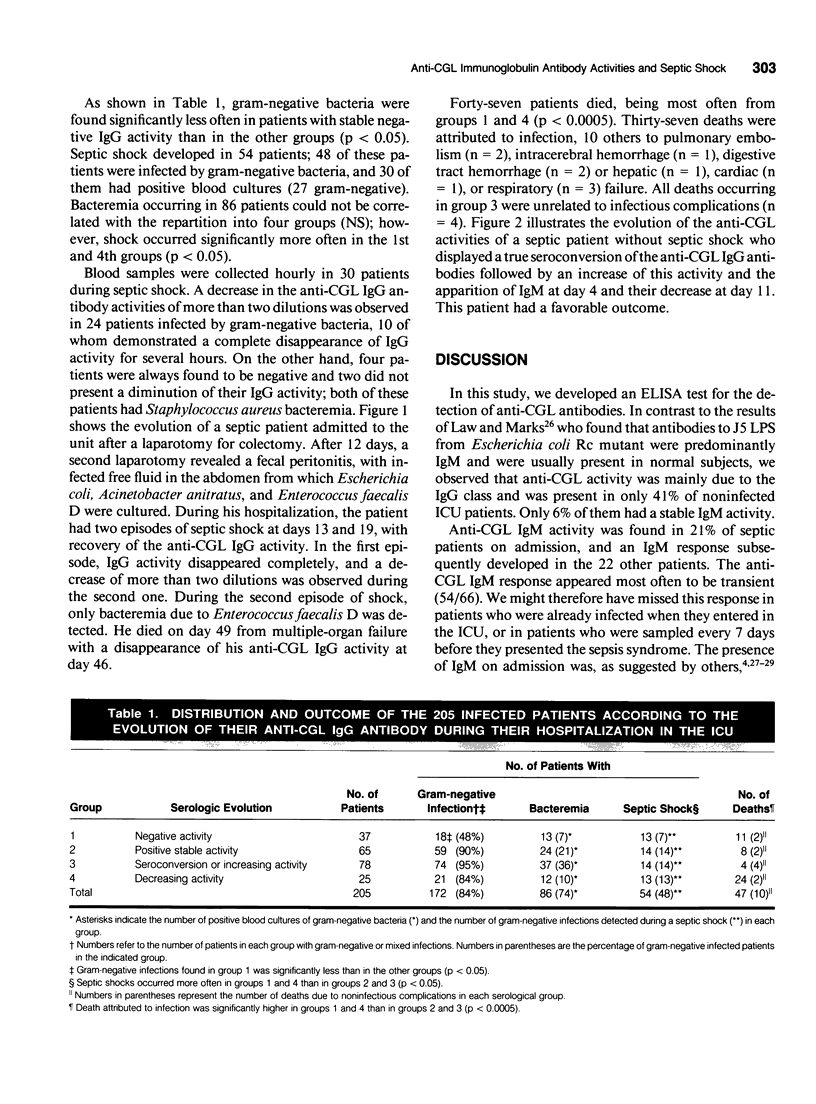
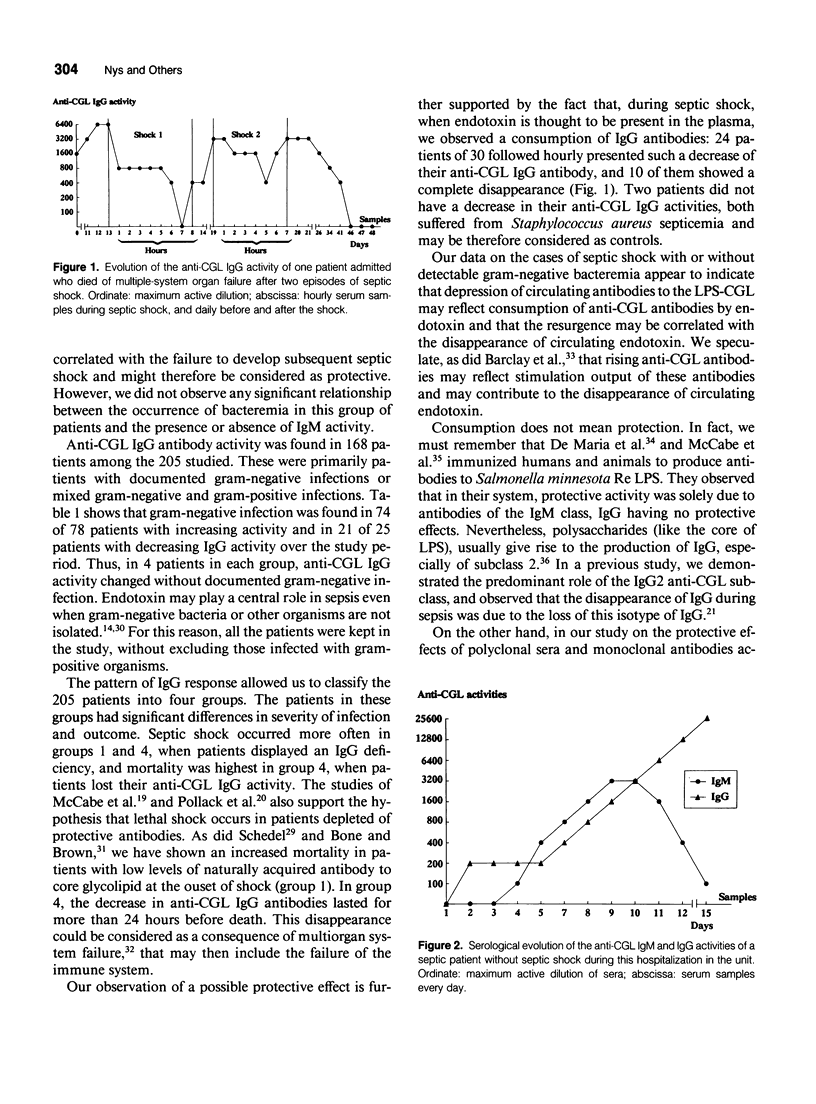
Abstract
OBJECTIVE: This study follows the sequential changes in anti-lipopolysaccharide antibodies in infected patients with and without septic shock. SUMMARY BACKGROUND DATA: A relation between high endogenous levels of anti-LPS antibodies and protection against bacteremia and septic shock in at-risk patient groups has been observed. However, information on the daily follow-up and kinetics of apparition or disappearance of anti-LPS antibody activities and their relations with the protective properties of the different immunoglobulin classes has not been clearly investigated. METHODS: Two hundred and five septic surgical patients were studied during their stay in the intensive care unit during a period of 3 years. Among these patients, septic shock developed in 54 and 47 died. A sensitive ELISA was used to study circulating IgM and IgG antibodies to the core glycolipid (CGL) region of Salmonella minnesota R595. The activities were measured each day when sepsis occurred and every hour during septic shock. RESULTS: Anti-CGL IgM activity was found in 32% of the septic patients. This response, however, most often appeared to be transient. A strong correlation was observed between the occurrence of septic shock and the absence of anti-CGL IgM activity on admission to the ICU (p < 0.02). Anti-CGL IgG activity was detected in 82% of the patients and better correlated with outcome for patients with high or rising activities during their hospitalization (p < 0.0005). In patients with septic shock or irreversible organ failure, a fall in the anti-CGL IgG activity was observed before death, suggesting that the IgG antibodies were consumed during this acute event. Therefore, the anti-CGL IgG activity measured by ELISA could be used as a marker of the evolution of the illness. CONCLUSIONS: Our observations demonstrate the interest to follow-up the evolution of the anti-CGL antibodies during sepsis. The fall of these antibodies during septic shock and in patients who died was an additional argument to perform, as an additive form, passive antibody therapy to decrease lethality in this group of patients.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barclay G. R., Scott B. B. Serological relationships between Escherichia coli and Salmonella smooth- and rough-mutant lipopolysaccharides as revealed by enzyme-linked immunosorbent assay for human immunoglobulin G antiendotoxin antibodies. Infect Immun. 1987 Nov;55(11):2706–2714. doi: 10.1128/iai.55.11.2706-2714.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barclay G. R., Scott B. B., Wright I. H., Rogers P. N., Smith D. G., Poxton I. R. Changes in anti-endotoxin-IgG antibody and endotoxaemia in three cases of gram-negative septic shock. Circ Shock. 1989 Oct;29(2):93–106. [PubMed] [Google Scholar]
