Abstract
Background
Subclinical mastitis, caused by many pathogens including Staphylococcus aureus (S. aureus) and Staphylococcus chromogenes (S. chromogenes), presents a major challenge to the dairy industry due to its associated economic losses and poor milk quality. The molecular regulatory mechanisms, including the role of small nucleolar RNAs (snoRNAs), of the host response to mastitis pathogens remain unclear. Therefore, this study investigated snoRNA expression and potential roles during subclinical mastitis. Milk somatic cells from cows with naturally occurring S. aureus (n = 14) and S. chromogenes (n = 3) subclinical mastitis, and healthy cows (n = 4) were subjected to transcriptome sequencing and bioinformatics analyses.
Results
We identified 255 expressed snoRNAs including 21 differentially expressed (DE) in S. aureus-positive cows and 20 DE in S. chromogenes-positive cows. Prediction of ribosomal RNA (rRNA) modification sites found several 18S rRNA and 28S rRNA modification (pseudouridylation and 2′-O-methylation) target sites essential for ribosome function for DE snoRNAs, such as SNORA79 (18S-1319, 28S-3001), SNORA1 (18S-1496, 28S-1747), suggesting their roles in translation and immune modulation during subclinical mastitis. Correlation analysis identified DE snoRNAs-mRNAs (from the same samples) pairs with majority of the correlated mRNAs (e.g., CXCL8, IL6R, IL2, IL1R, IL18R1, STAT3, NFKB2, MYD88, VEGFA, and CD40) having immune related functions. Functional enrichment of correlated genes of snoRNAs for S. aureus-positive group (regulation of defense/immune response, leukocyte differentiation, response to cytokine, NF-κB signaling pathway, JAK-STAT signaling pathway etc.) and S. chromogenes-positive group (e.g., regulation of defense response, response to cytokine, regulation of immune response, NF-κB signaling pathway, TNF signaling pathway, and JAK-STAT signaling pathway) revealed involvement in immune and inflammatory processes. Some functional terms were common to both pathogens (e.g., NF-κB, JAK-STAT signaling, immune system processes) and suggest common regulatory mechanisms used by both pathogens to contain infection. Furthermore, snoRNA-mRNA network construction identified 7 key (hub) snoRNAs each for S. aureus-positive group (SNORA66, novelsnoRNA_26_14905 (also denoted as novelSnoRNA_86), SNORD107, SNORA1, SNORA63, SNORA79, SNORA76) and S. chromogenes-positive group (SNORD18, SNORA79, SNORA46, U2-19, SNORA66, SNORD37, SNORD49) that correlated with the most protein coding genes (|r| > 0.9; ≥ 30 mRNAs). Functional enrichment of correlated genes of hub snoRNAs reveals their involvement in immune related functions (75% of enriched terms) and metabolic processes (20% of enriched terms).
Conclusion
These data suggest potential regulatory roles for the DE snoRNAs and in particular, the 14 hub snoRNAs during subclinical mastitis. This study presents the first evidence linking snoRNAs to bovine subclinical mastitis and offers new insights into the molecular mechanisms underlying subclinical mastitis caused by S. aureus and S. chromogenes.
Supplementary Information
The online version contains supplementary material available at 10.1186/s40104-025-01230-9.
Keywords: Dairy cattle, Ribosomal RNA, S. aureus, S. chromogenes, SnoRNA, Subclinical mastitis
Introduction
Mastitis, characterized by inflammation of the mammary gland, is the most prevalent and costly disease in dairy livestock globally [1]. Depending on the causal pathogen and degree of inflammation, mastitis can develop as a clinical, or subclinical infection. Subclinical mastitis caused by various pathogens, including Staphylococcus aureus (S. aureus) and Staphylococcus chromogens (S. chromogenes), lacks visible symptoms, making it difficult to detect, thus leading to substantial economic losses. Staphylococcus aureus as the most important mastitis pathogen has the ability to evade host immune cells, continually multiply within the host cells, and thereby causing frequent infections. On the other hand, in dairy production, S. chromogens is often considered a minor pathogen [2, 3]. Despite this, S. chromogenes is frequently isolated from cows with subclinical and clinical mastitis [4] and it is the most prevalent non-aureus staphylococci (NAS) in Canadian mastitis cases regardless of somatic cell count (SCC) levels [5]. Similar to S. aureus, S. chromogenes can penetrate the teat canal, attach to cells, and form biofilms, preventing removal during milking [4]. Thus, like S. aureus, S. chromogenes is becoming more prevalent and problematic.
High rate of S. chromogenes intra-mammary infection in udder quarters with high SCCs, persistent cases of subclinical mastitis, and more pronounced signs of clinical mastitis has been reported [4]. Given the high prevalence of subclinical mastitis and its impact on dairy production, including the devastating impacts of the development of antimicrobial/antibiotic resistance on animal, human, and environmental health, it is crucial to deepen our understanding of the molecular regulation and genetic basis of mastitis. This knowledge is essential for developing effective strategies for managing udder health, such as development of therapeutic solutions and genomic selection for mastitis resistance.
The epigenome including non-coding RNA (ncRNA) regulation, plays a crucial role in regulating gene expression and has been shown to regulate various livestock traits, including mammary gland health and production [6–9]. In particular, crucial roles of ncRNAs, including microRNAs (miRNAs), long ncRNAs (lncRNAs) and circular RNAs (circRNAs) have been reported for animal diseases [7, 10–12]. Among these, miRNAs and lncRNAs are the most extensively studied, while others like small nucleolar RNAs (snoRNAs) have received less attention despite being comparable to miRNAs and lncRNAs in abundance.
SnoRNAs, primarily located in the eukaryotic cell nuclei, are 60–300 nucleotides long and contain conserved structural elements. They are classified into two main types based on their structural components and biological roles: H/ACA snoRNAs (SNORA), which guide the pseudouridylation of nucleotides, and C/D box snoRNAs (SNORD), which are responsible for 2'-O-methylation. Recent data suggest that snoRNAs also modify small nuclear RNAs, transfer RNAs, and even messenger RNAs, and are associated with the pathogenesis of various malignancies, indicating their potential as prognostic biomarkers [13]. Studies have also confirmed that snoRNAs can perform miRNA-like functions by regulating post-transcriptional gene expression [14, 15]. In 2008, Ender and colleagues reported small RNAs derived from snoRNA ACA45 with miRNA-like functions by targeting CDC2L6 [16]. Also, Ono et al. [15] showed that while the C/D box snoRNA HBII-180C contains a 2′-O-methylation site, it also has an M-box region that allows it to function as a miRNA, inhibiting the mRNA and protein expression of target genes [15]. Huang et al. [17] and Asano-Inami et al. [18] found that SNORD50A and SNORD49 inhibited mRNA 3' processing by blocking the interaction between Fip1 (a component of cleavage and polyadenylation specificity factor) and the polyA site. This inhibition led to alterations in alternative polyadenylation (APA) profiles and transcript levels of various genes. Decreased expression of snoRNAs, including SNORD49, reduces the interaction between UBAP2L and G3BP1, thereby suppressing stress granules formation [18]. McCann et al. revealed that SNORA1 was significantly downregulated during stem cell differentiation, suggesting a potential role in the differentiation process [19]. The regulation of SNORA1 and other H/ACA snoRNAs may be crucial for proper cell differentiation, as these snoRNAs are involved in the modification and processing of rRNA, which is essential for ribosome biogenesis and overall cellular function. Taken together, these data highlight the importance of snoRNAs in cellular differentiation and their potential implications in developmental biology and disease states. Despite the demonstrated functions of snoRNAs in human diseases, their regulatory roles in livestock diseases including mastitis are yet to be elucidated. This study therefore aims to characterize snoRNA expression and their potential functions during bovine subclinical mastitis. To the best of our understanding, this is the first study to characterize snoRNA expression and their potential regulatory roles in bovine S. chromogenes and S. aureus subclinical mastitis.
Materials and methods
Ethics approval and consent for participation
The animal use procedures for this study adhered to the Canadian Council on Animal Care guidelines. Ethical approval was granted by the Animal Care and Ethics Committee of Agriculture and Agri-Food Canada (CIPA #570).
Farms and animal selection
Five commercial dairy farms in Quebec operating the tie-stall management system were selected for this study. Monthly dairy herd improvement (DHI) records (milk production) of lactating cows were monitored over a period of six months. The DHI records generated by Lactanet (www.lactanet.ca) were based on the analysis of monthly milk samples collected from each cow in the participating herds and included fat percentage (%), protein%, lactose, milk urea nitrogen and milk somatic cell counts (SCC) amongst others. Cows with consistently very high milk SCC (an indirect measure of mammary gland health) (> 350,000 cells/mL) or consistently very low milk SCC (< 100,000 cells/mL) for three consecutive months were recruited for the study.
Pathogen identification and milk sampling
The procedures for pathogen identification and milk sampling have been described previously [20]. In brief, to test for the presence of pathogens, 10 mL of milk samples were aseptically collected from each quarter of cows in the high SCC group or a composite milk sample (equal volumes from all four quarters) per cow in the low SCC group. The samples were kept on ice and sent to Biovet laboratories (https://www.biovet-inc.com/) on the same day for bacteriological analysis. The analysis included testing for mastitis-causing microorganisms such as non-fastidious bacteria and mesophilic microbes (e.g., Staphylococcus species, Escherichia species, Streptococcus species, Klebsiella species, Nocardia species, Aerococcus species, Micrococcus species, yeast species and many others). Fourteen cows from the high SCC group that tested positive to S. aureus only were selected to constitute the S. aureus-positive group or three cows positive to S. chromogenes only constituted the S. chromogenes-positive group. Four low SCC cows that tested negative for all mastitis pathogens tested served as the healthy control (HC) group. Cows that were positive to other pathogens or to multiple pathogens were excluded from the study. Subsequently, 200 mL of milk was collected from one positive quarter (even if multiple quarters were infected) of each cow in the S. aureus-positive and S. chromogenes-positive groups. For the HC group, a 200-mL composite sample (50 mL from each quarter) was collected from each cow. A second bacteriological test on 10 mL of milk from these samples was performed to confirm the initial results. Only samples with consistent findings from both tests were used for further analysis. Milk samples were immediately transported on ice to the laboratory for somatic cells isolation. Milk somatic cells were isolated by low speed centrifugation (1,500 × g for 15 min at 4 °C) followed by removal of the fat and whey layers. The somatic cells were then washed twice with phosphate buffered saline (PBS) by adding 40 mL of 1 × PBS and centrifuging at 1,500 × g for 15 min at 4 °C. Finally, the washed somatic cells were treated with TRIzol reagent (Qiagen Inc, Toronto, ON, Canada) and stored at −80 °C for further use.
RNA isolation and quality control
Total RNA was extracted from milk somatic cells with RNeasy Mini Kit (Qiagen Inc., Toronto, ON, Canada) according to the manufacturer’s protocol. Agilent Bioanalyzed 2100 (Agilent Technologies, Saint-Laurent, QC, Canada) was used to quantify RNA, and LabChip GXII (PerkinElmer Inc., Waltham, MA, USA) was used to assess RNA integrity. RNA samples with RIN (RNA integrity number) values above 7 were processed further.
Library preparation and sequencing
Ribosomal RNA was depleted from 125 ng of total RNA using the QIAseq FastSelect Kit (Qiagen Inc., Toronto, ON, Canada). Complementary DNA was synthesized using the NEBNext RNA First Strand Synthesis and NEBNext Ultra Directional RNA Second Strand Synthesis Modules (New England BioLabs, Whitby, ON, Canada). Library preparation was performed with the NEBNext Ultra II DNA Library Prep Kit for Illumina (New England BioLabs, Whitby, ON, Canada). Adapters and polymerase chain reaction primers were purchased from New England BioLabs. Library quantification was performed with the Kapa Illumina GA with Revised Primers-SYBR Fast Universal kit (Kapa Biosystems, Wilmington, MA, USA). The average fragment size was assessed using the LabChip GXII instrument (PerkinElmer, Woodbridge, ON, Canada).
The libraries were normalized and pooled (equimolar concentrations) and then denatured in 0.05 mol/L NaOH and neutralized using HT1 buffer. The pool was loaded at 200 pM on an Illumina NovaSeq 6000 lane using Xp protocol as per the manufacturer’s recommendations. The run was performed for 2 × 100 cycles (paired-end mode). A phiX library was used as a control and mixed with libraries at 1% level. Base calling was performed with Real-Time Analysis (version 3.4.4). Bcl2fastq2 v2.20 program was used to demultiplex samples and generate fastq reads. Sequencing was performed by Centre d'expertise et de services Génome Quebec (https://www.genomequebec.com/).
