution [20 mM K3Fe(CN)6/20 mM K4Fe(CN)6⋅3H2O/2 mM MgCl2/1 mg/ml of X-Gal in PBS, pH 7.4] at 37° for 12 h. For histological analysis, frozen sections (6 μm) were prepared from testes fixed in 4% PFA for 12 h and stained with hematoxylin and eosin.
PCR and Southern Blot Analysis.
To determine the germ line transmission of viral vector DNA, genomic DNA from the tail tip of offspring derived from male mouse transduced lentiviral vector was analyzed by PCR. We performed 30 cycles of PCR with lacZfor (5′-gcgccgaaatcccgaatctc-3′) and lacZrev (5′-tctccaggtagcgaaagcca-3′) primers to detect the lacZ transgene. Genomic DNA from heterozygous ROSA26, which carry a single copy of the lacZ transgene per diploid genome, was used as positive control (14). For KL2 transgene detection, we performed 30 cycles of PCR with KL2for and KL2rev primers described above. Wild-type genomic DNA was spiked with exogenous pLV-CMV-KL2 plasmid DNA to prepare a positive control, which carries a single copy of the KL2 gene per diploid genome. For Southern blot analysis, genomic DNA (10 μg) was digested with EcoRI, separated by electrophoresis in a 0.8% agarose gel, and blotted onto a nylon membrane before hybridization with the 32P-random-prime-labeled 434-bp NciI-BglI fragment of Sl cDNA.
Western Blot Analysis and Immunohistochemistry.
Lysates from 293T cells and testicular cells were subjected to SDS/PAGE under reducing conditions. Proteins transferred to the poly(vinylidene difluoride) (PVDF) membrane were probed by mouse anti-KL antibody (Santa Cruz Biotechnology), rabbit anti-calmegin antiserum (15), and rabbit anti-actin antibody (Sigma). Frozen sections (6 μm) prepared as above were also subjected to immunohistochemistry with rabbit anti-calmegin antiserum.
Microinsemination.
Spermatogenic cells were collected from recipient Sl/Sld male mice and frozen as described (16). The cells were thawed before injection, and then elongated spermatid and testicular spermatozoon were injected into wild-type B6D2F1 unfertilized oocytes. Treated eggs were cultivated for 48 h and the four- to eight-cell stage embryos were transferred to pseudopregnant females.
Results
Comparison of Transduction Efficiencies.
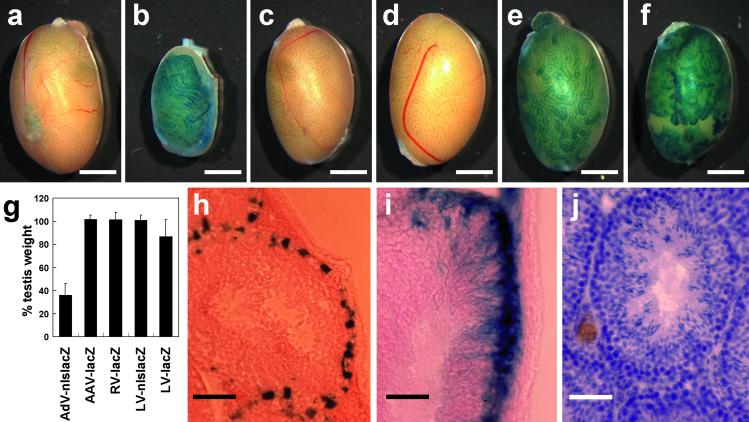
To study the efficiency and efficacy of gene transfer and expression in vivo in testis, we used recombinant adenoviral, AAV, retroviral, and lentiviral vectors containing the Escherichia coli lacZ gene with or without nls as a reporter under the control of the CMV promoter. Because it is difficult to equalize the concentration of different kinds of viral vectors, we prepared viral vector solutions as follows: AdV-CMV-nlslacZ at 1 × 1010 adenoviral particles/ml, AAV-CMV-lacZ at 1 × 1011 infectious unit (IU)/ml, RV-CMV-nlslacZ at 1 × 1010 IU/ml, LV-CMV-nlslacZ at 1 × 1010 IU/ml, and LV-CMV-lacZ at 1 × 1010 IU/ml. When we injected approximately 10 μl of viral vector solution containing 0.04% of trypan blue into seminiferous tubules through efferent ducts, more than 70% of seminiferous tubules were filled. We injected viral vectors into the right testis, and the left was used as a control. At 2 mo after injection of viral vectors, treated male mice were killed, and whole testes were stained with X-Gal (Fig. 1 a–f). In the testes injected with adenoviral vector, strong expression of the nlslacZ gene was observed (Fig. 1b); however, the testicular weight was significantly