Abstract
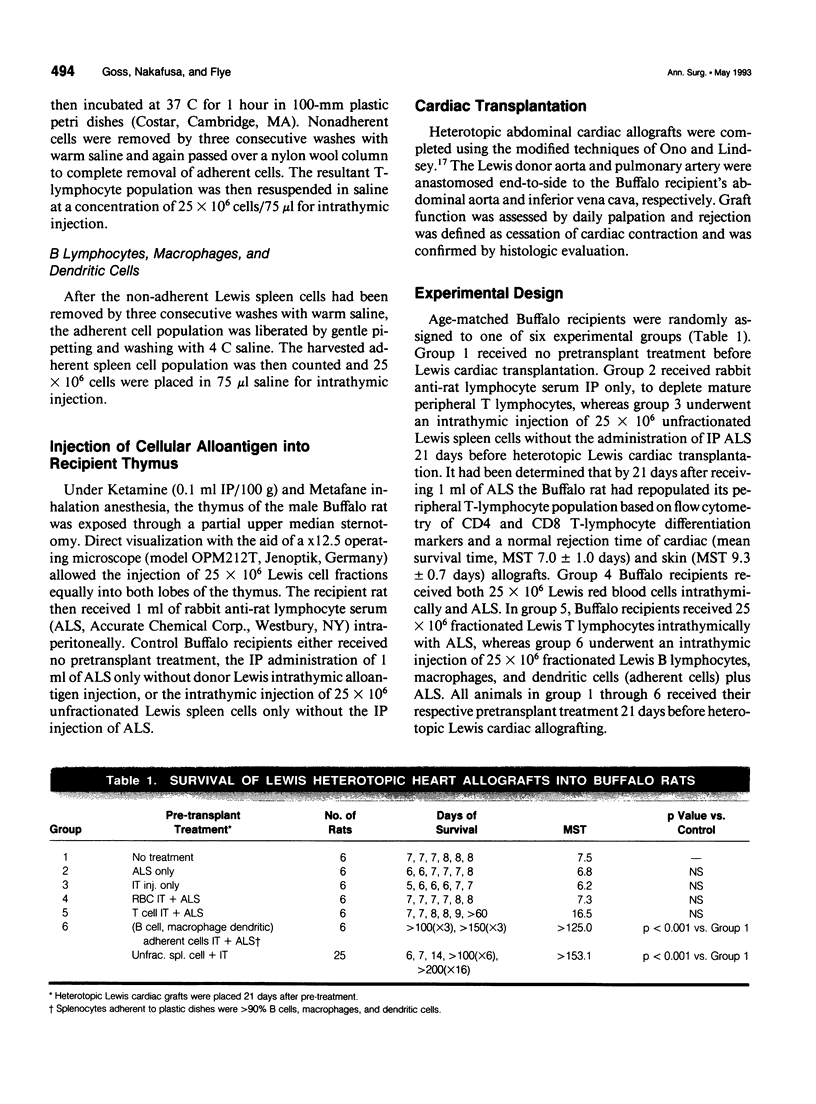
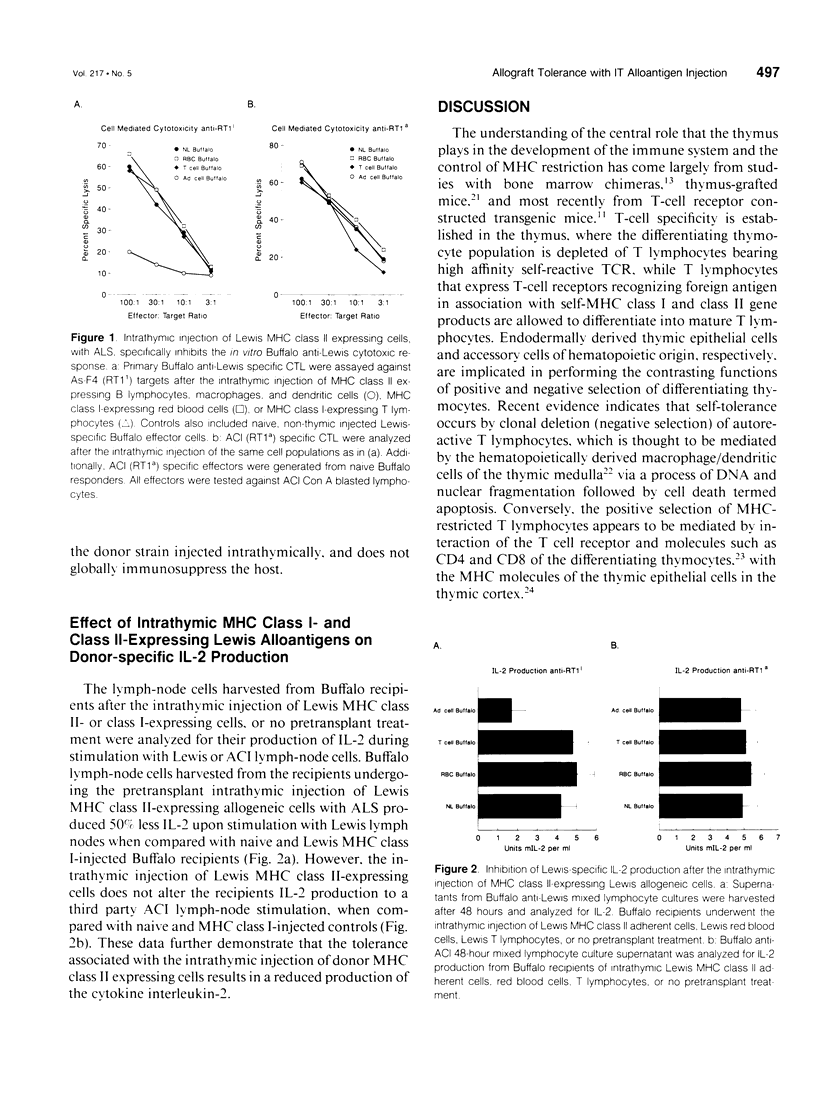
OBJECTIVE: This study determined the form of cellular donor MHC alloantigen necessary for the induction of intrathymic tolerance. BACKGROUND: The authors have achieved indefinite donor-specific tolerance, to a fully MHC-disparate rat heterotopic cardiac allograft, after the pretransplant intrathymic injection of unfractionated donor splenocytes and a single injection of rabbit anti-rat lymphocyte serum (ALS), without subsequent immunosuppression. METHODS: Male 4-12-week-old Buffalo (RT1b) rats underwent an intrathymic injection of either fractionated Lewis (RT1(1)) red blood cells (purified by Ficoll gradient) or T lymphocytes (purified by nylon wool column and plastic adherence), both of which express only MHC class I alloantigens, or B lymphocytes, macrophages, and dendritic cells (purified by plastic adherence) which express both MHC class I and class II alloantigens. At the completion of alloantigen injection the Buffalo recipient rats were given 1 ml of ALS intraperitoneally. Twenty-one days later a heterotopic Lewis heart was transplanted. RESULTS: The intrathymic injection of the fractions of Lewis MHC class I and class II expressing B lymphocytes, macrophages, and dendritic cells induced a donor-specific tolerance that resulted in indefinite Lewis cardiac allograft survival (MST > 125 days) in all recipients without further immunosuppression, whereas groups receiving MHC class I expressing red blood cell or T lymphocyte injections plus ALS rejected Lewis cardiac allografts with a MST of 7.3 and 16.5 days, respectively, thus indicating that the MHC class II expressing cell is necessary for the induction of intrathymic tolerance. Buffalo recipients with a long-term surviving Lewis cardiac allograft, after Lewis MHC class II expressing cells were still able to reject a third-party heterotopic ACI (RT1a) cardiac allograft in normal time (MST = 7.0 days), but did not reject a second Lewis cardiac allograft (MST > 100 days). Additionally, the intrathymic injection of MHC class II expressing cells resulted in decreased interleukin-2 (IL-2) production and an 80% decrease in in vitro donor-specific cell mediated cytotoxicity, whereas the cytolytic response to a third party was unaltered. CONCLUSION: Donor MHC class II, and not class I, expressing cells are the cells in donor splenocytes, injected intrathymically, responsible for the development of donor-specific allograft tolerance.