Bioinformatics analyses
RNA sequencing data was processed using nf-core/rnaseq analysis pipeline version 3.3 [21]. The steps in bioinformatics analysis are summarized in Fig. 1. Quality assessment of the sequences was performed with FastQC (version 0.11.9). Barcodes were removed with UMI-tools (https://github.com/CGATOxford/UMI-tools). Three prime adaptor sequences and 5′ adaptor contaminants and repeats were trimmed using Trim Galore! (version 0.6.5). Likewise, low-quality reads (i.e. reads shorter than 60 nucleotides or having a low Phred score of less than 20 for at least 50% of the bases) were removed. The high-quality trimmed reads were merged and mapped to the bovine reference genome (ARS-UCD1.2/Bostau9 genome) using STAR (https://github.com/alexdobin/STAR). Then reads of mRNAs and other classes of non-coding RNAs were removed. The General Feature Format (GFF) file was downloaded from the ARS-UCD1.2/Bostau9 genome database. The GFF file was modified to retain only entries corresponding to known snoRNAs by applying the grep command as follows: “grep snoRNA ARS-UCD1.2_RefSeq_other_rna.gff3 > ARS-UCD1.2_RefSeq_snoRNA.gff”. Subsequent modifications to the pipeline were implemented using Nextflow, particularly adjusting the trim_galore and feature counts modules for compatibility with the data. Box C/D and H/ACA box snoRNAs of the known snoRNAs were identified in the trimmed and filtered reads using snoDB (https://bioinfo-scottgroup.med.usherbrooke.ca/snoDB/) [22]. The remaining reads after annotating the known snoRNAs were used in identifying the novel snoRNAs using snoReport (version 2.0; https://joaovicers.github.io/snoreport2/), a tool that identifies box C/D and H/ACA box snoRNAs, using a combination of secondary structure prediction and machine learning [23]. The analysis of snoRNA expression differences between S. chromogenes-positive or S. aureus-positive group, and HC groups were conducted using DESeq2 (version 3.15) [24]. Lactation stage and treatment were included as batch factors for cows in the S. chromogenes-positive group while lactation stage, parity and treatment were included as batch factors for cows in the S. aureus-positive group during analysis. Statistically significant differentially expressed (DE) snoRNAs were defined as having a Benjamini and Hochberg [25] corrected false discovery rate (FDR) < 0.05 and log2 fold change (log2FC) > 1. Scripts used for the snoRNA analyses are available at https://github.com/EIAlab/snoRNA.
Fig. 1.
Flowchart of small nucleolar RNA bioinformatics analysis
snoRNA-rRNA modification site prediction
Ribosomal RNA modification site prediction of snoRNAs in S. aureus-positive and S. chromogenes-positive samples were determined with snoRNA target prediction tools as described previously [26, 27] and shown in Fig. 1. Briefly, ribosomal alignments of Bos taurus and human ribosomal sequences by type (5.8S, 18S and 28S) was performed using ClustalW embedded in the MEGA 11 software in Ubuntu 22.04 LTS (https://www.megasoftware.net/). To predict the modification sites in the ribosomal RNA sequence, consensus sequences were created by aligning 18S_Human (U13369.1:3657–5527) and 18S_Btaurus (NR_036642.1), 28S_Human (U13369.1:7935–12969) and 28S_Btaurus (DQ222453.1:4134–8675), and 5.8S_Human (U13369:6623–6779) and 5.8S_Btaurus (DQ222453.1:2946–3095) with UGENE software (v45.1, 64-bit version). MUSCLE algorithm and Levitsky consensus type were selected for the alignment. The consensus RNA sequence was used in RNAsnoop (version 2.5.1 [28]) and snoScan (version 1.0) [29] to predict the methylation site of H/ACA box (2′-O-ribose methylation; Nm) and guide C/D box (pseudouridylation; Ψ) snoRNA, respectively, using the default parameters. Also, snoDB (https://bioinfo-scottgroup.med.usherbrooke.ca/snoDB/), an up-to-date interactive database of human snoRNAs used to predict the function of the H/ACA box and C/D box snoRNAs, were used to further confirm the methylation sites of H/ACA box and C/D box snoRNAs in the studied samples.
Construction of snoRNA-mRNA co-expression networks and functional annotation of co-expressed genes
SnoRNA functions to regulate mRNA gene expression at the post transcriptional level. Therefore, Pearson correlation of the expression values of DE snoRNAs from S. aureus-positive and S. chromogenes-positive groups and DE mRNAs of the same groups [30] was conducted to identify DE mRNAs correlating positively or negatively with DE snoRNAs using Hmisc, a R studio software (Version 5.1.3), with the threshold of P < 0.05 and |r| > 0.9. The analysis of the mRNA expression from the same samples and in relation to the studied pathogens has been reported previously [30]. The mRNAs that correlated positively or negatively with snoRNAs were subjected to functional analysis to get further insights into the functions of snoRNAs. Functional enrichment (KEGG pathway and Gene Ontology (GO)) analysis was performed with ShinyGO (version 0.80) (http://bioinformatics.sdstate.edu/go/). Annotation terms with FDR < 0.05 were regarded as statistically significant and the correlation networks of snoRNA-mRNA were visualized with cytoscape software (version 3.9.1) (http://www.cytoscape.org/) [31]. The top 7 snoRNAs (according to degree values) that correlated with the most mRNAs were selected as hub snoRNAs using cytohubba (version 3.0) [32], a cytoscape plug in for the S. aureus-positive and S. chromogenes-positive groups. Functional enrichment analysis was performed for the DE mRNAs that correlated with the hub snoRNAs. Cytoscape was used to construct hub snoRNA-mRNA interaction network.
Real-time qPCR validation of snoRNA expression
The expression levels of four randomly selected snoRNA genes, comprising 3 DE and 1 non-DE snoRNAs, were analyzed using real-time qPCR to validate RNA-sequencing data (Supplemental Table S7). Primers for the genes were designed with Primer-BLAST (https://www.ncbi.nlm.nih.gov/tools/primer-blast/). For cDNA synthesis, 200 ng of total RNA per sample was used. The total RNA was extracted from blood samples (stored in PAXgene tubes, Qiagen Inc, Toronto, ON, Canada) from the same S. aureus positive cows and healthy cows used in snoRNA analysis. Take note that blood samples from the same animals were used for this validation because the RNA isolated from milk somatic cells were no longer available (finished). The total RNA was reverse transcribed using the SuperScript™ IV VILO™ Master Mix (Invitrogen, Waltham, Massachusetts, USA). The resulting cDNA was diluted 1:10 and used for gene-specific qPCR amplification. The 20 µL qPCR reaction mixture included 2 µL (10 ng) of cDNA, 5 µL Power SYBR® Green PCR Master Mix (Applied Biosystems, Waltham, Massachusetts, USA), 1 µL each of forward and reverse primers (500 nmol/L each), and 7 µL nuclease-free water. Real-time amplification was performed on a StepOnePlus™ instrument (Applied Biosystems, Waltham, Massachusetts, USA) using a standard cycling mode. The cycling protocol began with a 10 min enzyme activation at 95 °C, followed by 40 cycles of denaturation at 95 °C for 15 s and extension at 60 °C for 60 s. RPS9 was used as the reference gene to normalize gene expression levels, and the relative expression values were calculated using the 2−∆∆Ct method [33].
Results
SnoRNA expression profiles in milk somatic cells during subclinical mastitis
High-throughput RNA sequencing produced an average of 23.32 million, 16.68 million and 18.14 million raw reads per sample for S. aureus-positive, S. chromogenes-positive and HC groups, respectively (Supplemental Table S1). After removing adaptor and low-quality sequences, S. aureus-positive, S. chromogenes-positive and HC groups yielded an average of 17.25, 12.9 and 14.22 million clean reads (75%) per sample, respectively. The average GC content per sample was approximately 0.44% for each of the three groups. The clean reads were mapped to the bovine reference genome (ARS-UCD1.2). Uniquely mapped reads comprised 92.42% of the total clean reads (Supplemental Table S1). Principal component analysis (PCA) was conducted to assess the overall relationship between samples. The PCA plot revealed that most samples clustered into two main groups: the S. aureus-positive group and HC group (Supplemental Fig. S1 A) and the S. chromogenes-positive group and HC group (Supplemental Fig. S1B). The differences between S. chromogenes-positive and HC groups accounted for 36% of the variance, while the differences within groups accounted for 25% of the variance. Likewise, the differences between S. aureus-positive and HC groups accounted for 40% of the variance, while the differences within groups accounted for 15% of the variance demonstrating the reliability of the sample phenotypes for further analysis.
Differential snoRNA expression in response to S. aureus and S. chromogenes infection
A total of 255 snoRNAs were identified in the samples after filtering out reads with fewer than 10 read counts and reads mapping to mRNA and other ncRNA species. Among these, 233 were known snoRNAs and 20 were novel (Supplemental Table S2). The expression levels of snoRNAs were compared between the S. aureus-positive and HC groups, and between S. chromogenes-positive and HC groups to identify DE snoRNAs. A total of 21 snoRNAs (18 known and 3 novel) exhibited significant differential expression between the S. aureus-positive group and HC group (FDR < 0.05 and log2FC > 1; Supplemental Table S3A). Among the S. aureus-positive group DE snoRNAs, 11 were upregulated, and 10 were downregulated (Fig. 2A; Supplemental Table S3A). Several known snoRNAs including SNORD88, SNORA76, SNORA29, SNORA46, snoRD107, SNORA66, SNORA70, SNORA79, ACA64, SNORA76 and SNORA63 were significantly upregulated in the S. aureus-positive group (Fig. 2A; Supplemental Table S3A). In contrast, many known snoRNAs including U3, SNORA74, SNORD123, U2-30, SNORA58, U2-19, SNORA1 and three novel snoRNAs (novelsnoRNA_26_14905, novelsnoRNA_4_112140344 and novelsnoRNA_10_7030513) were significantly down-regulated in the S. aureus-positive group compared to the HC group (Fig. 2A; Supplemental Table S3B).
Fig. 2.
Differentially expressed (DE) snoRNAs identified in S. aureus-positive and S. chromogenes-positive groups. A Volcano plot showing DE snoRNAs between S. aureus-positive vs. control groups. B Volcano plot showing DE snoRNAs between S. chromogenes-positive vs. control groups. C Venn diagram showing the total number of DE snoRNAs that are specific and common to the two pathogens (S. aureus and S. chromogenes)
Comparing the S. chromogenes-positive group and HC group, 20 snoRNAs were significantly DE (FDR < 0.05 and log2FC > 1; Supplemental Table S3A). Among the S. chromogenes-positive group DE snoRNAs, 13 were upregulated and 7 were downregulated (Fig. 2B; Supplemental Table S3B). Several snoRNAs including SNORA64/SNORA10, SNORA70, SNORA68, SNORA79, SNORA50, SNORA17, SNORA29, novelsnoRNA_2_26696606, SNORA66, SNORA46, SCARNA4, ACA64 and SNORA63) were significantly up-regulated (Fig. 2B) while SNORD113/SNORD114, novelsnoRNA_26_14905, SNORD37, SNORD18, SNORA74, SNORD49 and U2-19 were significantly down-regulated (Fig. 2B) in the S. chromogenes-positive group. Additionally, ten snoRNAs (SNORA74, novelsnoRNA_26_14905, SNORA29, SNORA46, SNORA66, SNORA70, SNORA79, ACA64, U2-19 and SNORA63) were present in both S. aureus-positive group and S. chromogenes-positive group (Fig. 2C), suggesting these snoRNAs may play similar roles in the regulation of mastitis induced by both pathogens. Meanwhile 11 snoRNAs (U3, U5 snRNA, SNORD123, U2-30, novelsnoRNA_4_112140344, SNORA58, SNORA1, SNORD88, SNORD107, SNORA76 and SNORA3/SNORA45) were DE in S. aureus-positive group only and 10 snoRNAs (SNORD113/SNORD114, SNORD37, SNORD18, SNORD49, SNORA64/SNORA10, SNORA68, SNORA50, SNORA17, novelsnoRNA_2_26696606 and SCARNA4) were DE in S. chromogenes-positive group only (Fig. 2C). These findings suggest that the DE snoRNAs unique to each pathogen could regulate processes triggered in response to the degree of pathogenicity of each pathogen. Meanwhile, DE snoRNAs shared between the two pathogens highlight the important role of snoRNAs as key regulators of inflammatory responses caused by S. chromogenes and S. aureus.