- Baumgartner J. D., Glauser M. P. Controversies in the use of passive immunotherapy for bacterial infections in the critically ill patient. Rev Infect Dis. 1987 Jan-Feb;9(1):194–205. doi: 10.1093/clinids/9.1.194. [DOI] [PubMed] [Google Scholar]
- Baumgartner J. D., Glauser M. P., McCutchan J. A., Ziegler E. J., van Melle G., Klauber M. R., Vogt M., Muehlen E., Luethy R., Chiolero R. Prevention of gram-negative shock and death in surgical patients by antibody to endotoxin core glycolipid. Lancet. 1985 Jul 13;2(8446):59–63. doi: 10.1016/s0140-6736(85)90176-x. [DOI] [PubMed] [Google Scholar]
- Bone R. C., Fisher C. J., Jr, Clemmer T. P., Slotman G. J., Metz C. A., Balk R. A. A controlled clinical trial of high-dose methylprednisolone in the treatment of severe sepsis and septic shock. N Engl J Med. 1987 Sep 10;317(11):653–658. doi: 10.1056/NEJM198709103171101. [DOI] [PubMed] [Google Scholar]
- Bone R. C., Fisher C. J., Jr, Clemmer T. P., Slotman G. J., Metz C. A., Balk R. A. Sepsis syndrome: a valid clinical entity. Methylprednisolone Severe Sepsis Study Group. Crit Care Med. 1989 May;17(5):389–393. [PubMed] [Google Scholar]
- Bryan C. S., Reynolds K. L., Brenner E. R. Analysis of 1,186 episodes of gram-negative bacteremia in non-university hospitals: the effects of antimicrobial therapy. Rev Infect Dis. 1983 Jul-Aug;5(4):629–638. doi: 10.1093/clinids/5.4.629. [DOI] [PubMed] [Google Scholar]
- Carrico C. J., Meakins J. L., Marshall J. C., Fry D., Maier R. V. Multiple-organ-failure syndrome. Arch Surg. 1986 Feb;121(2):196–208. doi: 10.1001/archsurg.1986.01400020082010. [DOI] [PubMed] [Google Scholar]
- Cohen J. Anti-endotoxin immunotherapy in septic shock. J Antimicrob Chemother. 1986 Oct;18(4):436–439. doi: 10.1093/jac/18.4.436. [DOI] [PubMed] [Google Scholar]
- Danner R. L., Elin R. J., Hosseini J. M., Wesley R. A., Reilly J. M., Parillo J. E. Endotoxemia in human septic shock. Chest. 1991 Jan;99(1):169–175. doi: 10.1378/chest.99.1.169. [DOI] [PubMed] [Google Scholar]
- DeMaria A., Jr, Johns M. A., Berberich H., McCabe W. R. Immunization with rough mutants of Salmonella minnesota: initial studies in human subjects. J Infect Dis. 1988 Aug;158(2):301–311. doi: 10.1093/infdis/158.2.301. [DOI] [PubMed] [Google Scholar]
- Freeman R., Gould F. K. Prevention of fever and gram negative infection after open heart surgery by antiendotoxin. Thorax. 1985 Nov;40(11):846–848. doi: 10.1136/thx.40.11.846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galanos C., Lüderitz O., Westphal O. A new method for the extraction of R lipopolysaccharides. Eur J Biochem. 1969 Jun;9(2):245–249. doi: 10.1111/j.1432-1033.1969.tb00601.x. [DOI] [PubMed] [Google Scholar]