decreased (compare Fig. 1 a and b, approximately one-third of the control; see Fig. 1g). With the exception of adenoviral vectors, little effect on testicular weight could be detected by the other three vectors (Fig. 1g). Nuclear localized expression of nlslacZ in Sertoli cells and impaired spermatogenesis were confirmed in the frozen sections (data not shown). The data obtained with AdV-CMV-nlslacZ were consistent with the results reported before (17, 18). In the testes injected with AAV or retroviral vector, no cells stained with X-Gal could be found in either whole testis (Fig. 1 c and d) or frozen section (data not shown). Testes transduced with lentiviral vector expressed the nlslacZ and lacZ gene without impairing spermatogenesis (Fig. 1 e and f). When we stained frozen sections from lentiviral vector transduced testis, nlsLacZ expression along the basement membrane in clusters of seminiferous tubules (Fig. 1h) and cytoplasmic lacZ expression throughout the basement to the lumen could be observed (Fig. 1i). These staining patterns are characteristic of Sertoli cells, and reporter gene expression was observed over a period of 6 mo (data not shown). Morphologically normal spermatogenesis was observed in the testis injected with LV-CMV-nlslacZ (Fig. 1j).
Figure 1.
Transduction performance of viral vectors in testicular cells. We injected viral vectors into the seminiferous tubule of B6D2F1 wild-type testis and analyzed at 2 mo after injection. Whole testis was fixed and stained with X-Gal to observe lacZ reporter gene expression. (a) Wild-type control. (b) Adenoviral vector (AdV-CMV-nlslacZ). (c) Adeno-associated viral vector (AAV-CMV-lacZ). (d) Retroviral vector (RV-CMV-lacZ). (e) Lentiviral vector (LV-CMV-nlslacZ). (f) Lentiviral vector (LV-CMV-lacZ). (g) The values are percent testes weight (injected right testis/uninjected left testis, mean ± SD) at 2 mo after injection. AdV-CMV-nlslacZ; 36.1 ± 10.2 (n = 6), AAV-CMV-lacZ; 102.0 ± 3.5 (n = 3), RV-CMV-lacZ; 101.6 ± 6.4 (n = 4), LV-CMV-nlslacZ; 100.9 ± 4.6 (n = 7), LV-CMV-lacZ; 86.8 ± 14.8 (n = 4). (h and i) X-Gal-stained frozen testis cross sections. Nuclear localization and cytoplasmic diffusion of β-galactosidase in Sertoli cells were observed with LV-CMV-nlslacZ (h) and LV-CMV-lacZ (i), respectively. (j) Morphologically normal spermatogenesis was observed in the testis injected with LV-CMV-nlslacZ. [Bar = 2 mm (a–f) and 50 μm (h–j).]
Lack of Germ Line Transmission.
To investigate germline transmission of lentiviral DNA after intratesticular tubule injection, wild-type B6D2F1 male mice injected with approximately 10 μl of LV-CMV-nlslacZ lentiviral vector into both testes (1 × 108 IU/testis) were housed with wild-type B6D2F1 females for 3 mo. Recipient male mice could copulate within a few days after viral injection, and normal numbers of pups were born over a period of 3 mo (average litter size, 8.5 ± 1.7). Numbers of pups obtained from copulation in the first, second, and third months were 129, 125, and 136, respectively. A total of 390 pups obtained from 5 male mice were analyzed by PCR amplification of the lacZ gene, but none carried the lentiviral DNA (Table 1). As a positive control, we used heterozygous ROSA26 “knock-in” mouse DNA, which carries a single copy of the lacZ gene (14). We therefore conclude that transduction of testis by lentiviral vectors does not lead to germ line transmission.