Prediction of rRNA 2′-O-methylation and pseudouridylation binding sites on small nucleolar RNA
To understand the roles of snoRNAs in S. aureus and S. chromogenes subclinical mastitis, we identified the potential modification sites of rRNAs 2′-O-methylation and pseudouridylation binding sites on the DE snoRNAs. We discovered that from the 21 DE snoRNAs found in S. aureus-positive group (7 from the C/D subfamily, 13 from the H/ACA subfamily and 1 from snRNA subfamily), 17 of the DE snoRNAs could target 34 specific modification sites on rRNAs (14 modification sites on 18S rRNA and 20 modification sites on 28S rRNA; Table 1) while from the 20 DE snoRNAs found in S. chromogenes-positive group (6 from the C/D subfamily, and 14 from the H/ACA subfamily), 14 could target 29 specific modification sites on rRNAs (13 modification sites on 18S rRNA and 16 modification sites on 28S rRNAs; Table 2). Notably for S. aureus-positive group, three snoRNA C/D box family members were predicted to direct 2′-O-methylation at Um1911, Um3320, Cm451 Um3320 (SNORD123), Um4158 (SNORD107) and Am1434 (U3) on 28S rRNA (Table 1), while two snoRNAs are predicted to direct 2′-O-methylation at Am1200 and Am3289 (SNORD18), and 3261 (SNORD37) for the S. chromogenes-positive group (Table 2). The remaining 19 snoRNAs from S. aureus-positive group and 18 snoRNAs from S. chromogenes-positive group, are predicted to modify pseudouridylation sites on 18S and 28S rRNAs (Tables 1 and 2). For S. aureus and S. chromogenes groups, SnoDB results showed that SNORA66, SNORA63, SNORA1, SNORA3, SNORA79, SNORA46 and SNORD37 were involved in ribosome biogenesis, processing, transcription and translation regulation (Supplemental Table S4A and S4B). These results suggest that snoRNA could be potentially involved in regulating ribosomal RNA processing and translation during bovine subclinical mastitis.
Table 1.
Target modification sites for 17 snoRNAs binding found on 18S and 28S rRNAs for S. aureus-positive group
| snoRNA_ID | snoRNA box types | Target modification sites | |
|---|---|---|---|
| 18S rRNA (2′-O-methylation site) | 28S rRNA (2′-O-methylation site) | ||
| SNORD123 | C/D Box | - | Um1911, Um3320, Cm451, Um3320 |
| snoRD107 | C/D Box | - | Um4158 |
| U3 | C/D Box | - | Am1434 |
| 18S rRNA (Pseudouridylation site) | 28S rRNA (Pseudouridylation site) | ||
| SNORA70 | H/ACA | 1323 | 3011 |
| SNORA29 | H/ACA | 1329 | 4262 |
| ACA64 | H/ACA | 1333 | 267 |
| SNORA66 | H/ACA | 1323 | 4232 |
| U2-19 | H/ACA | 1359 | 2309 |
| SNORA63 | H/ACA | 688 | 3007 |
| SNORA74 | H/ACA | 596 | 263 |
| SNORA58 | H/ACA | 1608 | 2930 |
| SNORA76 | H/ACA | 1323 | 3534 |
| SNORA79 | H/ACA | 1319 | 3001 |
| SNORA76 | H/ACA | 1667 | 3463 |
| SNORA46 | H/ACA | 1319 | 941 |
| SNORA1 | H/ACA | 1496 | 1747 |
| U5 snRNA | snRNA | 1350 | 3617 |
Table 2.
Target modification sites for 14 snoRNAs binding found on 18S and 28S rRNAs for S. chromogenes-positive group
| snoRNA_ID | snoRNA box types | Target modification sites | |
|---|---|---|---|
| 18S rRNA (2′-O-methylation site) | 28S rRNA (2′-O-methylation site) | ||
| SNORD18 | C/D Box | - | Am1200 |
| SNORD37 | C/D Box | - | Am3289, 3261 |
| 18S rRNA (Pseudouridylation site) | 28S rRNA (Pseudouridylation site) | ||
| SNORA29 | H/ACA | 1329 | 4262 |
| SNORA50 | H/ACA | 1155 | 2954 |
| SNORA46 | H/ACA | 1154 | 941 |
| SNORA6 | H/ACA | 443 | 273 |
| SNORA17 | H/ACA | 945 | 239 |
| SNORA66 | H/ACA | 1323 | 4232 |
| SNORA63 | H/ACA | 1340, 688 | 239, 3007 |
| SNORA64/SNORA10 | H/ACA | 954 | 579 |
| SNORA74 | H/ACA | 596 | 263 |
| SNORA79 | H/ACA | 1319 | 3001 |
| ACA64 | H/ACA | 1333 | 267 |
| SNORA70 | H/ACA | 1323 | 3011 |
Identification of snoRNAs co-expressed protein-coding genes and functional enrichment
SnoRNAs and their associated mRNAs have been implicated in post-transcriptional processes that contribute to disease development. To investigate these relationships, we performed a correlation analysis to identify snoRNAs and their negatively and positively co-expressed protein-coding genes. Co-expressed mRNAs with correlation coefficients > 0.9 were retained for further functional analysis. A total of 3,550 co-expressed genes for the 21 DE snoRNAs from the S. aureus-positive vs. HC comparison (Supplemental Table S5A) and 1,316 co-expressed genes for the 20 DE snoRNAs from the S. chromogenes-positive group vs. HC comparison (Supplemental Table S5B), were identified. To further understand the function of the DE snoRNAs, KEGG and GO enrichment analyses were performed using SHINYGO. The S. aureus-positive group correlated genes were significantly enriched in 85 KEGG pathways (FDR < 0.05; Supplemental Table S6A) and 1,000 biological process (BP) GO terms (FDR < 0.05, Supplemental Table S6B). Among the top 20 KEGG pathways in the S. aureus-positive group are immune and disease pathways such as NF-κB signaling pathway, Chemokine signaling, JAK-STAT signaling, Cancer pathways, and TNF signaling, among others (Fig. 3A). Consistent with the KEGG pathways, the top 20 significant GO-BP terms involved in immune responses included regulation of defense response, leukocyte differentiation, and regulation of immune response, among others (Fig. 3B). In the S. chromogenes-positive group, the functional enrichment analysis of the DE snoRNAs correlated genes were significantly enriched in 93 KEGG pathways (FDR < 0.05; Supplemental Table S6C) and 1,000 biological process GO-BP terms (FDR < 0.05; Supplemental Table S6D). The top 20 significant KEGG pathways are involved in disease or immune functions including NF-κB signaling pathway, Osteoclast differentiation, TNF signaling pathway, COVID-19, and Chemokine signaling pathway, among others (Fig. 3C) while the top 20 significant GO-BP terms also involved in immune responses included regulation of defense response, response to cytokine, and regulation of immune response, etc. (Fig. 3D).
Fig. 3.
Functional pathway annotation and Gene Ontology Biological Processes terms of DE snoRNA correlated genes from S. aureus and S. chromogenes-positive groups. A The top 20 KEGG pathways with the largest gene ratios are plotted in order of gene ratio for S. aureus B. The top 20 GO biological processes terms with the largest gene ratios are plotted in order of gene ratio for S. aureus. C The top 20 KEGG pathways with the largest gene ratios are plotted in order of gene ratio for S. chromogenes. D The top 20 GO biological processes terms with the largest gene ratios are plotted in order of gene ratio for S. chromogenes. The size of the dots represent the number of genes in the significant DE gene list associated with the pathway and GO term and the color of the dots represent the P-adjusted values (FDR < 0.05)
Interestingly, among all the enriched terms, some disease and immune pathways (e.g., Notch signaling, Fc epsilon RI signaling, Leukocyte transendothelial migration, Neutrophil extracellular trap formation, and T cell receptor signaling) and GO-BP terms (such as cellular response to IL-6, positive regulation of neutrophil extravasation, antigen processing and presentation of peptide antigen via MHC class I, positive regulation of NIK/NF-κB signaling, T-helper 17 type immune response, etc.) were only found in S. aureus-positive group (Supplemental Table S6E, S5F) or S. chromogenes-positive group (KEGG pathways: IL-17 signaling, p53 signaling, Cell adhesion molecule and Platinum drug resistance etc. and GO-BP terms: negative regulation of cytokine production, negative regulation of interleukin-17 production, negative regulation of I-NF-κB kinase/NF-κB signaling, cellular response to interleukin-4, macrophage differentiation, regulation of Interleukin-12 production, etc. (Supplemental Table S6E, S5F)). Meanwhile, inflammatory and immune pathways common to the S. chromogenes-positive and S. aureus-positive groups included NOD-like receptor signaling, NF-κB, TNF signaling, chemokine signaling, JAK-STAT signaling, and cancer pathways, while the common GO-BP terms with immune related functions included immune system processes, defense processes, and regulation of cytokine production etc. (Supplemental Table S6E, S5 F).
Construction of snoRNA-mRNA correlation networks and functional annotation
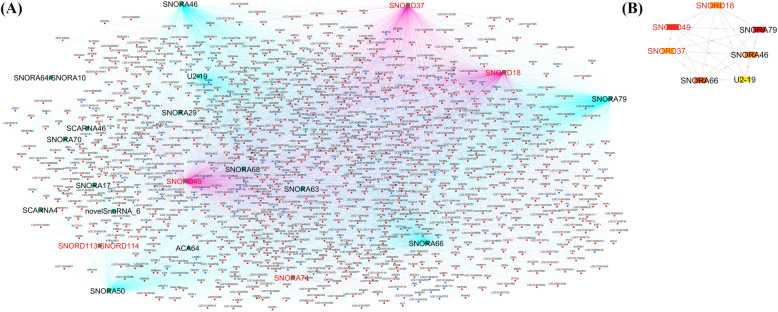
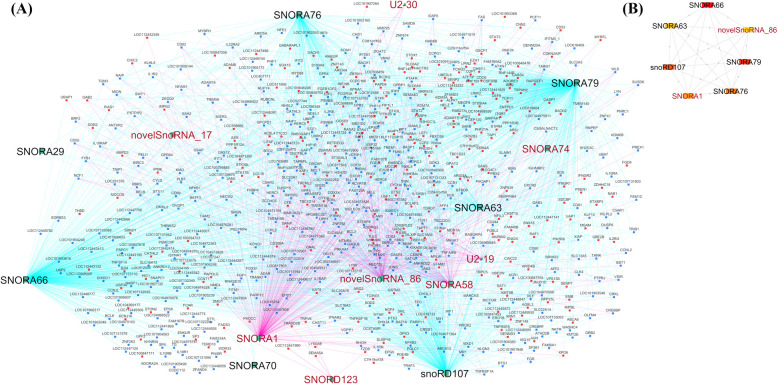
To identify snoRNA and mRNA co-expression patterns, snoRNA-mRNA correlation networks were constructed with Cytoscape software. In the S. aureus-positive group, 15 DE snoRNAs with correlations to two or more genes resulted in 15 networks (Fig. 4A). From the correlated networks, Cytohubba was further used to identify 7 top hub snoRNAs (snoRNAs correlated with ≥ 100 genes in the network) including upregulated SNORA66 (459 genes), SNORA79 (449 genes), SNORA76 (210 genes), SNORD107 (320 genes), SNORA63 (135 genes) and downregulated novelsnoRNA_26_14905 (117 genes) and SNORA1 (200 genes) (Fig. 4B). Similarly, 18 DE snoRNAs in the S. chromogenes-positive group meeting the same criteria resulted to 18 networks (Fig. 5A). From the correlated networks, Cytohubba was further used to identify 7 top hub snoRNAs (snoRNAs correlated with ≥ 30 genes) including upregulated SNORA79 (860 genes), SNORA46 (651 genes), U2-19 (56 genes), SNORA66 (798 genes) and downregulated SNORD37 (37 genes), SNORD49 (31 genes) and SNORD18 (81 genes) (Fig. 5B).
