Abstract
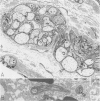
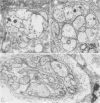
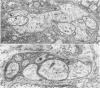
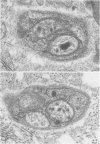
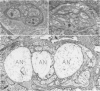
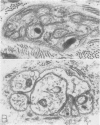
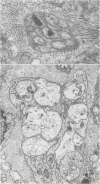
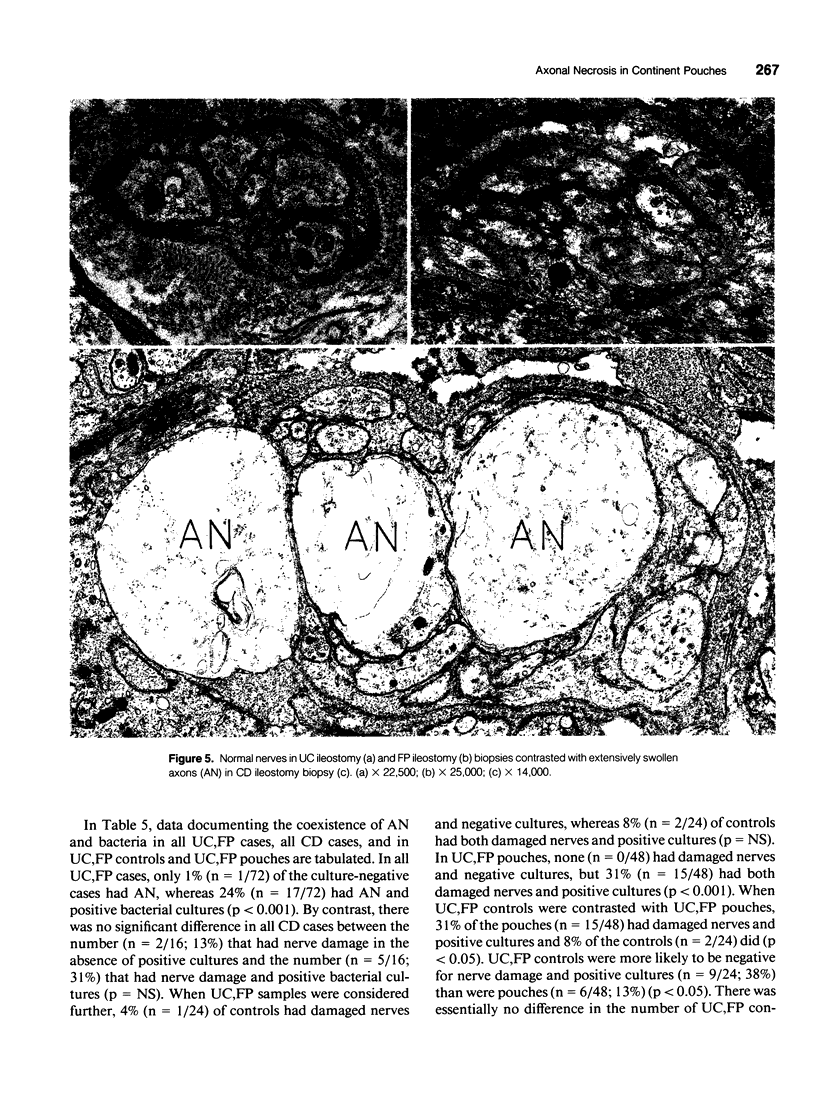
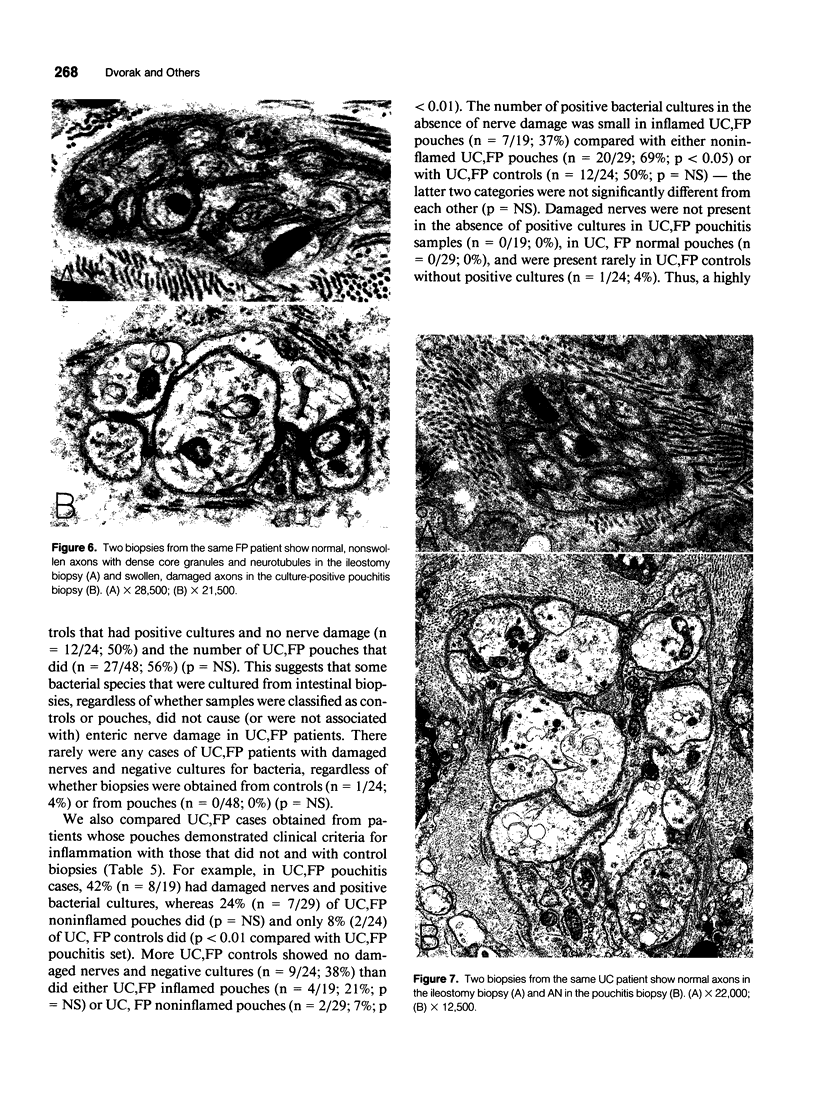
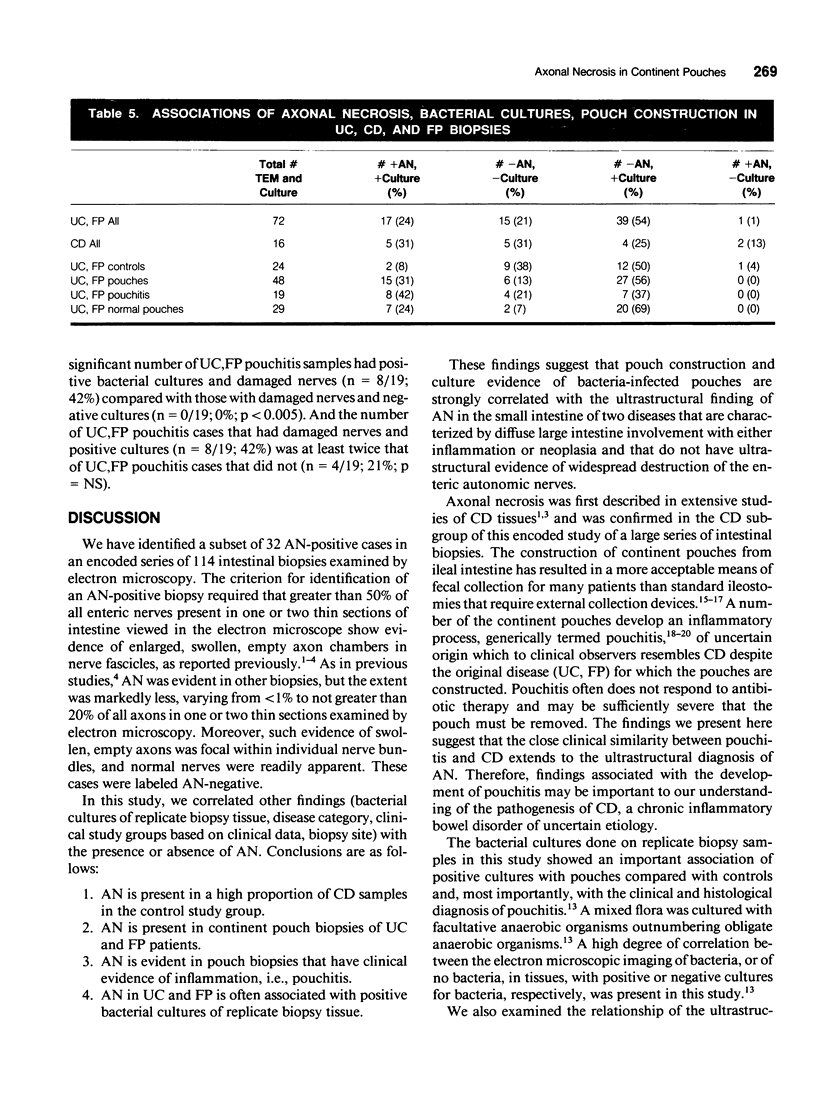
OBJECTIVE: Axonal necrosis was first described in samples of small intestine from patients with Crohn's disease (A.M. Dvorak et al. Hum Pathol 1980; 11:620-634). Clinically evident inflammation of continent ileal reservoirs (pouches) has clinical features that resemble Crohn's disease. Possible similarities in the pathogenesis of Crohn's disease and pouchitis were sought using ultrastructural and microbiologic tools to identify damaged enteric nerves and tissue bacteria. METHODS: An encoded ultrastructural and microbiologic study of replicate biopsies from 114 samples of human intestine was done. Biopsies from ileum, colon, conventional ileostomy or continent pouch were obtained from patients with ulcerative colitis, Crohn's disease, or familial polyposis and grouped into three clinical