Table 1.
Assessment of germ line transmission of LV-CMV-nlslacZ
| Recipient mouse no. | Percent tubules containing X-Gal stained cells | No. of litters | Total no. of pups | No. of pups carrying viral vector gene |
|---|---|---|---|---|
| 160 L | 77.7 (136/175) | 10 | 80 | 0 |
| R | 74.0 (108/146) | |||
| 161 L | 82.8 (144/174) | 10 | 89 | 0 |
| R | 63.3 (100/158) | |||
| 162 L | 73.2 (120/164) | 9 | 80 | 0 |
| R | 72.6 (106/146) | |||
| 163 L | 71.4 (100/140) | 8 | 64 | 0 |
| R | 52.8 (67/127) | |||
| 164 L | 76.1 (105/138) | 9 | 77 | 0 |
| R | 58.3 (77/132) | |||
| 70.2 ± 9.3 (575/847) | 46 | 390 | 0 |
Transduction of Sertoli Cells.
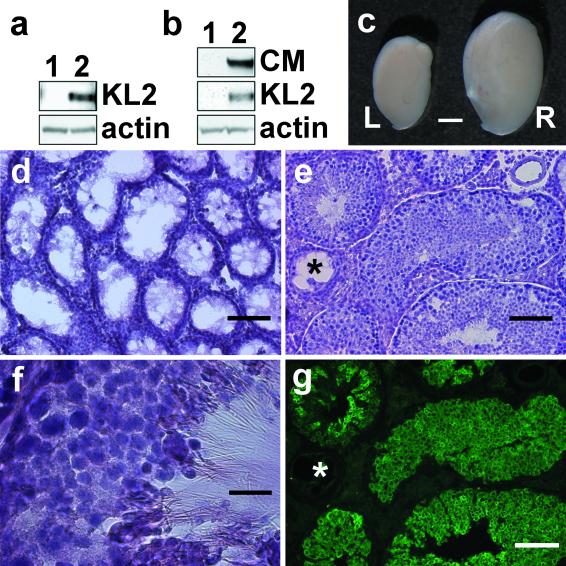
Because lentiviral vectors can efficiently transduce Sertoli cells, we next asked whether they can be used to correct the defect in Sl/Sld mutant mice. In these mice, Sertoli cell dysfunction results in disrupted spermatogenesis (8). However, some spermatogonia are present in a few seminiferous tubules in Sl/Sld mutant testis, and these germ cells can proliferate and differentiate into functional mature spermatozoa when transplanted into seminiferous tubules containing functional Sertoli cells (19). Because Sl/Sld mutant Sertoli cells express the soluble (KL1) but not the transmembrane form (KL2) of the c-kit ligand (20), we generated the LV-CMV-KL2 lentiviral vector to express the KL2 gene under the control of the CMV promoter. Recombinant KL2 protein can be detected as a 31-kDa protein (21) in transduced 293 T cells (Fig. 2a, lane 2). We injected ≈3 μl of the LV-CMV-KL2 lentiviral vector (3 × 107 IU) into testicular tubules of Sl/Sld mutant mice. At various time periods after treatment, we killed Sl/Sld mice and analyzed the restoration of spermatogenesis (Table 2 and Fig. 2 b–g). By Western blot analysis, expression of recombinant KL2 (31 kDa) and calmegin (93 kDa) was detected only in the treated testis (Fig. 2b, lane 2). Because calmegin is expressed only in differentiating germ cells (pachytene spermatocyte to spermatid) but not in Sertoli cells (22, 23), the existence of calmegin protein suggested restoration of spermatogenesis. Within 1 mo of LV-CMV-KL2 lentiviral vector injection, treated testis became almost twice the size of untreated testis and sustained the increased weight over a period of at least 5 mo (Table 2 and Fig. 2c). Histological analysis revealed morphologically normal spermatogenesis in nine of nine recipient testes (Fig. 2 d–g). Empty seminiferous tubules can be seen in the mutant mice (Fig. 2d), whereas in the transduced mice, a considerable number of filled seminiferous tubules can be detected (Fig. 2e). When we immunostained sections with anti-calmegin antibody, approximately 48% of seminiferous tubules in the treated testis contained the differentiating germ cells (Table 2 and Fig. 2g), whereas seminiferous tubules in wild-type and Sl/Sld contained 100% (100/100) and 0% (0/100), respectively. Mature spermatozoa (Fig. 2f) were also found in the seminiferous tubule lumen in six of six testes observed at 2 mo or later. A small amount of live and motile spermatozoa could be squeezed out from the epididymis of recipients 116 and 117.