Fig. 4.
snoRNA-mRNA network for S. chromogenes-positive group vs. control group constructed with Cytoscape software. A The differentially expressed (DE) snoRNAs connected with their corresponding correlated genes. The downregulated snoRNAs are in red, and the upregulated snoRNAs are in black. The edge colors denote the type of correlation between snoRNAs and mRNAs. The pink edges represent negative correlations between the DE snoRNAs and their correlated genes while the turquoise edges represent positive correlation between the DE snoRNAs and their correlated genes. The green nodes represent the DE snoRNAs. The red nodes represent upregulated mRNAs and the blue nodes represents downregulated mRNAs. B Top 7 hub genes in the correlation network identified by CytoHubba (a Cytoscape plugin). Genes with the highest number of connections in the network are considered hub genes. The image shows the degree of importance of the hub snoRNAs through a color scale of rectangular shape ranging from red to yellow. novelSnoRNA_6 is also denoted novelsnoRNA_2_26696606
Fig. 5.
snoRNA-mRNA network for S. aureus-positive group vs. control group constructed with Cytoscape software. A The differentially expressed (DE) snoRNAs connected with their corresponding correlated genes. The downregulated snoRNAs are in red, and the upregulated snoRNAs are in black. The edge colors denote the type of correlation between snoRNAs and mRNAs. The pink edges represent negative correlations between the DE snoRNAs and their correlated genes while the turquoise edges represent positive correlations between the DE snoRNAs and their correlated genes. The green nodes represent the DE snoRNAs. The red nodes represent upregulated mRNAs and the blue nodes represent downregulated mRNAs. B Top 7 hub genes in the correlation network identified by CytoHubba (a Cytoscape plugin). Genes with the highest number of connections in the network are considered hub genes. The image shows the degree of importance of the hub snoRNAs through a color scale of rectangular shape ranging from red to yellow. NovelSonRNA_17 is also denoted novelsnoRNA_4_112140344 and novelsonRNA_86 is also denoted novelsnoRNA_26_14905
The functional enrichment analysis of the genes correlated with the 7 hub snoRNAs in the S. aureus-positive group showed that the GO-BP terms were involved in immune and inflammatory responses, including regulation of defense response, leukocyte differentiation, response to cytokine, regulation of immune response, among others (Supplemental Table S6G). Similarly, the KEGG pathways for this group were also enriched in immune activation and inflammation processes, with key pathways such as Viral protein interaction with cytokine and cytokine receptors, Osteoclast differentiation, NF-κB signaling pathway, JAK-STAT signaling pathway, among others. (Supplemental Table S6H). The top 7 hub SNORNAs correlated genes in the S. chromogenes-positive group revealed their GO-BP terms with primarily immune-related functions, such as regulation of defense response, response to cytokine, regulation of response to external stimulus, cellular response to cytokine stimulus, regulation of immune response, and defense response etc. (Supplemental Table S6I). In line with the GO-BP terms, the KEGG pathways for the S. chromogenes-positive group involved in immune signaling and inflammatory responses, included Osteoclast differentiation, NF-κB signaling pathway, Viral protein interaction with cytokine and cytokine receptors, TNF signaling pathway, JAK-STAT signaling pathway, and Pathways in cancer etc. (Supplemental Table S6J). Notably, this reveals that the hub SNORNAs in S. aureus-positive and S. chromogenes-positive groups are potential regulators of inflammatory genes in bovine mastitis.
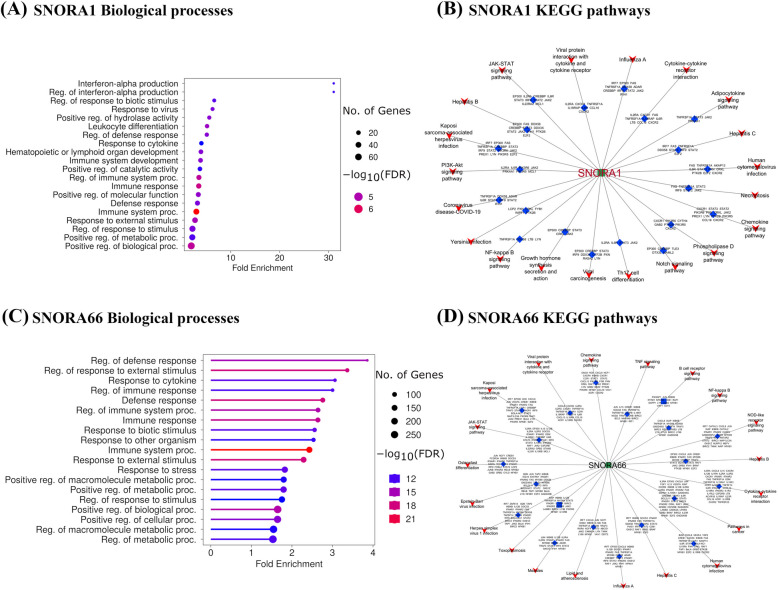
To further understand the roles of the hub snoRNAs, one downregulated snoRNA and one upregulated snoRNA with the highest number of correlated genes in the S. aureus-positive and S. chromogenes-positive snoRNA-mRNA networks were selected. In the S. aureus-positive group, SNORA1 (downregulated) showed correlations with 917 genes (Supplemental Fig. S2A), while SNORA66 (upregulated) was correlated with 1,174 genes (Supplemental Fig. S2B). The top 20 GO-BP terms enriched by genes co-expressed with downregulated SNORA1 were predominantly associated with immune functions, such as leukocyte differentiation, regulation of defense response, response to cytokine, immune system development among others. (Fig. 6A; Supplemental Table S6K). Likewise, over 95% of the top 20 KEGG pathways enriched for these co-expressed genes were involved in immune regulation and disease-related processes including JAK-STAT signaling pathway, P13-AKT signaling pathway, Notch signaling pathway, Chemokine signaling pathway, Th17 cell differentiation, amongst others (Fig. 6B; Supplemental Table S6L). For upregulated SNORA66, 75% of the top 20 GO-BP terms were related to immune functions (e.g., regulation of defense response, regulation of response to external stimulus, response to cytokine, and regulation of immune response (Fig. 6C; Supplemental Table S6M) while the remaining 25% were associated with metabolic and cellular processes (e.g., positive regulation of metabolic process, positive regulation of cellular processes, and regulation of macromolecule metabolic processes). Similarly, the pathways enriched for SNORA66 co-expressed genes, such as Chemokine signaling pathway, Osteoclast differentiation, NF-κB signaling pathway, JAK-STAT signaling pathway, B cell receptor signaling pathway etc. (Fig. 6D; Supplemental Table S6N) were predominantly linked to immune and inflammatory processes. These findings indicate that SNORA1 and SNORA66 may play significant roles in modulating immune responses to infection, highlighting their potential involvement in S. aureus subclinical mastitis.
Fig. 6.
Downregulated SNORA1 and Upregulated SNORA66-correlated mRNA enriched Gene Ontology (GO) biological processes terms for S. aureus-positive group. A The 20 GO biological processes with the largest gene ratios are plotted in order of gene ratio for SNORA1. The size of the dots represents the number of genes in the significant DE gene list associated with the GO term and the color of the dots represent the P-adjusted values (FDR < 0.05). B Cytoscape diagram showing the number of genes involved in the top 20 most significant KEGG pathways for SNORA1. The green rectangle shape represents the snoRNA, the blue diamond shape represents the mRNAs, and the red funnel shapes represent the significant pathways. C The 20 GO biological processes with the largest gene ratios were plotted in order of gene ratio for SNORA66. The size of the dots represent the number of genes in the significant DE gene list associated with the GO term and the color of the dots represent the P-adjusted values (FDR < 0.05). D Cytoscape diagram shows the number of genes involved in the top 20 most significant KEGG pathways for SNORA66. The green rectangle shape represents the snoRNA, the blue diamond shapes represent the mRNAs and the red funnel shapes represent the significant pathways
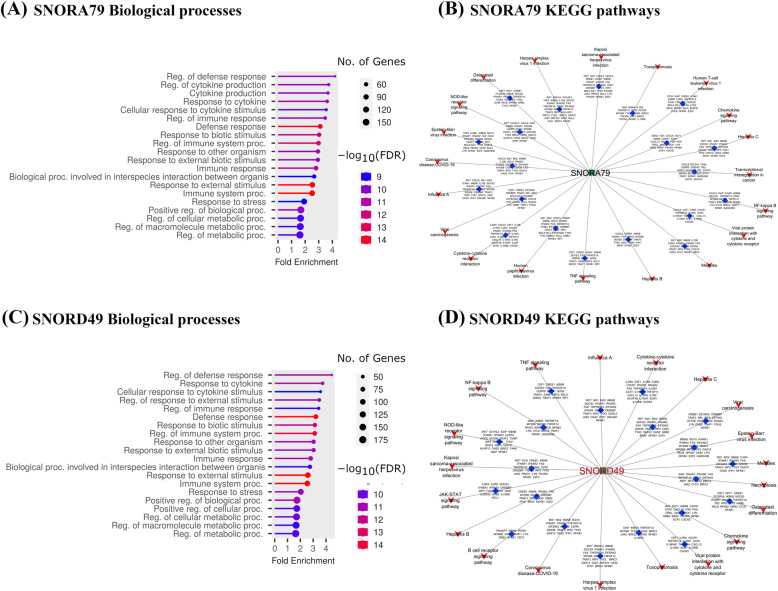
In the S. chromogenes-positive group, one downregulated snoRNA (SNORD49) and one upregulated snoRNA (SNORA79) with the highest number of co-expressed genes in the network were selected. SNORD49 correlated with 1,115 genes (Supplemental Fig. S3A) and SNORA79 correlated with 1,112 genes (Supplemental Fig. S3B). The top 20 enriched GO-BP terms and KEGG pathways for both SNORA79 (Fig. 7A, Supplemental Table S6O; Fig. 7B, Supplemental Table S6P) and SNORD49 (Fig. 7C, Supplemental Table S6Q; Fig. 7D, Supplemental Table S6R) were similar and predominantly related to immune responses including the regulation of defense response, cytokine production, immune response, cellular metabolic processes, among others for the GO-BP terms and NF-κB signaling pathway, TNF signaling pathway and Cytokine-cytokine receptor interaction, etc. for the KEGG pathways. These results suggest that the coordinated regulation of SNORD49 and SNORA79 contributes to a balanced immune response, allowing S. chromogenes to coexist with its host without eliciting excessive immune activation. This balance may underlie the pathogen’s milder clinical presentation and greater susceptibility to treatment compared to more virulent pathogens like S. aureus.
Fig. 7.
Upregulated SNORA79 and Downregulated SNORD49 correlated mRNAs enriched Gene Ontology (GO) biological processes terms for S. chromogenes-positive group. A The 20 GO biological processes with the largest gene ratios are plotted in order of gene ratio for SNORA79. The size of the dots represent the number of genes in the significant DE gene list associated with the GO term and the color of the dots represent the P-adjusted values (FDR < 0.05). B Cytoscape diagram shows the number of genes involved in the top 20 most significant KEGG pathways for SNORA79. The green rectangle shape represents the snoRNA, the blue diamond shapes represent the mRNAs, and the red funnel shapes represent the significant pathways. C The 20 GO biological processes with the largest gene ratios were plotted in order of gene ratio for SNORD49. The size of the dots represent the number of genes in the significant DE gene list associated with the GO term and the color of the dots represent the P-adjusted values (FDR < 0.05). D Cytoscape diagram shows the number of genes involved in the top 20 most significant KEGG pathways for SNORD49. The green rectangle shape represents the snoRNA, the blue diamond shapes represent the mRNAs and the red funnel shapes represent the significant pathways
Validation of DE snoRNAs using real time quantitative PCR
The real time qPCR expression results of one up-regulated DE snoRNA, two down-regulated DE snoRNAs and one non-DE snoRNA randomly selected to validate the RNA-Seq results are shown in Fig. 8. The qPCR relative expression results for the tested snoRNAs were similar to the RNA-Seq expression data for the two downregulated snoRNAs and the one non-DE snoRNA. However, the expression of the upregulated snoRNA was not significant as compared to the RNA-Seq results (Fig. 8).
Fig. 8.