study groups (control, normal pouch, pouchitis), based on clinical and endoscopic criteria. Biopsies were prepared for electron microscopy with standard methods; replicate biopsy samples were washed extensively before preparing cultures designed to identify aerobic as well as facultative and obligate anaerobic bacteria (Onderdonk et al. J Clin Microbiol 1992; 30:312-317). The ultrastructural diagnosis of damaged enteric nerves was based on previously published criteria for axonal necrosis (A.M. Dvorak and W. Silen. Ann Surg 1985; 201:53-63). Intergroup comparisons were tested for significance using Chi-square analysis. RESULTS: The highest incidence of axonal necrosis was present in Crohn's disease control biopsies (53%), regardless of whether bacteria were present (or not) in cultures of replicate biopsies. Axonal necrosis also occurred in more ulcerative colitis and familial polyposis biopsies (regardless of biopsy site) that had positive bacterial cultures than in those that did not (p < 0.001). In addition, axonal necrosis was documented in 42% of the pouch biopsies from ulcerative colitis and familial polyposis patients, particularly in those pouches that were found to be inflamed by clinical criteria and that also had positive bacterial cultures of the biopsied tissues. Control biopsies from patients with ulcerative colitis and familial polyposis had significantly less nerve damage than pouch biopsies in the presence of positive cultures (p < 0.01). Among the clinically inflamed pouches biopsied in ulcerative colitis or familial polyposis patients, we found that none had damaged enteric nerves when bacterial cultures were negative (p < 0.005). If the presence of axonal necrosis alone was compared with the presence of undamaged enteric nerves in all biopsies from patients with ulcerative colitis, a highly significant number of ulcerative colitis biopsies with axonal necrosis occurred in pouches (72%) compared with controls (p < 0.001). CONCLUSIONS: The ultrastructural finding of axonal necrosis in Crohn's disease confirms previous studies. The presence of damaged enteric nerves in patients with pouchitis provides ultrastructural support to the clinical impression of similarities between pouchitis and Crohn's disease. The association of damaged nerves and invasive bacteria in pouchitis suggests mechanistic similarities for the pathogenesis of Crohn's disease that requires further investigation.