- Kreger B. E., Craven D. E., McCabe W. R. Gram-negative bacteremia. IV. Re-evaluation of clinical features and treatment in 612 patients. Am J Med. 1980 Mar;68(3):344–355. doi: 10.1016/0002-9343(80)90102-3. [DOI] [PubMed] [Google Scholar]
- Law B. J., Marks M. I. Age-related prevalence of human serum IgG and IgM antibody to the core glycolipid of Escherichia coli strain J5, as measured by ELISA. J Infect Dis. 1985 Jun;151(6):988–994. doi: 10.1093/infdis/151.6.988. [DOI] [PubMed] [Google Scholar]
- Ledingham I. M., McArdle C. S. Prospective study of the treatment of septic shock. Lancet. 1978 Jun 3;1(8075):1194–1197. doi: 10.1016/s0140-6736(78)90979-0. [DOI] [PubMed] [Google Scholar]
- Marget W., Weiss M., Ruhland B. Lipid A antibody determinations using ELISA on patients at a children's hospital: a preliminary report. Infection. 1983 Mar-Apr;11(2):84–86. doi: 10.1007/BF01641072. [DOI] [PubMed] [Google Scholar]
- McCabe W. R., DeMaria A., Jr, Berberich H., Johns M. A. Immunization with rough mutants of Salmonella minnesota: protective activity of IgM and IgG antibody to the R595 (Re chemotype) mutant. J Infect Dis. 1988 Aug;158(2):291–300. doi: 10.1093/infdis/158.2.291. [DOI] [PubMed] [Google Scholar]
- McCabe W. R. Immunization with R mutants of S. Minnesota. I. Protection against challenge with heterologous gram-negative bacilli. J Immunol. 1972 Mar;108(3):601–610. [PubMed] [Google Scholar]
- McCabe W. R., Kreger B. E., Johns M. Type-specific and cross-reactive antibodies in gram-negative bacteremia. N Engl J Med. 1972 Aug 10;287(6):261–267. doi: 10.1056/NEJM197208102870601. [DOI] [PubMed] [Google Scholar]
- Meakins J. L., Wicklund B., Forse R. A., McLean A. P. The surgical intensive care unit: current concepts in infection. Surg Clin North Am. 1980 Feb;60(1):117–132. doi: 10.1016/s0039-6109(16)42038-4. [DOI] [PubMed] [Google Scholar]
- Nys M., Cloes J. M., Demonty J., Joassin L. Protective effects of polyclonal sera and of monoclonal antibodies active to Salmonella minnesota Re595 lipopolysaccharide during experimental endotoxemia. J Infect Dis. 1990 Nov;162(5):1087–1095. doi: 10.1093/infdis/162.5.1087. [DOI] [PubMed] [Google Scholar]
- Nys M., Damas P., Damas F., Joassin L., Demonty J. A direct enzyme-linked immunosorbent assay (ELISA) for antibodies to enterobacterial Re core glycolipid and lipid A. Results in healthy subjects and in patients infected by gram-negative bacteria. Med Microbiol Immunol. 1987;176(5):257–271. doi: 10.1007/BF00190532. [DOI] [PubMed] [Google Scholar]