Figure 2.
Restoration of spermatogenesis in Sl/Sld mutant testis transduced by lentiviral-mediated KL2 gene transfer. We prepared lentiviral vector, LV-CMV-KL2, to express membrane type c-kit receptor ligand (KL2). Western blot analysis after lentiviral gene transfer in vitro (a) and in vivo (b). (a) Recombinant KL2 expression was detected in 293T cells at 3 days after transduction (lane 1, untransduced; lane 2, transduced). (b) Recombinant KL2 expression and calmegin (CM) expression were detected in recipient Sl/Sld mutant testis at 4 wk after treatment (lane 1, Sl/Sld testis; lane 2, Sl/Sld testis injected with LV-CMV-KL2). (c) Macroscopic appearance of Sl/Sld testis (L) and LV-CMV-KL2 injected Sl/Sld testis (R). (d–g) Histological analysis of seminiferous tubules of Sl/Sld recipient mouse testes at 2 mo after LV-CMV-KL2 injection. Frozen sections were stained with hematoxylin/eosin (d–f). Sl/Sld mutant testis with disrupted spermatogenesis (d). The morphologically normal spermatogenesis occurred in the recipient Sl/Sld testis (e and f). The serial section of e was immunostained with anti-calmegin antibody (g). Asterisk indicates the seminiferous tubule in which spermatogenesis was not restored. [Bars = 2 mm (c), 100 μm (d, e, and g), and 20 μm (f).]
Table 2.
Restoration of spermatogenesis in SI/SId male mice after LV-CMV-KL2 injection
| Recipient SI/SId mouse no. | Days after operation | Uninjected left testis weight, mg | Injected right testis weight, mg | Weight change, % | Percent positive tubules stained with anti-calmegin antibody |
|---|---|---|---|---|---|
| 118 | 28 | 12 | 25 | 208 | 53.7 (65/121) |
| 119 | 28 | 10 | 21 | 210 | ND* |
| 115 | 56 | 10 | 30 | 300 | 58.1 (61/105) |
| 100 | 63 | 10 | 17 | 170 | 37.5 (60/160) |
| 204 | 85 | 11 | 27 | 245 | 42.5 (68/160) |
| 205 | 85 | 10 | 29 | 290 | 47.5 (57/120) |
| 99 | 107 | 12 | 32 | 267 | ND† |
| 116 | 142 | 11 | 22 | 200 | 51.6 (66/128) |
| 117 | 142 | 11 | 17 | 155 | 41.3 (43/104) |
| Average | 10.8 ± 0.8 | 24.4 ± 5.5 | 227 ± 51 | 47.5 ± 7.4 (420/898) |
Testes were subjected to Western blot analysis.
Testicular cells were collected and subjected to intracytoplasmic sperm injection.
Restoration of Spermatogenesis and Fertility.