Full text
PDF

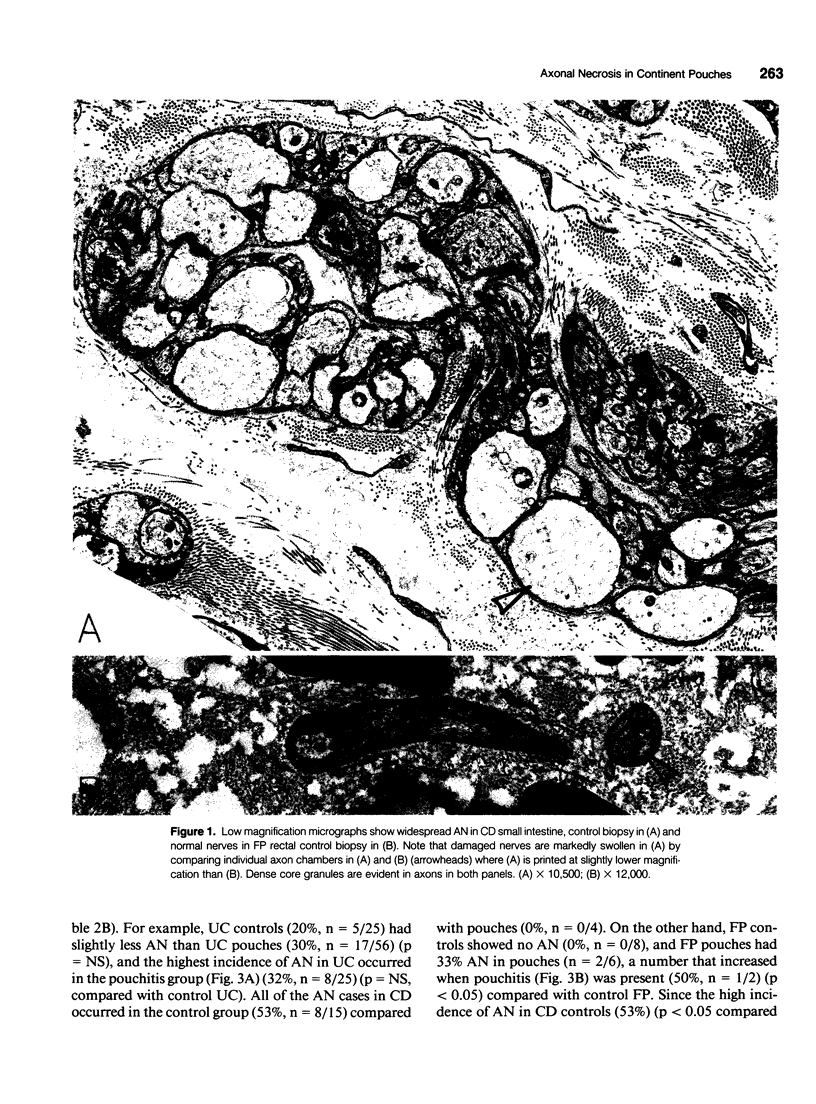
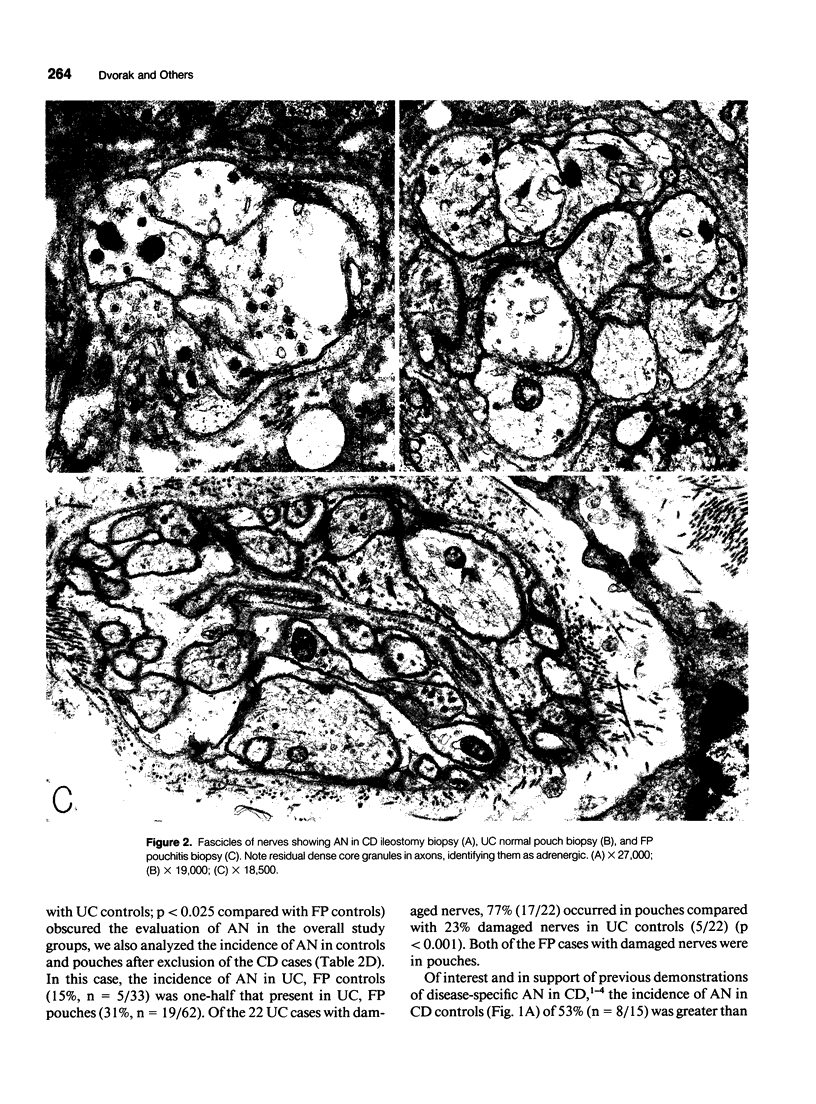
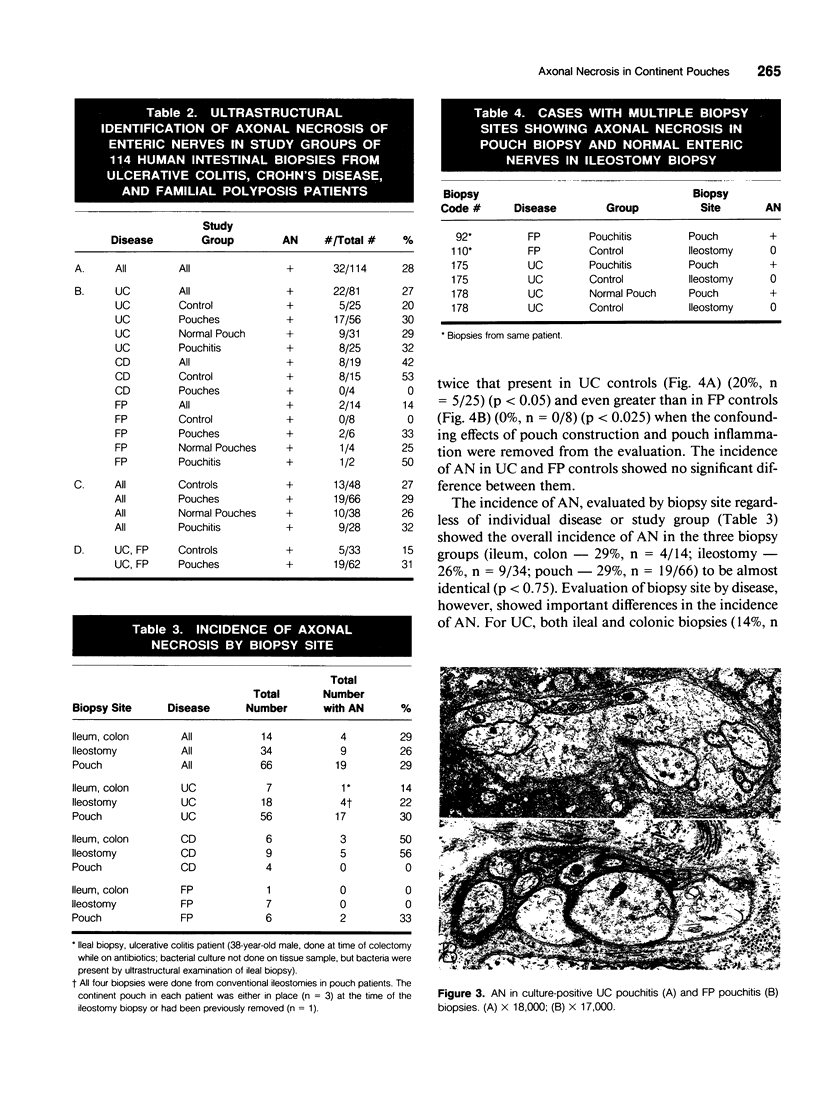
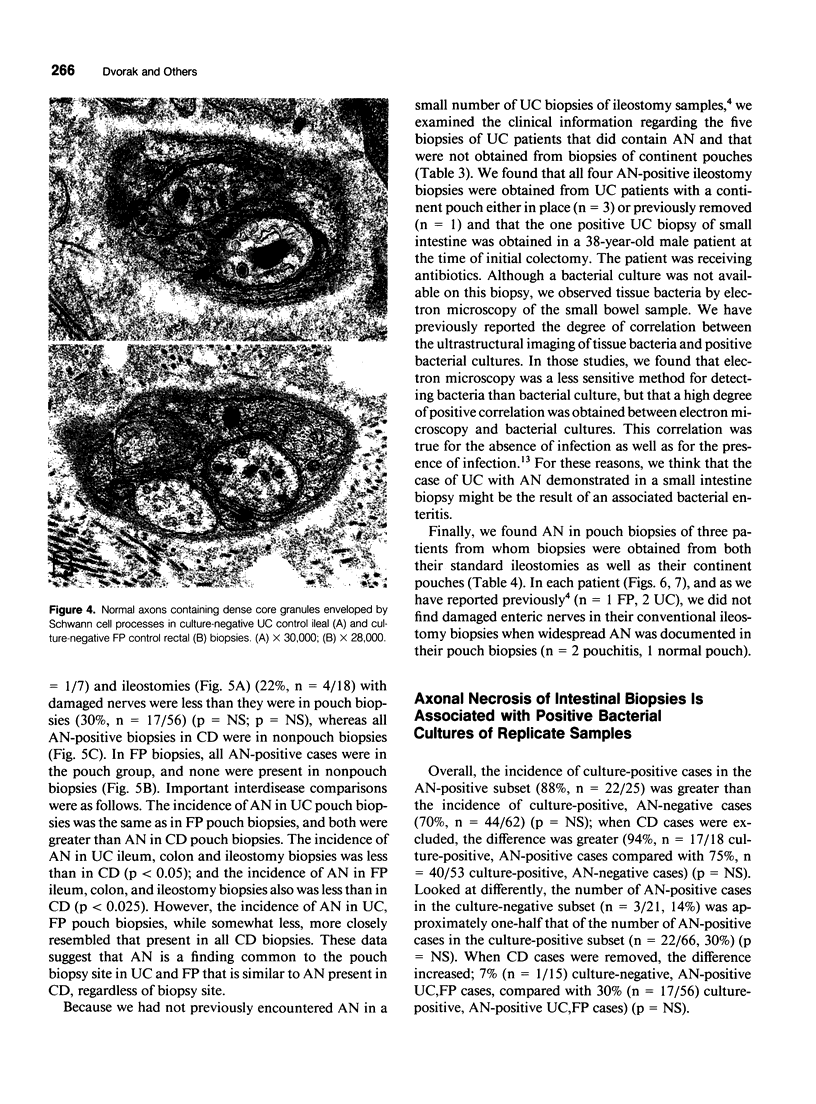


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Durack D. T., Sumi S. M., Klebanoff S. J. Neurotoxicity of human eosinophils. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1443–1447. doi: 10.1073/pnas.76.3.1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvorak A. M., McLeod R. S., Onderdonk A. B., Monahan-Earley R. A., Cullen J. B., Antonioli D. A., Morgan E., Blair J. E., Estrella P., Cisneros R. L. Human gut mucosal mast cells: ultrastructural observations and anatomic variation in mast cell-nerve associations in vivo. Int Arch Allergy Immunol. 1992;98(2):158–168. doi: 10.1159/000236180. [DOI] [PubMed] [Google Scholar]
- Dvorak A. M., McLeod R. S., Onderdonk A., Monahan-Earley R. A., Cullen J. B., Antonioli D. A., Morgan E., Blair J. E., Estrella P., Cisneros R. L. Ultrastructural evidence for piecemeal and anaphylactic degranulation of human gut mucosal mast cells in vivo. Int Arch Allergy Immunol. 1992;99(1):74–83. doi: 10.1159/000236338. [DOI] [PubMed] [Google Scholar]
- Dvorak A. M., Mihm M. C., Jr, Dvorak H. F. Degranulation of basophilic leukocytes in allergic contact dermatitis reactions in man. J Immunol. 1976 Mar;116(3):687–695. [PubMed] [Google Scholar]
