Abstract
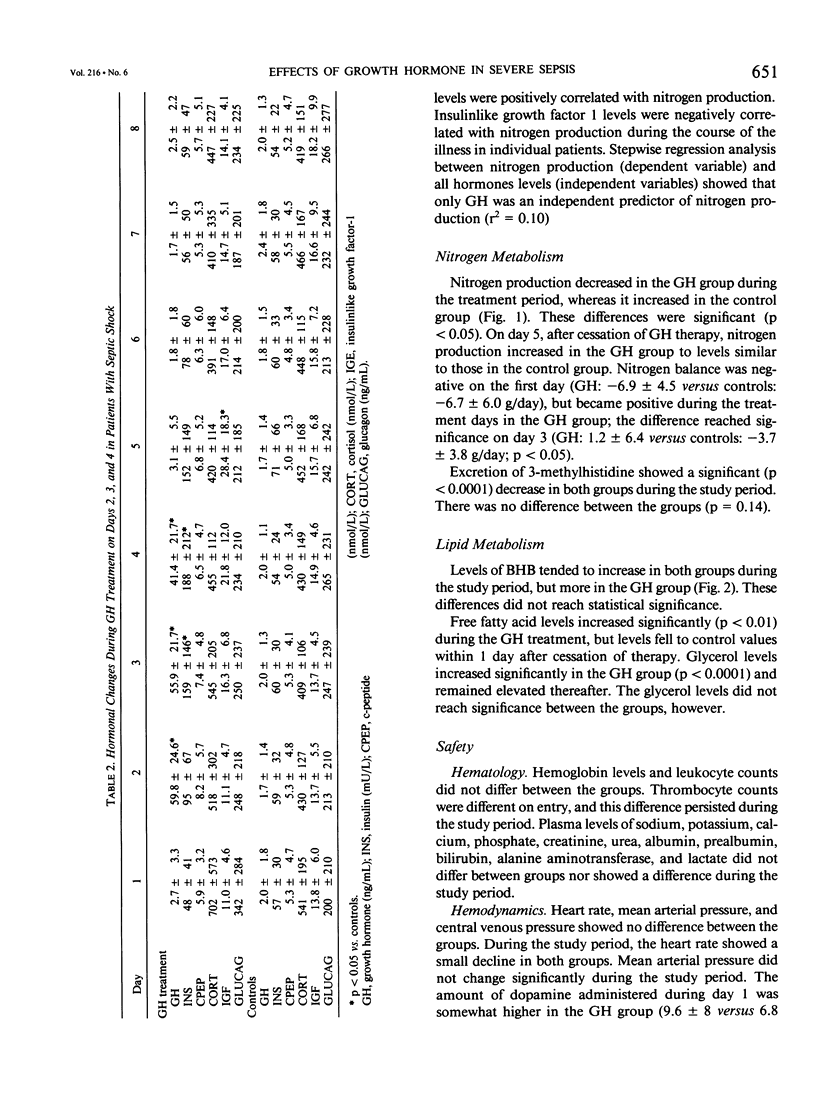
The objective of this study was to evaluate the safety and the effect of recombinant exogenous growth hormone (GH) on nitrogen production in patients with severe sepsis. It was designed as a prospective, randomized, placebo-controlled trial, and performed in the medical intensive care unit of a university hospital. Twenty patients admitted with septic shock and receiving standard parenteral nutrition served as subjects. Treatment consisted of GH 0.1 mg/kg/day or placebo administered as continuous intravenous infusion on the second, third, and fourth days after admission. The study period was eight days. During GH administration, nitrogen production decreased significantly in the GH group and increased in controls (p < 0.01). Nitrogen balance became slightly positive in the GH group during treatment: 1.2 +/- 6.4 versus controls -3.7 +/- 3.8 g/day (day 3) (p < 0.05). Within 24 hours after cessation of treatment, differences between GH and controls disappeared. 3-Methylhistidine excretion as a measure of absolute muscle breakdown declined during the study period, but did not differ between groups. The levels of insulin, insulinlike growth factor 1, glycerol, free fatty acids, and beta-hydroxybutyrate increased during treatment. Despite continuous intravenous administration, GH levels gradually declined during the 3 treatment days, indicating increased metabolic clearance. Side effects other than insulin resistance were not observed. Growth hormone administration reduces nitrogen production and improves nitrogen balance in patients with severe sepsis. These effects are not sustained after cessation of treatment.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albertsson-Wikland K., Edén S., Ahrén K., Isaksson O. Analysis of refractoriness to the effects of growth hormone on amino acid transport and protein synthesis in diaphragms of young normal rats. Endocrinology. 1980 Jan;106(1):298–305. doi: 10.1210/endo-106-1-298. [DOI] [PubMed] [Google Scholar]
- Albertsson-Wilkland K., Edén S., Isaksson O. Analysis of early responses to growth hormone on amino acid transport and protein synthesis in diaphragms of young normal rats. Endocrinology. 1980 Jan;106(1):291–297. doi: 10.1210/endo-106-1-291. [DOI] [PubMed] [Google Scholar]
- Buzby G. P., Knox L. S., Crosby L. O., Eisenberg J. M., Haakenson C. M., McNeal G. E., Page C. P., Peterson O. L., Reinhardt G. F., Williford W. O. Study protocol: a randomized clinical trial of total parenteral nutrition in malnourished surgical patients. Am J Clin Nutr. 1988 Feb;47(2 Suppl):366–381. doi: 10.1093/ajcn/47.2.366. [DOI] [PubMed] [Google Scholar]
- Crist D. M., Kraner J. C. Supplemental growth hormone increases the tumor cytotoxic activity of natural killer cells in healthy adults with normal growth hormone secretion. Metabolism. 1990 Dec;39(12):1320–1324. doi: 10.1016/0026-0495(90)90191-e. [DOI] [PubMed] [Google Scholar]