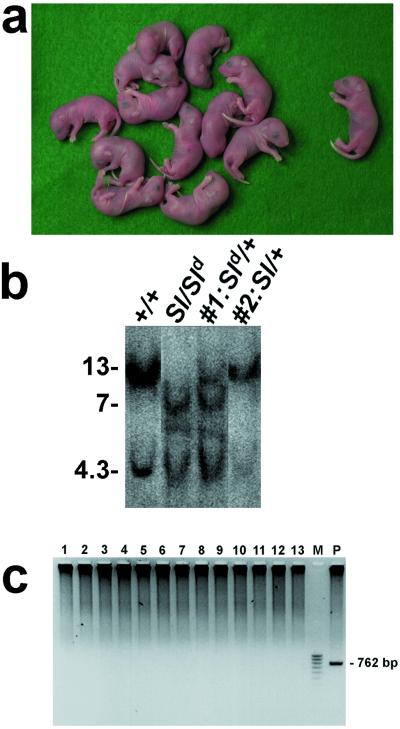
Although spermatogenesis can be restored in Sl/Sld mutant testis by lentiviral gene transfer, we could not collect a sufficient number of spermatozoa from the epididymis for normal in vitro fertilization (≈1 × 104 spermatozoa are required). Therefore we resorted to intracytoplasmic injection of spermatogenic cells, a widespread microinsemination technique for male infertility treatment (24). We collected late-stage spermatid and spermatozoon from LV-CMV-KL2 treated Sl/Sld mutant testis and injected them into unfertilized wild-type B6D2F1 oocytes (Table 3). A total of 90 oocytes were injected, and 59 of them developed normally in vitro. We obtained 13 progeny by transferring these embryos into pseudopregnant females (Fig. 3a). To confirm the inheritance of both Sl deletion and Sld mutation, we analyzed the genomic DNA from the weanlings by Southern blot analysis (Fig. 3b). The wild-type locus (+) shows 13- and 4.3-kb bands. The Sl locus gives no bands, whereas the Sld locus gives 7- and 4.3-kb bands, respectively. Sl and Sld haplotypes of the donor germ cells were segregated during meiosis and transmitted to offspring. Among the 13 offspring, 6 mice carried the wild-type and Sld locus, and 7 mice carried the wild-type and Sl locus, as shown in #1 (Sld/+) and #2 (Sl/+), respectively (Fig. 3b). None proved to be transgenic for lentiviral vector carrying KL2 gene (Fig. 3c), thus establishing that, whereas there is correction of the Sertoli cells, there is no germline transmission of the transgene.
Table 3.
Production of offspring from LV-CMV-KL2-treated Sl/SId male mouse by microinsemination
| Type of testicular cells | No. of eggs treated | No. of embryos transferred* | No. of pups | No. of pups carrying viral vector gene |
|---|---|---|---|---|
| Spermatid | 72 | 43 | 12 | 0 |
| Spermatozoon | 18 | 16 | 1 | 0 |
| Total | 90 | 59 | 13 | 0 |
Embryos were cultured for 48 hr and transferred into a pseudopregnant female.
Figure 3.
Offspring obtained from LV-CMV-KL2-treated Sl/Sld male mice. Testicular cells were collected from recipient Sl/Sld testis injected with LV-CMV-KL2 lentiviral vector and subjected to microinsemination. (a) Newborn pups obtained from recipient Sl/Sld male mice by lentiviral gene transfer followed by the intracytoplasmic sperm injection technique. (Left) Twelve pups were from elongated spermatid, and (Right) one pup was from a testicular spermatozoon. (b) Southern blot analysis of EcoRI-digested DNAs from wild-type, Sl/Sld, and two pups shown in a. The wild-type locus produces 13- and 4.3-kbp bands, the Sl locus produces no bands, and the Sld locus produces 7- and 4.3-kbp bands. Pups #1 and #2 were diagnosed as Sld/+ and Sl/+, respectively. (c) PCR amplification of 762 bp of the KL2 cDNA fragment showed no germ line transmission of the lentiviral vector gene. M, 100-bp ladder marker; P, wild-type genomic DNA containing one copy of the viral gene was used as positive control.
Discussion
Currently four main viral vectors are available for in vivo gene delivery (25). We injected all four viral vectors into seminiferous tubules and investigated their transduction performances in Sertoli cells. Adenoviral vectors displayed strong expression but had deleterious effects on spermatogenesis. Additionally, the lack of long-term expression because of immune problems will be a handicap. However, Kanatsu-Shinohara et al., using lower viral titers, succeeded in expressing the transgene over 3 mo with decreased transduction efficiency (26). No transduction of seminiferous tubules could be observed with AAV vectors (Fig. 1c). When we injected vesicular stomatitis virus G envelope protein-pseudotyped retroviral and lentiviral vectors, efficient gene delivery into Sertoli cells was obtained only with the lentiviral but not with the retroviral vector. This result is consistent with the fact that retroviral vectors require recipient cell division during their transduction, whereas lentiviral vectors can transduce nondividing cells (25, 27).