- Dvorak A. M., Monahan R. A., Osage J. E., Dickersin G. R. Crohn's disease: transmission electron microscopic studies. II. Immunologic inflammatory response. Alterations of mast cells, basophils, eosinophils, and the microvasculature. Hum Pathol. 1980 Nov;11(6):606–619. doi: 10.1016/s0046-8177(80)80072-4. [DOI] [PubMed] [Google Scholar]
- Dvorak A. M., Osage J. E., Monahan R. A., Dickersin G. R. Crohn's disease: transmission electron microscopic studies. III. Target tissues. Proliferation of and injury to smooth muscle and the autonomic nervous system. Hum Pathol. 1980 Nov;11(6):620–634. doi: 10.1016/s0046-8177(80)80073-6. [DOI] [PubMed] [Google Scholar]
- Dvorak A. M., Silen W. Differentiation between Crohn's disease and other inflammatory conditions by electron microscopy. Ann Surg. 1985 Jan;201(1):53–63. [PMC free article] [PubMed] [Google Scholar]
- Dvorak A. M. Ultrastructural evidence for release of major basic protein-containing crystalline cores of eosinophil granules in vivo: cytotoxic potential in Crohn's disease. J Immunol. 1980 Jul;125(1):460–462. [PubMed] [Google Scholar]
- Espersen F., Jarløv J. O., Jensen C., Skov P. S., Norn S. Staphylococcus aureus peptidoglycan induces histamine release from basophil human leukocytes in vitro. Infect Immun. 1984 Dec;46(3):710–714. doi: 10.1128/iai.46.3.710-714.