Full text
PDF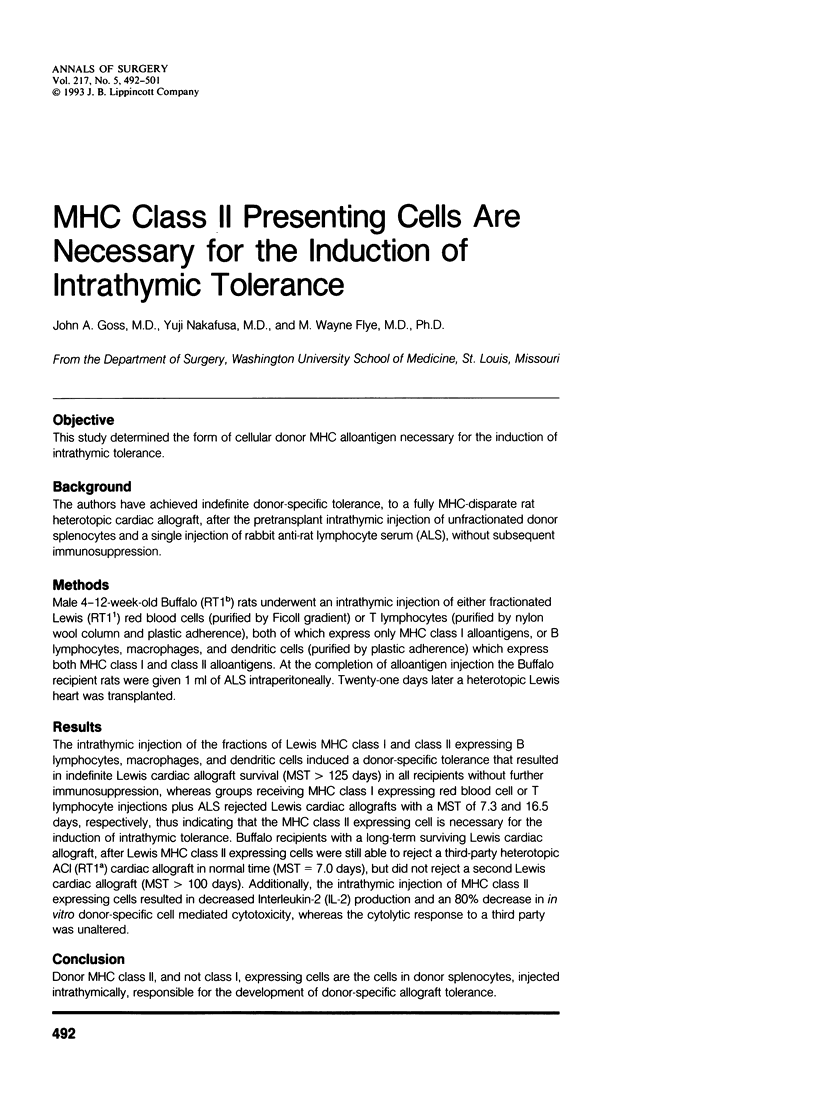





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bach F. H., Sachs D. H. Current concepts: immunology. Transplantation immunology. N Engl J Med. 1987 Aug 20;317(8):489–492. doi: 10.1056/NEJM198708203170807. [DOI] [PubMed] [Google Scholar]
- Bellgrau D. Induction of cytotoxic T cell precursors in vivo. Role of T helper cells. J Exp Med. 1983 May 1;157(5):1505–1515. doi: 10.1084/jem.157.5.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink P. J., Bevan M. J. H-2 antigens of the thymus determine lymphocyte specificity. J Exp Med. 1978 Sep 1;148(3):766–775. doi: 10.1084/jem.148.3.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink P. J., Shimonkevitz R. P., Bevan M. J. Veto cells. Annu Rev Immunol. 1988;6:115–137. doi: 10.1146/annurev.iy.06.040188.000555. [DOI] [PubMed] [Google Scholar]
- Fuchs E. J., Matzinger P. B cells turn off virgin but not memory T cells. Science. 1992 Nov 13;258(5085):1156–1159. doi: 10.1126/science.1439825. [DOI] [PubMed] [Google Scholar]
- Gallimore P. H. Viral DNA in transformed cells. II. A study of the sequences of adenovirus 2 DNA IN NINE LINES OF TRANSFORMED RAT CELLS USING SPECIFIC FRAGMENTS OF THE VIRAL GENOME;. J Mol Biol. 1974 Oct 15;89(1):49–72. doi: 10.1016/0022-2836(74)90162-4. [DOI] [PubMed] [Google Scholar]
- Heeg K., Wagner H. Induction of peripheral tolerance to class I major histocompatibility complex (MHC) alloantigens in adult mice: transfused class I MHC-incompatible splenocytes veto clonal responses of antigen-reactive Lyt-2+ T cells. J Exp Med. 1990 Sep 1;172(3):719–728. doi: 10.1084/jem.172.3.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahan B. D. Cyclosporine. N Engl J Med. 1989 Dec 21;321(25):1725–1738. doi: 10.1056/NEJM198912213212507. [DOI] [PubMed] [Google Scholar]
- Kappler J. W., Roehm N., Marrack P. T cell tolerance by clonal elimination in the thymus. Cell. 1987 Apr 24;49(2):273–280. doi: 10.1016/0092-8674(87)90568-x. [DOI] [PubMed] [Google Scholar]
- Kast W. M., Van Twuyver E., Mooijaart R. J., Verveld M., Kamphuis A. G., Melief C. J., De Waal L. P. Mechanism of skin allograft enhancement across an H-2 class I mutant difference. Evidence for involvement of veto cells. Eur J Immunol. 1988 Dec;18(12):2105–2108. doi: 10.1002/eji.1830181238. [DOI] [PubMed] [Google Scholar]
- Kiziroglu F., Miller R. G. In vivo functional clonal deletion of recipient CD4+ T helper precursor cells that can recognize class II MHC on injected donor lymphoid cells. J Immunol. 1991 Feb 15;146(4):1104–1112. [PubMed] [Google Scholar]
- Langrehr J. M., Dull K. E., Ochoa J. B., Billiar T. R., Ildstad S. T., Schraut W. H., Simmons R. L., Hoffman R. A. Evidence that nitric oxide production by in vivo allosensitized cells inhibits the development of allospecific CTL. Transplantation. 1992 Mar;53(3):632–640. doi: 10.1097/00007890-199203000-00027. [DOI] [PubMed] [Google Scholar]