- Nys M., Joassin L., Somzee A., Demonty J. Enzyme-linked immunosorbent assay for immunoglobulin G subclass antibodies specific for enterobacterial Re core glycolipid in healthy individuals and in patients infected by gram-negative bacteria. J Clin Microbiol. 1988 May;26(5):857–862. doi: 10.1128/jcm.26.5.857-862.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker M. M., Parrillo J. E. Septic shock. Hemodynamics and pathogenesis. JAMA. 1983 Dec 23;250(24):3324–3327. [PubMed] [Google Scholar]
- Pollack M., Huang A. I., Prescott R. K., Young L. S., Hunter K. W., Cruess D. F., Tsai C. M. Enhanced survival in Pseudomonas aeruginosa septicemia associated with high levels of circulating antibody to Escherichia coli endotoxin core. J Clin Invest. 1983 Dec;72(6):1874–1881. doi: 10.1172/JCI111150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schedel I. New aspects in the treatment of gram-negative bacteraemia and septic shock. Infection. 1988;16(1):4–7. doi: 10.1007/BF01646920. [DOI] [PubMed] [Google Scholar]
- Wiles J. B., Cerra F. B., Siegel J. H., Border J. R. The systemic septic response: does the organism matter? Crit Care Med. 1980 Feb;8(2):55–60. doi: 10.1097/00003246-198002000-00001. [DOI] [PubMed] [Google Scholar]
- Young L. S., Stevens P., Ingram J. Functional role of antibody against "core" glycolipid of Enterobacteriaceae. J Clin Invest. 1975 Oct;56(4):850–861. doi: 10.1172/JCI108164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yount W. J., Dorner M. M., Kunkel H. G., Kabat E. A. Studies on human antibodies. VI. Selective variations in subgroup composition and genetic markers. J Exp Med. 1968 Mar 1;127(3):633–646. doi: 10.1084/jem.127.3.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler E. J., Fisher C. J., Jr, Sprung C. L., Straube R. C., Sadoff J. C., Foulke G. E., Wortel C. H., Fink M. P., Dellinger R. P., Teng N. N. Treatment of gram-negative bacteremia and septic shock with HA-1A human monoclonal antibody against endotoxin. A randomized, double-blind, placebo-controlled trial. The HA-1A Sepsis Study Group. N Engl J Med. 1991 Feb 14;324(7):429–436. doi: 10.1056/NEJM199102143240701. [DOI] [PubMed] [Google Scholar]
- Ziegler E. J., McCutchan J. A., Fierer J., Glauser M. P., Sadoff J. C., Douglas H., Braude A. I. Treatment of gram-negative bacteremia and shock with human antiserum to a mutant Escherichia coli. N Engl J Med. 1982 Nov 11;307(20):1225–1230. doi: 10.1056/NEJM198211113072001. [DOI] [PubMed] [Google Scholar]
- Ziegler E. J. Protective antibody to endotoxin core: the emperor's new clothes? J Infect Dis. 1988 Aug;158(2):286–290. doi: 10.1093/infdis/158.2.286. [DOI] [PubMed] [Google Scholar]
- van Deventer S. J., Buller H. R., ten Cate J. W., Sturk A., Pauw W. Endotoxaemia: an early predictor of septicaemia in febrile patients. Lancet. 1988 Mar 19;1(8586):605–609. doi: 10.1016/s0140-6736(88)91412-2. [DOI] [PubMed] [Google Scholar]