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaston J. S., Life P. F., Bailey L. C., Bacon P. A. In vitro responses to a 65-kilodalton mycobacterial protein by synovial T cells from inflammatory arthritis patients. J Immunol. 1989 Oct 15;143(8):2494–2500. [PubMed] [Google Scholar]
- Gleich G. J., Frigas E., Loegering D. A., Wassom D. L., Steinmuller D. Cytotoxic properties of the eosinophil major basic protein. J Immunol. 1979 Dec;123(6):2925–2927. [PubMed] [Google Scholar]
- Goetzl E. J., Chernov T., Renold F., Payan D. G. Neuropeptide regulation of the expression of immediate hypersensitivity. J Immunol. 1985 Aug;135(2 Suppl):802s–805s. [PubMed] [Google Scholar]
- Klebanoff S. J., Agosti J. M., Jörg A., Waltersdorph A. M. Comparative toxicity of the horse eosinophil peroxidase-H2O2-halide system and granule basic proteins. J Immunol. 1989 Jul 1;143(1):239–244. [PubMed] [Google Scholar]
- Knutson L., Ahrenstedt O., Odlind B., Hällgren R. The jejunal secretion of histamine is increased in active Crohn's disease. Gastroenterology. 1990 Apr;98(4):849–854. doi: 10.1016/0016-5085(90)90006-m. [DOI] [PubMed] [Google Scholar]
- Koch C., Andersen P., Boëtius Hertz J., Høiby N., Kappelgaard E., Møller N. E., Norn S., Pedersen M., Petersen P. S., Stahl Skov P. Studies on hypersensitivity to bacterial antigens in intrinsic asthma. Allergy. 1982 Apr;37(3):191–201. doi: 10.1111/j.1398-9995.1982.tb01896.x. [DOI] [PubMed] [Google Scholar]