Real-time qPCR expression results compared with snoRNA differential expression results. *P < 0.05, **P < 0.01, ***P < 0.001, ns Non-significant
Discussion
Through transcriptome analysis of milk somatic cells from cows with naturally occurring subclinical mastitis due to S. aureus and S. chromogenes, 255 snoRNAs (233 known and 22 novel; Supplemental Table S2) were identified. Out of the identified snoRNAs, 21 were DE (11 upregulated and 10 downregulated) in S. aureus-positive group (Fig. 2A) whereas 20 were DE in S. chromogenes-positive group (Fig. 2B). SnoRNAs are crucial for rRNA modification and essential for ribosome function, as highlighted in previous studies [13, 14]. In our study, 2′-O-methylation modification sites on 18S rRNA and 28S rRNA were observed for majority of the DE snoRNAs in S. aureus-positive and S. chromogenes-positive groups (Tables 1 and 2). Interestingly, there were also some snoRNAs having modification sites common between the two groups on both 18S and 28S rRNA pseudouridylation sites, such as SNORA29 (18S-1323, 28S-4262); SNORA66 (18S-1323, 28S-4232); SNORA74 (18S-596, 28S-263) etc. (Tables 1 and 2). Moreso, some snoRNAs were found to target the same modification sites on 18S rRNA, such as SNORA70, SNORA66 and SNORA76 (18S-1323) for S. aureus-positive group (Table 1). These findings are consistent with human studies, where snoRNAs like SNORA63, SNORA70, and SNORA1 have been shown to guide chemical modifications of rRNA processing [34, 35] and control translation processes such as initiation, elongation, and termination [19, 36, 37], suggesting their vital role during subclinical mastitis.
The observed DE snoRNAs in the S. aureus-positive group (including U3, U5 snRNA, SNORA74, SNORA66, SNORD123, SNORA1 and novelsnoRNA_26_14905 (log2FC > 1 or log2FC < −1) among others), and in the S. chromogenes-positive group (including SNORD113/SNORD114, SNORA66, SNORD49 and SNORA46, etc. (log2FC > 1 or log2FC < −1)) are consistent with findings from previous research [38–42]. For instance, Li and colleagues found upregulated expression of SNORA66 in human patients with diffuse large B-cell lymphomas [42]. Ferreira and colleagues reported that SNORD123 was silenced in various cancers due to hypermethylation of their host gene-associated CpG islands, indicating a novel subclass of non-coding RNAs targeted in tumorigenesis [40]. In another study, snoRNA expression data from eight major cancers revealed that SNORD123 contributed to high classification accuracy, with the support vector machines classifier showing a Matthew's correlation coefficient of 0.881 for identifying cancer samples suggesting its importance in understanding tumor biology and developing targeted therapies [41]. In a recent study, overexpression of SNORA76 was identified as a potential biomarker for early detection of clear cell renal cell carcinoma from urine-derived extracellular vesicles [38]. Furthermore, SNORA76 was found to be upregulated in gallbladder cancer compared to adjacent non-tumor tissues [39] and significantly downregulated in metastatic vs. non-metastatic prostate cancer xenograft models [43]; SNORD37 was found to be downregulated in non-small cell lung cancer [44] while SNORA64 was identified as significantly upregulated in pancreatic cancer patients [45] and acute myeloid leukemia patients [46]. Overall, these findings suggest the involvement of these snoRNAs in disease processes and consequently in S. aureus and S. chromogenes subclinical mastitis. Furthermore, 10 DE snoRNAs identified in this study were found to be common between the two pathogens, including SNORA66, SNORA70, SNORA29, and ACA64, suggesting involvement in common biological processes. Meanwhile some snoRNAs displayed pathogen-specific expression patterns, such as SNORD123, SNORA58, and SNORA76 in S. aureus-positive group and SNORA50, SNORA68, and SNORD49 in S. chromogenes-positive group (Fig. 2C), suggesting differential regulatory mechanisms between S. aureus and S. chromogenes-induced mastitis.
To further explore the roles of the DE snoRNAs, the negative and positive correlated DE mRNAs from each group (correlation coefficient > 0.9; Supplemental Table S4A and S4B) were subjected to functional enrichment. Both positive and negative correlations were observed, and which reflects the dual roles of snoRNAs in gene regulation. These roles include their positive involvement in rRNA processing and biogenesis as well as their non-canonical regulatory functions, such as repressing the expression of specific mRNAs or reflecting a feedback-loop [16]. In both S. aureus-positive and S. chromogenes-positive cows, the DE snoRNAs were correlated with several immune and inflammatory genes (e.g., IL1R, IL18R1, STAT3, NFKB2, MYD88, VEGFA and CD40), all of which have been reported in several studies to be associated to mastitis [47–53]. As evident in our study, the enrichment analysis of the correlated genes showed that the top 20 KEGG pathways and GO-BP were enriched in immune and inflammatory functions including NF-κB signaling, TNF signaling, immune system processes and defense responses, among others (Fig. 3A and B). These pathways and biological processes terms have been associated to the immune response to mastitis pathogens through analysis of different biological molecules like mRNA, miRNA and lncRNA [7, 30, 54], thus supporting the involvement of snoRNAs in the host immune response to S. aureus and S. chromogenes induced mastitis.
For the S. aureus-positive group, some enriched pathways and biological processes terms (Supplemental Table S6E, S5F) are indicative of S. aureus’s reliance on complex regulatory pathways (e.g., Leukocyte transendothelial migration, and Neutrophil extracellular trap (NET) formation) to enhance immune activation and establish infection. Generally, neutrophils which make up to 75% of the leukocytes in milk from cows with mastitis, exhibit anti-inflammatory activity through three main mechanisms: phagocytosis, degranulation, and the release of NETs. NETs are large, extracellular, web-like structures made up of cytosolic and granule proteins organized on a scaffold of decondensed chromatin and their dysregulation can lead to tissue damage, contributing to the development of immune-related diseases [55].
For the S. chromogenes-positive group, the correlated genes (including IFNG, IL12B, etc.) were involved in immune and cellular pathways such as IL-17 signaling, p53 signaling, Cell adhesion molecule pathways etc., while some GO-BP terms were mostly involved in negative regulation of the immune processes such as negative regulation of cytokine production, negative regulation of IL-17 production, cellular response to IL-4, and macrophage differentiation among others (Supplemental Table S6E, S5F). Among the correlated genes involved in these processes, the IFNG gene encodes interferon-gamma (IFN-γ), a critical cytokine in the immune system. Interferon-gamma is a small but highly potent molecule widely utilized in clinical treatments due to its role in modulating immune responses and enhancing resistance to infections. During Staphylococcus infections, IFN-γ is produced by capsular polysaccharide-stimulated T cells, amplifying the harmful effects on resistant Staphylococcus strains [56]. Interferon-gamma has been used to manage acute bovine mastitis during the periparturient period. Its application induces functional changes in lymphocytes and phagocytic cells within the mammary gland, leading to decreased mastitis severity [57]. In this study, the negative regulation of the IL-17 pathway suggests that it may be a strategy to keep inflammation under control [58]. It is generally considered that interleukin 17 pathway could activate antimicrobial peptides and neutrophil activating molecules [59]. Also, they could improve host innate and adaptive defense by modulating the gene expression patterns of mammary gland epithelial cells [59, 60]. These results reflect S. chromogenes’s lower virulence and the milder immune activation it induces.
Interestingly, we observed that some of the significant pathways and GO terms were found in the S. aureus-positive group only or the S. chromogenes positive group only. This observation is not surprising as it is a well-established fact that most of the mastitis infections due to S. aureus develop to a more severe and prolonged infection while those due to S. chromogenes are milder [61–64] and could explain the differences in the observed pathways. Moreover, Wang and colleagues reported mostly positive and higher gene set scores for immune-related pathways and GO terms in S. aureus-positive cows supporting a more aggressive profile which also aligns with S. aureus higher virulence and robust immune evasion strategies that allow it to persist in the mammary gland and cause severe infections [30]. Meanwhile, mostly negative and lower gene set scores for the corresponding terms were reported in relation to S. chromogenes-positive cows supporting a less aggressive profile [30]. Likewise, several KEGG pathways and biological processes terms related to immune and inflammatory functions identified (e.g., NOD-like receptor signaling, NF-κB signaling, TNF signaling, Chemokine signaling, JAK-STAT signaling, defense responses, and regulation of cytokine production; Supplemental Table S6E, S5F) were associated to both S. aureus and S. chromogens suggesting that both pathogens belonging to the staphylococci genus use some common mechanisms to manipulate the host immune response to establish subclinical mastitis and persist within the host.
To gain further insights into the functions of DE snoRNAs, a cytoscape plugin was used to construct snoRNA-mRNA co-expressed networks for the two pathogens (Supplemental Table S4). Majority of the correlated genes have immune functions suggesting that snoRNAs may play a role in their regulation. In particular, further identification of hub snoRNAs (Figs. 4A, B and 5A, B) support a role of the DE snoRNAs in regulating many genes in the networks. Furthermore, the functional enrichment analysis of the correlated genes of the 7 hub snoRNAs in S. chromogenes-positive group (SNORD18, SNORA79, SNORA46, U2-19, SNORA66, SNORD37, SNORD49) revealed involvement in immune pathways (NF-κB, JAK-STAT, IL-17, TNF-signaling pathways; Supplemental Table S6G) and mostly negative immune and cellular biological processes (e.g., negative regulation of immune response, negative regulation of leucocyte activation, negative regulation of inflammatory responses; Supplemental Table S6H). This further buttresses a mild host immune response to the infection by S. chromogenes. Piccart et al. found that dairy heifers inoculated with S. chromogenes strains in the mammary gland evoked a mild local host response [65]. Similarly, Simojoki and colleague revealed that S. chromogenes infected primiparous cows had minor tissue damage and the infection was eradicated in few days [63]. However, since S. chromogenes is the most NAS pathogen frequently isolated from milk and skin, the prevalence and re-occurrence of the infection may be more problematic [63], requiring further investigation.
In contrast to S. chromogenes-positive group, the S. aureus-positive group correlated genes of the 7 hub snoRNAs (SNORA66, novelsnoRNA_26_14905, SNORD107, SNORA1, SNORA63, SNORA79 and SNORA76) were mostly involved in positive regulation of immune and inflammatory responses including positive regulation of immune system process, positive regulation of cytokine production, positive regulation of leucocyte differentiation, regulation of autophagy, NF-κB, JAK-STAT and TNF-signaling pathways, among others (Supplemental Table S6I, S5J). The positive regulation of the immune responses could explain S. aureus pathogenicity to evade both innate and adaptive immune responses thereby causing localized or systemic infections [66]. S. aureus has the ability to internalize, multiply and persist in bovine mammary gland thereby making S. aureus mastitis infection difficult to eradicate [67]. Among the mechanisms underlying the intracellular survival of S. aureus is its ability to induce autophagy formation. In this study, STAT3 reported to regulate autophagy [68] was enriched in autophagy GO-BP term. Following infection with S. aureus, the activation of STAT3 occurs later than other signaling pathways, such as ERK and mTOR, leading to the delayed suppression of TFEB, a key regulator of lysosomal biogenesis and function. This delayed STAT3 activation results in a weakened lysosomal function in macrophages, impairing their ability to degrade and clear the bacteria. By inhibiting TFEB activity, STAT3 facilitates the survival of S. aureus within macrophages, allowing the bacteria to evade the host’s immune response [68]. This study highlights the strategic manipulation of host cell pathways by S. aureus to prevent bacterial clearance and promote persistent infection. These findings suggest the potential roles of the 7 hub snoRNAs in regulating inflammatory responses during mastitis. However, while our correlation and enrichment analyses suggest that certain snoRNAs may be involved in immune-related pathways during subclinical mastitis, the regulatory direction and mechanism remain to be validated. Most snoRNAs are known to function in trans [13], but their emerging roles in mRNA regulation remain to be investigated. Similarly, we observed strong correlations between some snoRNAs and immune-related genes. However, future studies involving snoRNA perturbation (e.g., knockdown or overexpression) are essential to confirm the regulatory roles of these snoRNAs, and establish causality.
The correlated genes (e.g., JAK2, SOCS3, PIK3AP1, PTK2B; Supplemental Fig. S2–S3) of the 7 hub SNORNAs for S. aureus-positive group and S. chromogenes-positive group were also enriched in metabolic processes and pathways related to milk synthesis, such as response to lipid, prostaglandin metabolic process, prolactin signaling pathway and JAK-STAT signaling pathway, among others (Supplemental Table S6K–R). The JAK-STAT pathway plays central roles in regulating cytokine signaling in the mammary gland with strong association with mammary gland development and milk production [69]. This pathway is crucial for blood cell differentiation and the regulation of casein gene expression during lactation [70, 71]. For instance, JAK-STAT-associated proteins, regulated by prolactin receptor, help maintain a balance between growth hormone function and milk protein synthesis [72]. The interplay between lipid-related processes and immune responses during subclinical mastitis caused by S. aureus or S. chromogenes could lead to reduced milk fat content, altered milk composition, and a shift in energy allocation towards supporting immune functions [30]. These findings highlight potential regulatory mechanisms underlying the compromised milk production observed in cows with subclinical mastitis, emphasizing the complex molecular interactions driving these changes.