Do Lentiviral Vectors Transduce Germ Cells?
This is an important concern for the clinical application of gene therapy. At least 90% of the cells in testis are germ cells, and 95% of them are differentiating cells (28). No evidence of spermatogenic cell transduction was observed with the lentiviral vector by histological and mating experiments (Tables 1 and 3). In contrast, Nagano et al. (29) have reported that vesicular stomatitis virus G envelope protein pseudotyped retroviral vectors can transduce male mouse germ cells in vitro, and some of the offspring carry the transgene. Because Sertoli cells cover spermatogenic cells and remove particles in the seminiferous tubule lumen by active endocytosis (30), the viral vector may not reach spermatogenic cells in vivo. The blood–testis barrier, because of inter-Sertoli cell tight junction, is also helpful in preventing viral infection of spermatogonia, which are localized at the peripheral part of the seminiferous tubule. Although no animal studies have shown germ line transmission after in vivo viral vector treatment (31–34), further large-scale and detailed studies may be required, because it is difficult to totally rule out the possibility of germ line transmission.
Rescue of Infertility.
Although transduction by lentiviral vectors containing KL2 restored spermatogenesis, and testicular spermatozoa were able to generate normal pups by the assisted fertilization technique (Fig. 3a), we could not obtain pups by normal mating. Possible explanations are: (i) the number of Sertoli cells transduced by the lentiviral vector might not be enough; (ii) the mating period was not long enough, because 5.5–8 months were required to acquire in vivo fertility when Sl/Sld spermatogonia cells were transplanted into adult W/Wv testis (19); and (iii) the CMV promoter was used in this study, whereas endogenous c-kit ligand expression is tightly regulated by differentiation and stage-specific manner (21). Because recovery of in vivo fertility was reported in W/Wv male mice with the restoration of spermatogenesis in 66 and 25% of seminiferous tubules in a pair of testes (19), our values (48% of tubules) are comparable. Intracytoplasmic injection of spermatogenic cells is a powerful assisted fertilization technology applicable for oligo- or azoospermia (24). In fertility clinics, the epididymal spermatozoon, the testicular spermatozoon, and spermatid are routinely used. However, this microinsemination technique is not beneficial for male infertility patients with totally disrupted spermatogenesis (24). Therefore, spermatozoa obtained by restoration of spermatogenesis offer a viable approach.
We propose that lentiviral vector-mediated gene transfer to Sertoli cells is not only useful to study spermatogenesis but also may become an option for male infertility treatment. No human equivalent to the Steel mutation has been documented, but recent studies revealed genes such as claudin-11, protein C inhibitor, and tyro-3 families are essential for Sertoli cell function to support normal spermatogenesis (35–37). Lack of these genes resulted in disrupted spermatogenesis similar to the Sertoli cell only syndrome. Gene therapy approaches, along with germ cell transplantation and assisted fertilization techniques, offer a potential novel treatment for male infertility.
Acknowledgments
We thank Dr. M. Weitzman (The Salk Institute) for the AAV2 vector and Dr. Y. Matsui (Osaka Medical Center for Maternal and Child Health, Osaka) for Sl cDNA. M.I. is supported by the Wayne and Gladys Valley Foundation, V. Tergaonkar is supported by a fellowship from the Leukemia and Lymphoma Society. I.M.V. is an American Cancer Society Professor of Molecular Biology and is supported by grants from The National Institutes of Health, the March of Dimes, the Wayne and Gladys Valley Foundation, and the H. N. and Frances C. Berger Foundation.