- Kock N. G., Darle N., Hultén L., Kewenter J., Myrvold H., Philipson B. Ileostomy. Curr Probl Surg. 1977 Aug;14(8):1–52. doi: 10.1016/s0011-3840(77)80065-8. [DOI] [PubMed] [Google Scholar]
- Lehrer R. I., Szklarek D., Barton A., Ganz T., Hamann K. J., Gleich G. J. Antibacterial properties of eosinophil major basic protein and eosinophil cationic protein. J Immunol. 1989 Jun 15;142(12):4428–4434. [PubMed] [Google Scholar]
- Lowman M. A., Benyon R. C., Church M. K. Characterization of neuropeptide-induced histamine release from human dispersed skin mast cells. Br J Pharmacol. 1988 Sep;95(1):121–130. doi: 10.1111/j.1476-5381.1988.tb16555.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden M. V., Farthing M. J., Nicholls R. J. Inflammation in ileal reservoirs: 'pouchitis'. Gut. 1990 Mar;31(3):247–249. doi: 10.1136/gut.31.3.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norn S., Stahl Skov P., Kock C., Andersen P., Pedersen M., Tønnesen P., Pedersen P. S., Møller N. E., Hertz J., Høiby N. Intrinsic asthma and bacterial histamine release. Agents Actions. 1982 Apr;12(1-2):101–102. doi: 10.1007/BF01965116. [DOI] [PubMed] [Google Scholar]