Furthermore, the roles of the top downregulated or upregulated hub snoRNAs in each of the pathogen groups were investigated through functional analyses. In the S. aureus-positive group, the correlated genes of the 2 hub SNORNAs (upregulated SNORA66 and downregulated SNORA1; Fig. 6) including inflammatory cytokines (e.g., CXCL8, IL1R, IL-10R, IFNGR1; Supplemental Fig. S2A) and chemokine (e.g., STAT3, IL6R, IL2, JAK2; Supplemental Fig. S2B) genes show involvement in immune responses and pathways like JAK-STAT signaling pathway, P13K-AKT signaling pathway, Th17 differentiation, Leukocyte differentiation, Interferon alpha production, Positive regulation of interleukin 6 production and neutrophil chemotaxis and extravasation etc. (Supplemental Table S6K–N). The genes correlated with downregulated SNORA1 (e.g., JAK2 and IL6R) have been shown to stimulate inflammation during subclinical mastitis as discussed in several reviews [54, 60, 73, 74]. For instance, IL-6 receptor (IL6R) is a receptor that transmits the effects of IL-6, a cytokine involved in promoting inflammation [60]. Bochniarz et al. found upregulated expression of IL-6 in Streptococcus spp. infected cows, suggesting that it is an important cytokine that could be used for early detection of subclinical mastitis [75]. JAK-2 is one of the key members in JAK-STAT signaling pathway and the activation of this gene is critical in bovine mastitis resistance. In contrast, the correlated chemokines and cytokines genes (e.g., CXCL8 and IL-10R) of upregulated SNORA66 are mostly involved in the protection against pathogens infecting bovine mammary glands through recruiting leukocytes from blood into the mammary tissue [76–79]. For instance, IL-10 could exhibit immune-stimulatory properties, aiding in the clearance of infectious and noninfectious particles while minimizing inflammation [80, 81]. CXCL8 (also known as interleukin-8) plays a pivotal role in the immune response by directing the migration and activation of neutrophils. It is the most potent neutrophil-attracting chemokine to infections and tissue injuries. In the S. chromogenes positive group, the correlated genes of the two hub SNORNAs (downregulated SNORD49 and upregulated SNORA79; Fig. 7) suggest they may mediate the upregulation of various pro-inflammatory genes (e.g., CXCL8, IKBKB, TRAF3, NFKB1; Supplemental Fig. S3) which are involved in bridging innate and adaptive immunity by regulating inflammation locally and systematically via the recruitment of neutrophils and macrophages to the site of infection [73] via the IL-17 signaling pathway and p53 signaling pathway among others (Supplemental Table 6O–R). These genes have been reported to increase in response to bovine mastitis pathogens [30, 82–84].
This study provides valuable data for understanding the snoRNAs regulatory mechanisms behind subclinical mastitis caused by S. aureus and S. chromogenes. However, extrapolation of our results needs to be careful, due to the relatively small sample sizes. While we used stringent thresholds to identify robustly expressed snoRNAs (read counts ≥ 10 in all samples and log2FC > 1) and a software (DESeq2 [24]) that is specifically designed to handle small and unbalanced RNA-seq datasets, further validation of the functional roles of the identified snoRNAs using larger and more balanced datasets is necessary to confirm our findings. Moreover, experimental validation of snoRNA-mRNA interactions and predicted rRNA modification sites (e.g., via mass spectrometry) is essential and represents an important direction for future functional characterization.
Conclusion
This study explored the roles of dysregulated snoRNAs in subclinical mastitis caused by S. aureus and S. chromogenes. In this study, 2′-O-methylation and pseudouridylation binding sites on 18S rRNA and 28S rRNA were observed for majority of the DE snoRNAs in S. aureus-positive and S. chromogenes-positive groups, suggesting snoRNA potential roles in rRNA biogenesis, translation and processing during bovine subclinical mastitis. Likewise, the DE snoRNAs were associated with key immune and inflammatory pathways and processes including NF-κB, JAK-STAT, TNF signaling pathways and leucocyte activation, among others. Moreover, correlated mRNAs in snoRNA-mRNA networks for the two pathogens have mostly immune related functions and correlated genes of the hub snoRNAs identified for S. aureus-positive and S. chromogenes-positive groups suggest important roles in regulating immune and inflammatory pathways and processes through overrepresentation in IL-17 signaling pathway and p53 signaling pathway, etc., and JAK-STAT signaling pathway, P13K-AKT signaling pathway, Th17 differentiation pathway and Leukocyte differentiation pathway, among others, respectively.
Supplementary Information
Supplementary Material 1. Table S1 General whole genome snoRNA sequence statistics per sample.
Supplementary Material 2. Table S2 List of identified snoRNAs expressed in samples.
Supplementary Material 3. Table S3A List of differntially expressed snoRNAs in Staphylococcus aureus versus control group. Table S3B List of differntially expressed snoRNAs in Staphylococcus chromogenes versus control group.
Supplementary Material 4. Table S4A Summary of snoRNA function and their rRNA target modification site in Stahylococcus aureus versus control group. Table S4B Summary of snoRNA function and their rRNA target modification site in Stahylococcus chromogenes versus control group.
Supplementary Material 5. Table S5A Correlation anaysis of differentially expressed (DE) snoRNAs and DE mRNAs in Staphylococcus aureus versus control group. Table S5B Correlation anaysis of differentially expressed (DE) snoRNAs and DE mRNAs in Staphylococcus chromogenes versus control group.
Supplementary Material 6. Table S6A Functional enrichment Gene Ontology Biological processes of differentially expressed snoRNA correlated genes of Staphylococcus aureus versus control group. Table S6B Functional enrichment Gene Ontology Biological processes of differentially expressed snoRNA correlated genes of Staphylococcus chromogenes versus control group. Table S6C Functional enrichment KEGG pathway analysis of differentially expressed snoRNA correlated genes of Staphylococcus aureus versus control group. Table S6D Functional enrichment KEGG pathway analysis of differentially expressed snoRNA correlated genes of Staphylococcus chromogenes versus control group. Table S6E Functional enrichment KEGG pathway analysis of differentially expressed snoRNA correlated genes specific to Staphylococcus aureus, Staphylococcus chromogenes and Staphylococcus aureus versus Staphylococcus chromogenes. Table S6F Functional enrichment Gene Ontology Biological processes terms of differentially expressed snoRNA correlated genes specific to Staphylococcus aureus, Staphylococcus chromogenes and Staphylococcus aureus versus Staphylococcus chromogenes. Table S6G Functional enrichment KEGG pathways of 7 hub snoRNAs correlated genes of Staphylococcus chromogenes versus control group. Table S6H Functional enrichment Gene Ontology Biological processes of 7 hub snoRNAs correlated genes of Staphylococcus chromogenes versus control group. Table S6I Functional enrichment Gene Ontology Biological processes of 7 hub snoRNAs correlated genes of Staphylococcus aureus versus control group. Table S6J Functional enrichment KEGG pathways of 7 hub snoRNAs correlated genes of Staphylococcus aureus versus control group. Table S6K Functional enrichment Gene Ontology Biological processes of SNORA1 correlated genes of Staphylococcus aureus versus control group. Table S6L Functional enrichment KEGG pathway analysis of differentially expressed SNORA1 correlated genes of Staphylococcus aureus versus control group. Table S6M Functional enrichment Gene Ontology Biological processes of SNORA66 correlated genes of Staphylococcus aureus versus control group. Table S6N Functional enrichment KEGG pathway analysis of differentially expressed SNORA66 correlated genes of Staphylococcus aureus versus control group. Table S6O Functional enrichment Gene Ontology Biological processes of SNORA79 correlated genes of Staphylococcus chromogenes versus control group. Table S6P Functional enrichment KEGG pathway analysis of differentially expressed SNORA79 correlated genes of Staphylococcus aureus versus control group. Table S6Q Functional enrichment Gene Ontology Biological processes of SNORD49 correlated genes of Staphylococcus chromogenes versus control group. Table S6R Functional enrichment KEGG pathway analysis of differentially expressed SNORD49 correlated genes of Staphylococcus aureus versus control group.
Supplementary Material 7. Table S7 Genes and their primer sequences used for real-time qPCR validation of RNA-sequencing results.
Supplementary Material 8. Fig. S1 Principal component analysis plot showing the sample cluster per group. Fig. S2 Correlation network between hub snoRNAs and target DE genes for Staphylococcus aureus versus the control. Fig. S3 Correlation network between hub snoRNAs and target DE genes for Staphylococcus chromogenes versus the control.
Acknowledgements
The participation of the dairy farms involved in this study and the Fonds de recherche du Québec scholarship awarded to Faith Omonijo by the Quebec Government are gratefully acknowledged.
Abbreviations
- AML
Acute myeloid leukemia
- APA
Alternative polyadenylation
- CDC2L6
Cyclin-dependent kinase 6
- CXCL8
Chemokine (C-X-C motif) ligand 8
- CXCR1
Chemokine receptor 1
- CXCR2
Chemokine receptor 2
- DE
Differentially expressed
- DHI
Dairy herd improvement
- DNA
Deoxyribonucleic acid
- FDR
False discovery rate
- G3BP1
Ras‐GTPase‐activating protein (GAP)‐binding protein 1
- GFF
General feature format
- GO
Gene Ontology
- HC
Healthy control
- IFNGR1
Interferon gamma receptor 1
- IKBKB
Inhibitor of nuclear factor kappa B kinase subunit beta
- IL-17
Interleukin 17
- IL1R
Interleukin receptor 1
- IL6R
Interleukin receptor 6
- I-NF-κB kinase/NF-κB
I-κB kinase (IKK) and Nuclear Factor kappa B (NF-κB)
- JAK2
Janus kinase 2
- JAK-STAT
Janus kinase/signal transducers and activators of transcription
- KEGG
Kyoto Encyclopedia of Genes and Genomes
- Log2FC
Log2 fold change
- LncRNAs
Long non coding RNAs
- MEGA
Molecular Evolutionary Genetics Analysis
- MHC
Major histocompatibility complex
- miRNAs
MicroRNAs
- mRNAs
Messenger RNAs
- MUSCLE
Multiple sequence comparison by log-expectation
- NAS
Non-aureus staphylococci
- ncRNAs
Non-coding RNAs
- NET
Neutrophil extracellular trap formation
- NF-κB
Nuclear factor kappa B
- ON
Ontario
- P13-AKT
Phosphatidylinositol 3-kinase (PI3K) and protein kinase B
- PBS
Phosphate buffered saline
- PCA
Principal component analysis
- PCR
Polymerase chain reaction
- PIK3AP1
Phosphoinositide-3-kinase adaptor protein 1
- PTK2B
Protein tyrosine kinase 2 beta
- QC
Quebec
- RIN
RNA integrity number
- RNA
Ribonucleic acid
- rRNA
Ribosomal RNA
- SCC
Somatic cell count
- SCS
Somatic cell score
- snoRNA
Small nucleolar RNA
- SOCS3
Suppressor of cytokine signaling 3
- STAR
Spliced transcripts alignment to a reference
- STAT3
Subunit signal transducers and activators of transcription 3
- TNF
Tumor necrosis factor
- TNFRSF1A
Tumor necrosis factor receptor superfamily member 1A
- TRAF1
Tumor necrosis factor receptor associated factor 1
- TRAF3
Tumor necrosis factor receptor associated factor 3
- UBAP2L
Ubiquitin associated protein 2 like
- UMI
Unique molecular identifiers
Authors’ contributions
EMI-A conceptualized and designed the study, supervised the sampling process, laboratory procedures, bioinformatics analyses and contributed to drafting the manuscript. XZ participated in the study design. MW and EMI-A conducted the field sampling. DG and ML developed the analyses pipelines and performed the transcriptome analyses. FAO and SG performed the laboratory analyses. FAO was responsible for differential expression analysis, snoRNA-mRNA correlation analysis, functional enrichment analysis and rRNA analysis, and made significant contributions to drafting the manuscript. EMI-A and XZ contributed to data interpretation. All authors reviewed and approved the final version of the manuscript.
Funding
Agriculture and Agri-Food Canada funded this research (grant number J-002223).
Data availability
The raw read sequences analyzed in this study have been deposited in NCBI Sequence Read Archive (SRA) under the Bio Projects PRJNA878880 and PRJNA967255.