- Dahn M. S., Lange M. P., Jacobs L. A. Insulinlike growth factor 1 production is inhibited in human sepsis. Arch Surg. 1988 Nov;123(11):1409–1414. doi: 10.1001/archsurg.1988.01400350123019. [DOI] [PubMed] [Google Scholar]
- Dahn M. S., Mitchell R. A., Smith S., Lange M. P., Whitcomb M. P., Kirkpatrick J. R. Altered immunologic function and nitrogen metabolism associated with depression of plasma growth hormone. JPEN J Parenter Enteral Nutr. 1984 Nov-Dec;8(6):690–694. doi: 10.1177/0148607184008006690. [DOI] [PubMed] [Google Scholar]
- Dempsey D. T., Mullen J. L., Buzby G. P. The link between nutritional status and clinical outcome: can nutritional intervention modify it? Am J Clin Nutr. 1988 Feb;47(2 Suppl):352–356. doi: 10.1093/ajcn/47.2.352. [DOI] [PubMed] [Google Scholar]
- Douglas R. G., Humberstone D. A., Haystead A., Shaw J. H. Metabolic effects of recombinant human growth hormone: isotopic studies in the postabsorptive state and during total parenteral nutrition. Br J Surg. 1990 Jul;77(7):785–790. doi: 10.1002/bjs.1800770722. [DOI] [PubMed] [Google Scholar]
- Frayn K. N. Hormonal control of metabolism in trauma and sepsis. Clin Endocrinol (Oxf) 1986 May;24(5):577–599. doi: 10.1111/j.1365-2265.1986.tb03288.x. [DOI] [PubMed] [Google Scholar]
- Fryburg D. A., Gelfand R. A., Barrett E. J. Growth hormone acutely stimulates forearm muscle protein synthesis in normal humans. Am J Physiol. 1991 Mar;260(3 Pt 1):E499–E504. doi: 10.1152/ajpendo.1991.260.3.E499. [DOI] [PubMed] [Google Scholar]
- Gluckman P. D., Douglas R. G., Ambler G. R., Breier B. H., Hodgkinson S. C., Koea J. B., Shaw J. H. The endocrine role of insulin-like growth factor I. Acta Paediatr Scand Suppl. 1991;372:97–106. doi: 10.1111/j.1651-2227.1991.tb17981.x. [DOI] [PubMed] [Google Scholar]
- Gore D. C., Honeycutt D., Jahoor F., Wolfe R. R., Herndon D. N. Effect of exogenous growth hormone on whole-body and isolated-limb protein kinetics in burned patients. Arch Surg. 1991 Jan;126(1):38–43. doi: 10.1001/archsurg.1991.01410250042006. [DOI] [PubMed] [Google Scholar]
- Gottardis M., Benzer A., Koller W., Luger T. J., Pühringer F., Hackl J. Improvement of septic syndrome after administration of recombinant human growth hormone (rhGH)? J Trauma. 1991 Jan;31(1):81–86. doi: 10.1097/00005373-199101000-00015. [DOI] [PubMed] [Google Scholar]
- Hoffer L. J. Nutritional status affects renal 3-methylhistidine handling in humans. Metabolism. 1990 Jul;39(7):744–748. doi: 10.1016/0026-0495(90)90111-o. [DOI] [PubMed] [Google Scholar]
- Horber F. F., Marsh H. M., Haymond M. W. Differential effects of prednisone and growth hormone on fuel metabolism and insulin antagonism in humans. Diabetes. 1991 Jan;40(1):141–149. doi: 10.2337/diab.40.1.141. [DOI] [PubMed] [Google Scholar]