Abbreviations
- AdV
adenovirus
- CMV
cytomegalovirus
- X-Gal
5-bromo-4-chloro-3-indolyl β-d-galactoside
- nls
nuclear localization signal
- IU
infectious unit
References
- 1.Hull M G, Glazener C M, Kelly N J, Conway D I, Foster P A, Hinton R A, Coulson C, Lambert P A, Watt E M, Desai K M. Br Med J. 1985;291:1693–1697. doi: 10.1136/bmj.291.6510.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Greenberg S H, Lipshultz L I, Wein A J. J Urol. 1978;119:507–510. doi: 10.1016/s0022-5347(17)57531-x. [DOI] [PubMed] [Google Scholar]
- 3.Sharpe R. In: Physiology of Reproduction. Knobil E, Neill J D, editors. New York: Raven; 1994. pp. 1363–1434. [Google Scholar]
- 4.Ross M H. Am J Anat. 1977;148:49–55. doi: 10.1002/aja.1001480105. [DOI] [PubMed] [Google Scholar]
- 5.Streilein J W. Science. 1995;270:1158–1159. doi: 10.1126/science.270.5239.1158. [DOI] [PubMed] [Google Scholar]
- 6.Russell L D, Steinberger A. Biol Reprod. 1989;41:571–577. doi: 10.1095/biolreprod41.4.571. [DOI] [PubMed] [Google Scholar]
- 7.Kierszenbaum A L. Endocr Rev. 1994;15:116–134. doi: 10.1210/edrv-15-1-116. [DOI] [PubMed] [Google Scholar]
- 8. Besmer, P., Manova, K., Duttlinger, R., Huang, E. J., Packer, A., Gyssler, C. & Bachvarova, R. F. (1993) Dev. Suppl. 125–137. [PubMed]
- 9.Miyake S, Makimura M, Kanegae Y, Harada S, Sato Y, Takamori K, Tokuda C, Saito I. Proc Natl Acad Sci USA. 1996;93:1320–1324. doi: 10.1073/pnas.93.3.1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grifman M, Trepel M, Speece P, Gilbert L B, Arap W, Pasqualini R, Weitzman M D. Mol Ther. 2001;3:964–975. doi: 10.1006/mthe.2001.0345. [DOI] [PubMed] [Google Scholar]
- 11.Naviaux R K, Costanzi E, Haas M, Verma I M. J Virol. 1996;70:5701–5705. doi: 10.1128/jvi.70.8.5701-5705.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Follenzi A, Ailles L E, Bakovic S, Geuna M, Naldini L. Nat Genet. 2000;25:217–222. doi: 10.1038/76095. [DOI] [PubMed] [Google Scholar]
- 13.Ogawa T, Arechaga J M, Avarbock M R, Brinster R L. Int J Dev Biol. 1997;41:111–122. [PubMed] [Google Scholar]
- 14.Soriano P. Nat Genet. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- 15.Ikawa M, Nakanishi T, Yamada S, Wada I, Kominami K, Tanaka H, Nozaki M, Nishimune Y, Okabe M. Dev Biol. 2001;240:254–261. doi: 10.1006/dbio.2001.0462. [DOI] [PubMed] [Google Scholar]
- 16.Ogura A, Matsuda J, Asano T, Suzuki O, Yanagimachi R. J Assist Reprod Genet. 1996;13:431–434. doi: 10.1007/BF02066177. [DOI] [PubMed] [Google Scholar]
- 17.Blanchard K T, Boekelheide K. Biol Reprod. 1997;56:495–500. doi: 10.1095/biolreprod56.2.495. [DOI] [PubMed] [Google Scholar]
- 18.Scobey M, Bertera S, Somers J, Watkins S, Zeleznik A, Walker W. Endocrinology. 2001;142:948–954. doi: 10.1210/endo.142.2.7948. [DOI] [PubMed] [Google Scholar]
- 19.Ogawa T, Dobrinski I, Avarbock M R, Brinster R L. Nat Med. 2000;6:29–34. doi: 10.1038/71496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Flanagan J G, Chan D C, Leder P. Cell. 1991;64:1025–1035. doi: 10.1016/0092-8674(91)90326-t. [DOI] [PubMed] [Google Scholar]