- Onderdonk A. B., Dvorak A. M., Cisneros R. L., McLeod R. S., Antionoli D., Silen W., Blair J. E., Monahan-Earley R. A., Cullen J., Cohen Z. Microbiologic assessment of tissue biopsy samples from ileal pouch patients. J Clin Microbiol. 1992 Feb;30(2):312–317. doi: 10.1128/jcm.30.2.312-317.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patella V., Casolaro V., Björck L., Marone G. Protein L. A bacterial Ig-binding protein that activates human basophils and mast cells. J Immunol. 1990 Nov 1;145(9):3054–3061. [PubMed] [Google Scholar]
- Polla B. S. A role for heat shock proteins in inflammation? Immunol Today. 1988 May;9(5):134–137. doi: 10.1016/0167-5699(88)91199-1. [DOI] [PubMed] [Google Scholar]
- Shanahan F., Denburg J. A., Fox J., Bienenstock J., Befus D. Mast cell heterogeneity: effects of neuroenteric peptides on histamine release. J Immunol. 1985 Aug;135(2):1331–1337. [PubMed] [Google Scholar]
- Steinhoff M. M., Kodner I. J., DeSchryver-Kecskemeti K. Axonal degeneration/necrosis: a possible ultrastructural marker for Crohn's disease. Mod Pathol. 1988 May;1(3):182–187. [PubMed] [Google Scholar]
- Tytgat G. N., van Deventer S. J. Pouchitis. Int J Colorectal Dis. 1988 Nov;3(4):226–228. doi: 10.1007/BF01660720. [DOI] [PubMed] [Google Scholar]
- Young J. D., Peterson C. G., Venge P., Cohn Z. A. Mechanism of membrane damage mediated by human eosinophil cationic protein. Nature. 1986 Jun 5;321(6070):613–616. doi: 10.1038/321613a0. [DOI] [PubMed] [Google Scholar]