- Isley W. L., Underwood L. E., Clemmons D. R. Dietary components that regulate serum somatomedin-C concentrations in humans. J Clin Invest. 1983 Feb;71(2):175–182. doi: 10.1172/JCI110757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Z. M., He G. Z., Zhang S. Y., Wang X. R., Yang N. F., Zhu Y., Wilmore D. W. Low-dose growth hormone and hypocaloric nutrition attenuate the protein-catabolic response after major operation. Ann Surg. 1989 Oct;210(4):513–525. doi: 10.1097/00000658-198910000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jørgensen J. O., Blum W. F., Møller N., Ranke M. B., Christiansen J. S. Short-term changes in serum insulin-like growth factors (IGF) and IGF binding protein 3 after different modes of intravenous growth hormone (GH) exposure in GH-deficient patients. J Clin Endocrinol Metab. 1991 Mar;72(3):582–587. doi: 10.1210/jcem-72-3-582. [DOI] [PubMed] [Google Scholar]
- Jørgensen J. O., Flyvbjerg A., Lauritzen T., Orskov H., Christiansen J. S. Subcutaneous degradation of biosynthetic human growth hormone in growth hormone deficient patients. Acta Endocrinol (Copenh) 1988 May;118(1):154–158. doi: 10.1530/acta.0.1180154. [DOI] [PubMed] [Google Scholar]
- Kimbrough T. D., Shernan S., Ziegler T. R., Scheltinga M., Wilmore D. W. Insulin-like growth factor-I response is comparable following intravenous and subcutaneous administration of growth hormone. J Surg Res. 1991 Dec;51(6):472–476. doi: 10.1016/0022-4804(91)90167-k. [DOI] [PubMed] [Google Scholar]
- Knaus W. A., Draper E. A., Wagner D. P., Zimmerman J. E. APACHE II: a severity of disease classification system. Crit Care Med. 1985 Oct;13(10):818–829. [PubMed] [Google Scholar]
- Kostyo J. L., Reagan C. R. The biology of growth hormone. Pharmacol Ther B. 1976;2(3):591–604. doi: 10.1016/0306-039x(76)90009-x. [DOI] [PubMed] [Google Scholar]
- Long C. L., Dillard D. R., Bodzin J. H., Geiger J. W., Blakemore W. S. Validity of 3-methylhistidine excretion as an indicator of skeletal muscle protein breakdown in humans. Metabolism. 1988 Sep;37(9):844–849. doi: 10.1016/0026-0495(88)90118-7. [DOI] [PubMed] [Google Scholar]
- Okamura K., Okuma T., Tabira Y., Miyauchi Y. Effect of administered human growth hormone on protein metabolism in septic rats. JPEN J Parenter Enteral Nutr. 1989 Sep-Oct;13(5):450–454. doi: 10.1177/0148607189013005450. [DOI] [PubMed] [Google Scholar]
- Ponting G. A., Halliday D., Teale J. D., Sim A. J. Postoperative positive nitrogen balance with intravenous hyponutrition and growth hormone. Lancet. 1988 Feb 27;1(8583):438–440. doi: 10.1016/s0140-6736(88)91232-9. [DOI] [PubMed] [Google Scholar]
- Roeder R. A., Hossner K. L., Sasser R. G., Gunn J. M. Regulation of protein turnover by recombinant human insulin-like growth factor-I in L6 myotube cultures. Horm Metab Res. 1988 Nov;20(11):698–700. doi: 10.1055/s-2007-1010920. [DOI] [PubMed] [Google Scholar]