Declarations
Ethics approval and consent to participate
Animal use procedures were approved in accordance with the guidelines of the Canadian Council on Animal Care, and ethical approval to conduct the study was provided by the Animal Care and Ethics Committee of Agriculture and Agri‑Food Canada (approval #570).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Dego OK. Bovine mastitis: part I. In: Aral F, Payan-Carreira R, Quaresma M, editors. Animal reproduction in veterinary medicine. Rijeka: IntechOpen; 2020. p. 93483.
- 2.Niedziela DA, Murphy MP, Grant J, Keane OM, Leonard FC. Clinical presentation and immune characteristics in first-lactation Holstein-Friesian cows following intramammary infection with genotypically distinct Staphylococcus aureus strains. J Dairy Sci. 2020;103(9):8453–66. [DOI] [PubMed] [Google Scholar]
- 3.Cheng WN, Han SG. Bovine mastitis: risk factors, therapeutic strategies, and alternative treatments—A review. Asian-Aust J Anim Sci. 2020;33(11):1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Buck J, Ha V, Naushad S, Nobrega DB, Luby C, Middleton JR, et al. Non-aureus staphylococci and bovine udder health: current understanding and knowledge gaps. Front Vet Sci. 2021;8:658031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Condas LA, De Buck J, Nobrega DB, Carson DA, Naushad S, De Vliegher S, et al. Prevalence of non-aureus staphylococci species causing intramammary infections in Canadian dairy herds. J Dairy Sci. 2017;100(7):5592–612. [DOI] [PubMed] [Google Scholar]
- 6.Ibeagha-Awemu EM, Yu Y. Consequence of epigenetic processes on animal health and productivity: is additional level of regulation of relevance? Anim Front. 2021;11(6):7–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Do DN, Dudemaine PL, Mathur M, Suravajhala P, Zhao X, Ibeagha-Awemu EM. miRNA regulatory functions in farm animal diseases, and biomarker potentials for effective therapies. Int J Mol Sci. 2021;22(6):3080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang M, Ibeagha-Awemu EM. Impacts of epigenetic processes on the health and productivity of livestock. Front Genet. 2020;11:613636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ibeagha-Awemu EM, Zhao X. Epigenetic marks: regulators of livestock phenotypes and conceivable sources of missing variation in livestock improvement programs. Front Genet. 2015;6:302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mattick JS, Amaral PP, Carninci P, Carpenter S, Chang HY, Chen L-L, et al. Long non-coding RNAs: definitions, functions, challenges and recommendations. Nature Rev Mol Cell Biol. 2023;24(6):430–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oyelami FO, Usman T, Suravajhala P, Ali N, Do DN. Emerging roles of noncoding RNAs in bovine mastitis diseases. Pathog. 2022;11(9):1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Do DN, Suravajhala P. Editorial: Role of non-coding RNAs in Animals. Anim (Basel). 2023;13(5):805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bratkovič T, Božič J, Rogelj B. Functional diversity of small nucleolar RNAs. Nucleic Acids Res. 2020;48(4):1627–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang Zh, Du YP, Wen JT, Lu BF, Zhao Y. snoRNAs: functions and mechanisms in biological processes, and roles in tumor pathophysiology. Cell Death Discov. 2022;8(1):259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ono M, Yamada K, Avolio F, Scott MS, van Koningsbruggen S, Barton GJ, et al. Analysis of human small nucleolar RNAs (snoRNA) and the development of snoRNA modulator of gene expression vectors. Mol Biol Cell. 2010;21(9):1569–84. [DOI] [PMC free article] [PubMed]
- 16.Ender C, Krek A, Friedländer MR, Beitzinger M, Weinmann L, Chen W, et al. A human snoRNA with microRNA-like functions. Mol Cell. 2008;32(4):519–28. [DOI] [PubMed] [Google Scholar]
- 17.Huang C, Shi J, Guo Y, Huang W, Huang S, Ming S, et al. A snoRNA modulates mRNA 3′ end processing and regulates the expression of a subset of mRNAs. Nucleic Acids Res. 2017;45(15):8647–60. [DOI] [PMC free article] [PubMed]
- 18.Asano-Inami E, Yokoi A, Sugiyama M, Hyodo T, Hamaguchi T, Kajiyama H. The association of UBAP2L and G3BP1 mediated by small nucleolar RNA is essential for stress granule formation. Commun Biol. 2023;6(1):415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McCann KL, Kavari SL, Burkholder AB, Phillips BT, Hall Traci MT. H/ACA snoRNA levels are regulated during stem cell differentiation. Nucleic Acids Res. 2020;48(15):8686–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang M, Bissonnette N, Laterrière M, Dudemaine PL, Gagné D, Roy JP, et al. Methylome and transcriptome data integration reveals potential roles of DNA methylation and candidate biomarkers of cow Streptococcus uberis subclinical mastitis. J Anim Sci Biotechnol. 2022;13:136. [DOI] [PMC free article] [PubMed]
- 21.Ewels PA, Peltzer A, Fillinger S, Patel H, Alneberg J, Wilm A, et al. The nf-core framework for community-curated bioinformatics pipelines. Nat Biotechol. 2020;38(3):276–8. [DOI] [PubMed] [Google Scholar]
- 22.Bergeron D, Paraqindes H, Fafard-Couture É, Deschamps-Francoeur G, Faucher-Giguère L, Bouchard-Bourelle P, et al. snoDB 2.0: an enhanced interactive database, specializing in human snoRNAs. Nucleic Acids Res. 2022;51(D1):D291–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Araujo Oliveira JV, Costa F, Backofen R, Stadler PF, Machado Telles ME, Hertel J. SnoReport 2.0: new features and a refined support vector machine to improve snoRNA identification. BMC Bioinform. 2016;17(Suppl 18):464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B Methodol. 1995;57(1):289–300. [Google Scholar]
- 26.Chen X, Deng Z, Yu D, Zhang X, An Z, Wu W, et al. Genome-wide identification and analysis of small nucleolar RNAs and their roles in regulating latex regeneration in the rubber tree (Hevea brasiliensis). Front Plant Sci. 2021;12:731484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jorjani H, Kehr S, Jedlinski DJ, Gumienny R, Hertel J, Stadler PF, et al. An updated human snoRNAome. Nucleic Acids Res. 2016;44(11):5068–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tafer H, Kehr S, Hertel J, Hofacker IL, Stadler PF. RNAsnoop: efficient target prediction for H/ACA snoRNAs. Bioinform. 2010;26(5):610–6. [DOI] [PubMed] [Google Scholar]
- 29.Lowe TM, Eddy SR. A computational screen for methylation guide snoRNAs in yeast. Science. 1999;283(5405):1168–71. [DOI] [PubMed] [Google Scholar]
- 30.Wang M, Yang N, Laterrière M, Gagné D, Omonijo F, Ibeagha-Awemu EM. Multi-omics integration identifies regulatory factors underlying bovine subclinical mastitis. J Anim Sci Biotechnol. 2024;15:46. [DOI] [PMC free article] [PubMed]
- 31.Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13(11):2498–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chin CH, Chen SH, Wu HH, Ho CW, Ko MT, Lin CY. cytoHubba: identifying hub objects and sub-networks from complex interactome. BMC Syst Biol. 2014;8(Suppl 4):S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods. 2001;25(4):402–8. [DOI] [PubMed] [Google Scholar]
- 34.Penzo M, Clima R, Trerè D, Montanaro L. Separated Siamese twins: intronic small nucleolar RNAs and matched host genes may be altered in conjunction or separately in multiple cancer types. Cells. 2020;9(2):387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Deryusheva S, Talhouarne GJS, Gall JG. “Lost and Found”: snoRNA annotation in the Xenopus genome and implications for evolutionary studies. Mol BiolEvol. 2019;37(1):149–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang Y, Liu J, Jia C, Li T, Wu R, Wang J, et al. Systematic identification and evolutionary features of rhesus monkey small nucleolar RNAs. BMC Genom. 2010;11:61. [DOI] [PMC free article] [PubMed]
- 37.Guerrieri AN, Zacchini F, Onofrillo C, Di Viggiano S, Penzo M, Ansuini A, et al. DKC1 Overexpression induces a more aggressive cellular behavior and increases intrinsic ribosomal activity in immortalized mammary gland cells. Cancers. 2020;12(12):3512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grützmann K, Salomo K, Krüger A, Lohse-Fischer A, Erdmann K, Seifert M, et al. Identification of novel snoRNA-based biomarkers for clear cell renal cell carcinoma from urine-derived extracellular vesicles. Biol Direct. 2024;19(1):38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Qin Y, Meng L, Fu Y, Quan Z, Ma M, Weng M, et al. SNORA74B gene silencing inhibits gallbladder cancer cells by inducing PHLPP and suppressing Akt/mTOR signaling. Oncotarget. 2017;8(12):19980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ferreira HJ, Heyn H, Moutinho C, Esteller M. CpG island hypermethylation-associated silencing of small nucleolar RNAs in human cancer. RNA Biol. 2012;9(6):881–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pan X, Chen L, Feng K-Y, Hu X-H, Zhang Y-H, Kong X-Y, et al. Analysis of expression pattern of snoRNAs in different cancer types with machine learning algorithms. Int J Mol Sci. 2019;20(9):2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li MW, Huang FX, Xie ZC, Hong HY, Xu QY, Peng ZG. Identification of three small nucleolar RNAs (snoRNAs) as potential prognostic markers in diffuse large B-cell lymphoma. Cancer Med. 2023;12(3):3812–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Crea F, Quagliata L, Michael A, Liu HH, Frumento P, Azad AA, et al. Integrated analysis of the prostate cancer small-nucleolar transcriptome reveals SNORA55 as a driver of prostate cancer progression. Mol Oncol. 2016;10(5):693–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bagheri A, Khorshid HRK, Mowla SJ, Mohebbi HA, Mohammadian A, Yaseri M, et al. Altered miR-223 expression in sputum for diagnosis of non-small cell lung cancer. Avicenna J Med Biotechnol. 2017;9(4):189. [PMC free article] [PubMed] [Google Scholar]
- 45.Alfardan R. Small nuclear RNA 64 (snoRNA64): A novel tumor biomarker for pancreatic cancer. Baghdad Sci. J. 2023;20(5):31.