- 21.Vincent S, Segretain D, Nishikawa S, Nishikawa S I, Sage J, Cuzin F, Rassoulzadegan M. Development (Cambridge, UK) 1998;125:4585–4593. doi: 10.1242/dev.125.22.4585. [DOI] [PubMed] [Google Scholar]
- 22.Watanabe D, Sawada K, Koshimizu U, Kagawa T, Nishimune Y. Mol Reprod Dev. 1992;33:307–312. doi: 10.1002/mrd.1080330312. [DOI] [PubMed] [Google Scholar]
- 23.Ikawa M, Wada I, Kominami K, Watanabe D, Toshimori K, Nishimune Y, Okabe M. Nature (London) 1997;387:607–611. doi: 10.1038/42484. [DOI] [PubMed] [Google Scholar]
- 24.Silber S J. Hum Reprod. 1998;13 Suppl. 1:208–218. doi: 10.1093/humrep/13.suppl_1.208. [DOI] [PubMed] [Google Scholar]
- 25.Somia N, Verma I M. Nat Rev Genet. 2000;1:91–99. doi: 10.1038/35038533. [DOI] [PubMed] [Google Scholar]
- 26.Kanatsu-Shinohara M, Ogura A, Ikegawa M, Inoue K, Ogonuki N, Tashiro K, Toyokuni S, Honjo T, Shinohara T. Proc Natl Acad Sci USA. 2002;99:1383–1388. doi: 10.1073/pnas.022646399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Naldini L, Verma I M. Adv Virus Res. 2000;55:599–609. doi: 10.1016/s0065-3527(00)55020-9. [DOI] [PubMed] [Google Scholar]
- 28.Huckins C. Anat Rec. 1971;169:533–557. doi: 10.1002/ar.1091690306. [DOI] [PubMed] [Google Scholar]
- 29.Nagano M, Brinster C J, Orwig K E, Ryu B Y, Avarbock M R, Brinster R L. Proc Natl Acad Sci USA. 2001;98:13090–13095. doi: 10.1073/pnas.231473498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morales C, Clement Y, Hermo L. Am J Anat. 1985;173:203–217. doi: 10.1002/aja.1001730305. [DOI] [PubMed] [Google Scholar]
- 31.Ye X, Gao G P, Pabin C, Raper S E, Wilson J M. Hum Gene Ther. 1998;9:2135–2142. doi: 10.1089/hum.1998.9.14-2135. [DOI] [PubMed] [Google Scholar]
- 32.Roehl H H, Leibbrandt M E, Greengard J S, Kamantigue E, Glass W G, Giedlin M, Boekelheide K, Johnson D E, Jolly D J, Sajjadi N C. Hum Gene Ther. 2000;11:2529–2540. doi: 10.1089/10430340050208000. [DOI] [PubMed] [Google Scholar]
- 33.Hall S J, Bar-Chama N, Ta S, Gordon J W. Hum Gene Ther. 2000;11:1705–1712. doi: 10.1089/10430340050111359. [DOI] [PubMed] [Google Scholar]
- 34.Nagano M, Shinohara T, Avarbock M R, Brinster R L. FEBS Lett. 2000;475:7–10. doi: 10.1016/s0014-5793(00)01606-9. [DOI] [PubMed] [Google Scholar]
- 35.Gow A, Southwood C M, Li J S, Pariali M, Riordan G P, Brodie S E, Danias J, Bronstein J M, Kachar B, Lazzarini R A. Cell. 1999;99:649–659. doi: 10.1016/s0092-8674(00)81553-6. [DOI] [PubMed] [Google Scholar]
- 36.Uhrin P, Dewerchin M, Hilpert M, Chrenek P, Schofer C, Zechmeister-Machhart M, Kronke G, Vales A, Carmeliet P, Binder B R, Geiger M. J Clin Invest. 2000;106:1531–1539. doi: 10.1172/JCI10768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lu Q, Gore M, Zhang Q, Camenisch T, Boast S, Casagranda F, Lai C, Skinner M K, Klein R, Matsushima G K, et al. Nature (London) 1999;398:723–728. doi: 10.1038/19554. [DOI] [PubMed] [Google Scholar]