- Shaw J. H., Wildbore M., Wolfe R. R. Whole body protein kinetics in severely septic patients. The response to glucose infusion and total parenteral nutrition. Ann Surg. 1987 Mar;205(3):288–294. doi: 10.1097/00000658-198703000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder D. K., Clemmons D. R., Underwood L. E. Dietary carbohydrate content determines responsiveness to growth hormone in energy-restricted humans. J Clin Endocrinol Metab. 1989 Oct;69(4):745–752. doi: 10.1210/jcem-69-4-745. [DOI] [PubMed] [Google Scholar]
- Streat S. J., Beddoe A. H., Hill G. L. Aggressive nutritional support does not prevent protein loss despite fat gain in septic intensive care patients. J Trauma. 1987 Mar;27(3):262–266. doi: 10.1097/00005373-198703000-00006. [DOI] [PubMed] [Google Scholar]
- Teerlink T., de Boer E. Determination of 3-methylhistidine in urine by high-performance liquid chromatography using pre-column derivatization with 9-fluorenylmethyl chloroformate. J Chromatogr. 1989 Jul 21;491(2):418–423. doi: 10.1016/s0378-4347(00)82860-1. [DOI] [PubMed] [Google Scholar]
- Ward H. C., Halliday D., Sim A. J. Protein and energy metabolism with biosynthetic human growth hormone after gastrointestinal surgery. Ann Surg. 1987 Jul;206(1):56–61. doi: 10.1097/00000658-198707000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilmore D. W., Moylan J. A., Jr, Bristow B. F., Mason A. D., Jr, Pruitt B. A., Jr Anabolic effects of human growth hormone and high caloric feedings following thermal injury. Surg Gynecol Obstet. 1974 Jun;138(6):875–884. [PubMed] [Google Scholar]
- Wroblewski V. J., Masnyk M., Becker G. W. Proteolytic cleavage of human growth hormone (hGH) by rat tissues in vitro: influence on the kinetics of exogenously administered hGH. Endocrinology. 1991 Jul;129(1):465–474. doi: 10.1210/endo-129-1-465. [DOI] [PubMed] [Google Scholar]
- Young V. R., Munro H. N. Ntau-methylhistidine (3-methylhistidine) and muscle protein turnover: an overview. Fed Proc. 1978 Jul;37(9):2291–2300. [PubMed] [Google Scholar]
- Zaloga G. P., Chernow B. The multifactorial basis for hypocalcemia during sepsis. Studies of the parathyroid hormone-vitamin D axis. Ann Intern Med. 1987 Jul;107(1):36–41. doi: 10.7326/0003-4819-107-1-36. [DOI] [PubMed] [Google Scholar]
- Zapf J., Froesch E. R. Pathophysiological and clinical aspects of the insulin-like growth factors. Horm Res. 1986;24(2-3):160–165. doi: 10.1159/000180555. [DOI] [PubMed] [Google Scholar]
- Ziegler T. R., Young L. S., Ferrari-Baliviera E., Demling R. H., Wilmore D. W. Use of human growth hormone combined with nutritional support in a critical care unit. JPEN J Parenter Enteral Nutr. 1990 Nov-Dec;14(6):574–581. doi: 10.1177/0148607190014006574. [DOI] [PubMed] [Google Scholar]