- 46.Warner WA, Spencer DH, Trissal M, White BS, Helton N, Ley TJ, et al. Expression profiling of snoRNAs in normal hematopoiesis and AML. Blood Adv. 2018;2(2):151–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wallemacq H, Bedoret D, Pujol J, Desmet C, Drion P-V, Farnir F, et al. CD40 triggering induces strong cytotoxic T lymphocyte responses to heat-killed Staphylococcus aureus immunization in mice: a new vaccine strategy for staphylococcal mastitis. Vaccine. 2012;30(12):2116–24. [DOI] [PubMed] [Google Scholar]
- 48.Liu Y, Zhang J, Zhou YH, Jiang YN, Zhang W, Tang XJ, et al. IL-6/STAT3 signaling pathway is activated in plasma cell mastitis. Int J Clin Exp Pathol. 2015;8(10):12541–8. [PMC free article] [PubMed] [Google Scholar]
- 49.Yan S, Zhang C, Ji X, Wu G, Huang X, Zhang Y, et al. MSC-ACE2 Ameliorates Streptococcus uberis-induced inflammatory injury in mammary epithelial cells by upregulating the IL-10/STAT3/SOCS3 pathway. Front Immunol. 2022;13:870780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang M, Bissonnette N, Laterrière M, Dudemaine PL, Gagné D, Roy JP, et al. Gene co-expression in response to Staphylococcus aureus infection reveals networks of genes with specific functions during bovine subclinical mastitis. J Dairy Sci. 2023;106(8):5517–36. [DOI] [PubMed] [Google Scholar]
- 51.Lin X, Zhao Z, Cai Y, He Y, Wang J, Liu N, et al. MyD88 deficiency in mammary epithelial cells attenuates lipopolysaccharide (LPS)-induced mastitis in mice. Biochem Biophys Res Commun. 2024;739:150569. [DOI] [PubMed] [Google Scholar]
- 52.Zhao J, Xu L, Lv L, Wang L, Wang X, Liang C, et al. Network pharmacology and in vivo and in vitro experiments to determine the mechanism behind the effects of Jiawei Yanghe decoction via TLR4/Myd88/NF-κB against mastitis. Heliyon. 2023;9(11):e21219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang M, Bissonnette N, Laterrière M, Gagné D, Dudemaine PL, Roy JP, et al. Genome-wide DNA methylation and transcriptome integration associates DNA methylation changes with bovine subclinical mastitis caused by Staphylococcus chromogenes. Int J Mol Sci. 2023;24(12):10369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hasankhani A, Bakherad M, Bahrami A, Shahrbabak HM, Pecho RDC, Shahrbabak MM. Integrated analysis of inflammatory mRNAs, miRNAs, and lncRNAs elucidates the molecular interactome behind bovine mastitis. Sci Rep. 2023;13:13826. [DOI] [PMC free article] [PubMed]
- 55.Jiang L, Sun H, Gu F, He J, Zhao F, Liu J. Blood neutrophil extracellular traps: a novel target for the assessment of mammary health in transition dairy cows. J Anim Sci Biotechnol. 2022;13:131. [DOI] [PMC free article] [PubMed]
- 56.Gao MQ, Zhang R, Yang Y, Luo Y, Jiang M, Zhang Y, et al. A subchronic feeding safety evaluation of transgenic milk containing human β-defensin 3 on reproductive system of C57BL/6J mouse. Food Chem Toxicol. 2018;115:198–204. [DOI] [PubMed] [Google Scholar]
- 57.Sordillo LM, Babiuk LA. Controlling acute Escherichia coli mastitis during the periparturient period with recombinant bovine interferon gamma. Vet Microbiol. 1991;28(2):189–98. [DOI] [PubMed] [Google Scholar]
- 58.Amatya N, Garg AV, Gaffen SL. IL-17 Signaling: The Yin and the Yang. Trends Immunol. 2017;38(5):310–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Roussel P, Cunha P, Porcherie A, Petzl W, Gilbert FB, Riollet C, et al. Investigating the contribution of IL-17A and IL-17F to the host response during Escherichia coli mastitis. Vet Res. 2015;46(1):56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vitenberga-Verza Z, Pilmane M, Šerstņova K, Melderis I, Gontar Ł, Kochański M, et al. Identification of inflammatory and regulatory cytokines IL-1α-, IL-4-, IL-6-, IL-12-, IL-13-, IL-17A-, TNF-α-, and IFN-γ-producing cells in the milk of dairy cows with subclinical and clinical mastitis. Pathog. 2022;11(3):372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tremblay YDN, Lamarche D, Chever P, Haine D, Messier S, Jacques M. Characterization of the ability of coagulase-negative staphylococci isolated from the milk of Canadian farms to form biofilms. J Dairy Sci. 2013;96(1):234–46. [DOI] [PubMed] [Google Scholar]
- 62.Pyörälä S, Taponen S. Coagulase-negative staphylococci-emerging mastitis pathogens. Vet Microbiol. 2009;134(1–2):3–8. [DOI] [PubMed] [Google Scholar]
- 63.Simojoki H, Orro T, Taponen S, Pyörälä S. Host response in bovine mastitis experimentally induced with Staphylococcus chromogenes. Vet Microbiol. 2009;134(1):95–9. [DOI] [PubMed] [Google Scholar]
- 64.Tomazi T, Gonçalves JL, Barreiro JR, Arcari MA, dos Santos MV. Bovine subclinical intramammary infection caused by coagulase-negative staphylococci increases somatic cell count but has no effect on milk yield or composition. J Dairy Sci. 2015;98(5):3071–8. [DOI] [PubMed] [Google Scholar]
- 65.Piccart K, Verbeke J, De Visscher A, Piepers S, Haesebrouck F, De Vliegher S. Local host response following an intramammary challenge with Staphylococcus fleurettii and different strains of Staphylococcus chromogenes in dairy heifers. Vet Res. 2016;47(1):56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Viganò S, Perreau M, Pantaleo G, Harari A. Positive and negative regulation of cellular immune responses in physiologic conditions and diseases. Clin Dev Immunol. 2012;2012:485781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Karauzum H, Datta SK. Adaptive immunity against staphylococcus aureus. Curr Top Microbiol Immunol. 2017;409:419–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rao S, Xu T, Xia Y, Zhang H. Salmonella and S. aureus escape from the clearance of macrophages via controlling TFEB. Front Microbiol. 2020;11:573844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Arun SJ, Thomson PC, Sheehy PA, Khatkar MS, Raadsma HW, Williamson P. Targeted analysis reveals an important role of JAK-STAT-SOCS genes for milk production traits in Australian dairy cattle. Front Genet. 2015;6:342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Briscoe J, Guschin D, Müller M. Signal Transduction: Just another signalling pathway. Curr Biol. 1994;4(11):1033–5. [DOI] [PubMed] [Google Scholar]
- 71.Groner B, Gouilleux F. Prolactin-mediated gene activation in mammary epithelial cells. Curr Opin Genet Dev. 1995;5(5):587–94. [DOI] [PubMed] [Google Scholar]
- 72.Sigl T, Meyer H, Wiedemann S. Gene expression analysis of protein synthesis pathways in bovine mammary epithelial cells purified from milk during lactation and short-term restricted feeding. J Anim Physiol Anim Nutr. 2014;98(1):84–95. [DOI] [PubMed] [Google Scholar]
- 73.Saleem A, Saleem Bhat S, Omonijo FA, Ganai NA, Ibeagha-Awemu EM, Mudasir Ahmad S. Immunotherapy in mastitis: state of knowledge, research gaps and way forward. Vet Q. 2024;44(1):1–23. [DOI] [PMC free article] [PubMed]
- 74.Stotts MJ, Zhang Y, Zhang S, Michal JJ, Velez J, Bothe H, et al. Alternative polyadenylation events in epithelial cells sense endometritis progression in dairy cows. J Integr Agric. 2023;22(6):1820–32. [Google Scholar]
- 75.Bochniarz M, Ziomek M, Szczubiał M, Dąbrowski R, Wochnik M, Kurek Ł, et al. Interleukin-6 as a milk marker of clinical and subclinical intramammary infections (IMI) in cows caused by Streptococcus spp. Animals. 2024;14(7):1100. [DOI] [PMC free article] [PubMed]
- 76.Islam MA, Takagi M, Fukuyama K, Komatsu R, Albarracin L, Nochi T, et al. Transcriptome analysis of the inflammatory responses of bovine mammary epithelial cells: exploring immunomodulatory target genes for bovine mastitis. Pathog. 2020;9(3):200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Varzandian B, Ghaderi-Zefrehei M, Hosseinzadeh S, Sayyadi M, Taghadosi V, Varzandian S. An investigation on the expression level of interleukin 10 (IL-10) in the healthy and mastitic holstein cows and the bioinformatics analysis of nucleosome profile. Anim Biotechnol. 2017;28(4):294–300. [DOI] [PubMed] [Google Scholar]
- 78.Gangur V, Birmingham NP, Thanesvorakul S. Chemokines in health and disease. Vet Immunol Immunopathol. 2002;86(3–4):127–36. [DOI] [PubMed] [Google Scholar]
- 79.Cambier S, Gouwy M, Proost P. The chemokines CXCL8 and CXCL12: molecular and functional properties, role in disease and efforts towards pharmacological intervention. Cell Mol Immunol. 2023;20(3):217–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Couper KN, Blount DG, Riley EM. IL-10: the master regulator of immunity to infection. J Immunol. 2008;180(9):5771–7. [DOI] [PubMed] [Google Scholar]
- 81.Asadullah K, Sterry W, Volk H. Interleukin-10 therapy—review of a new approach. Pharmacol Rev. 2003;55(2):241–69. [DOI] [PubMed] [Google Scholar]
- 82.Kawecka-Grochocka E, Zalewska M, Rzewuska M, Kościuczuk E, Ząbek T, Sakowski T, et al. Expression of cytokines in dairy cattle mammary gland parenchyma during chronic staphylococcal infection. Vet Res. 2021;52:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pereira HP, Verardo LL, Weller MMDCA, Sbardella AP, Munari DP, de Paiva Daibert RM, et al. Going further post-RNA-seq: In silico functional analyses revealing candidate genes and regulatory elements related to mastitis in dairy cattle. J Dairy Res. 2021;88(3):286–92. [DOI] [PubMed] [Google Scholar]
- 84.Song N, Wang X, Gui L, Raza SHA, Luoreng Z, Zan L. MicroRNA-214 regulates immunity-related genes in bovine mammary epithelial cells by targeting NFATc3 and TRAF3. Mol Cell Probes. 2017;35:27–33. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material 1. Table S1 General whole genome snoRNA sequence statistics per sample.
Supplementary Material 2. Table S2 List of identified snoRNAs expressed in samples.
Supplementary Material 3. Table S3A List of differntially expressed snoRNAs in Staphylococcus aureus versus control group. Table S3B List of differntially expressed snoRNAs in Staphylococcus chromogenes versus control group.
Supplementary Material 4. Table S4A Summary of snoRNA function and their rRNA target modification site in Stahylococcus aureus versus control group. Table S4B Summary of snoRNA function and their rRNA target modification site in Stahylococcus chromogenes versus control group.
Supplementary Material 5. Table S5A Correlation anaysis of differentially expressed (DE) snoRNAs and DE mRNAs in Staphylococcus aureus versus control group. Table S5B Correlation anaysis of differentially expressed (DE) snoRNAs and DE mRNAs in Staphylococcus chromogenes versus control group.
Supplementary Material 6. Table S6A Functional enrichment Gene Ontology Biological processes of differentially expressed snoRNA correlated genes of Staphylococcus aureus versus control group. Table S6B Functional enrichment Gene Ontology Biological processes of differentially expressed snoRNA correlated genes of Staphylococcus chromogenes versus control group. Table S6C Functional enrichment KEGG pathway analysis of differentially expressed snoRNA correlated genes of Staphylococcus aureus versus control group. Table S6D Functional enrichment KEGG pathway analysis of differentially expressed snoRNA correlated genes of Staphylococcus chromogenes versus control group. Table S6E Functional enrichment KEGG pathway analysis of differentially expressed snoRNA correlated genes specific to Staphylococcus aureus, Staphylococcus chromogenes and Staphylococcus aureus versus Staphylococcus chromogenes. Table S6F Functional enrichment Gene Ontology Biological processes terms of differentially expressed snoRNA correlated genes specific to Staphylococcus aureus, Staphylococcus chromogenes and Staphylococcus aureus versus Staphylococcus chromogenes. Table S6G Functional enrichment KEGG pathways of 7 hub snoRNAs correlated genes of Staphylococcus chromogenes versus control group. Table S6H Functional enrichment Gene Ontology Biological processes of 7 hub snoRNAs correlated genes of Staphylococcus chromogenes versus control group. Table S6I Functional enrichment Gene Ontology Biological processes of 7 hub snoRNAs correlated genes of Staphylococcus aureus versus control group. Table S6J Functional enrichment KEGG pathways of 7 hub snoRNAs correlated genes of Staphylococcus aureus versus control group. Table S6K Functional enrichment Gene Ontology Biological processes of SNORA1 correlated genes of Staphylococcus aureus versus control group. Table S6L Functional enrichment KEGG pathway analysis of differentially expressed SNORA1 correlated genes of Staphylococcus aureus versus control group. Table S6M Functional enrichment Gene Ontology Biological processes of SNORA66 correlated genes of Staphylococcus aureus versus control group. Table S6N Functional enrichment KEGG pathway analysis of differentially expressed SNORA66 correlated genes of Staphylococcus aureus versus control group. Table S6O Functional enrichment Gene Ontology Biological processes of SNORA79 correlated genes of Staphylococcus chromogenes versus control group. Table S6P Functional enrichment KEGG pathway analysis of differentially expressed SNORA79 correlated genes of Staphylococcus aureus versus control group. Table S6Q Functional enrichment Gene Ontology Biological processes of SNORD49 correlated genes of Staphylococcus chromogenes versus control group. Table S6R Functional enrichment KEGG pathway analysis of differentially expressed SNORD49 correlated genes of Staphylococcus aureus versus control group.
Supplementary Material 7. Table S7 Genes and their primer sequences used for real-time qPCR validation of RNA-sequencing results.
Supplementary Material 8. Fig. S1 Principal component analysis plot showing the sample cluster per group. Fig. S2 Correlation network between hub snoRNAs and target DE genes for Staphylococcus aureus versus the control. Fig. S3 Correlation network between hub snoRNAs and target DE genes for Staphylococcus chromogenes versus the control.
Data Availability Statement
The raw read sequences analyzed in this study have been deposited in NCBI Sequence Read Archive (SRA) under the Bio Projects PRJNA878880 and PRJNA967255.