- Lo D., Sprent J. Identity of cells that imprint H-2-restricted T-cell specificity in the thymus. Nature. 1986 Feb 20;319(6055):672–675. doi: 10.1038/319672a0. [DOI] [PubMed] [Google Scholar]
- MacDonald H. R., Hengartner H., Pedrazzini T. Intrathymic deletion of self-reactive cells prevented by neonatal anti-CD4 antibody treatment. Nature. 1988 Sep 8;335(6186):174–176. doi: 10.1038/335174a0. [DOI] [PubMed] [Google Scholar]
- Marrack P., Kushnir E., Born W., McDuffie M., Kappler J. The development of helper T cell precursors in mouse thymus. J Immunol. 1988 Apr 15;140(8):2508–2514. [PubMed] [Google Scholar]
- Medawar P. B. The behaviour and fate of skin autografts and skin homografts in rabbits: A report to the War Wounds Committee of the Medical Research Council. J Anat. 1944 Oct;78(Pt 5):176–199. [PMC free article] [PubMed] [Google Scholar]
- Miller R. G. An immunological suppressor cell inactivating cytotoxic T-lymphocyte precursor cells recognizing it. Nature. 1980 Oct 9;287(5782):544–546. doi: 10.1038/287544a0. [DOI] [PubMed] [Google Scholar]
- Miller R. G., Phillips R. A. Reduction of the in vitro cytotoxic lymphocyte response produced by in vivo exposure to semiallogeneic cells: recruitment or active suppression? J Immunol. 1976 Nov;117(5 PT2):1913–1921. [PubMed] [Google Scholar]
- Nikolić-Zugić J., Bevan M. J. Role of self-peptides in positively selecting the T-cell repertoire. Nature. 1990 Mar 1;344(6261):65–67. doi: 10.1038/344065a0. [DOI] [PubMed] [Google Scholar]
- Odorico J. S., Barker C. F., Posselt A. M., Naji A. Induction of donor-specific tolerance to rat cardiac allografts by intrathymic inoculation of bone marrow. Surgery. 1992 Aug;112(2):370–377. [PubMed] [Google Scholar]
- Ono K., Lindsey E. S. Improved technique of heart transplantation in rats. J Thorac Cardiovasc Surg. 1969 Feb;57(2):225–229. [PubMed] [Google Scholar]
- Rammensee H. G., Fink P. J., Bevan M. J. Functional clonal deletion of class I-specific cytotoxic T lymphocytes by veto cells that express antigen. J Immunol. 1984 Nov;133(5):2390–2396. [PubMed] [Google Scholar]
- Rosenberg A. S., Mizuochi T., Sharrow S. O., Singer A. Phenotype, specificity, and function of T cell subsets and T cell interactions involved in skin allograft rejection. J Exp Med. 1987 May 1;165(5):1296–1315. doi: 10.1084/jem.165.5.1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salaün J., Bandeira A., Khazaal I., Calman F., Coltey M., Coutinho A., Le Douarin N. M. Thymic epithelium tolerizes for histocompatibility antigens. Science. 1990 Mar 23;247(4949 Pt 1):1471–1474. doi: 10.1126/science.247.4949.1471. [DOI] [PubMed] [Google Scholar]
- Schwartz R. H. Acquisition of immunologic self-tolerance. Cell. 1989 Jun 30;57(7):1073–1081. doi: 10.1016/0092-8674(89)90044-5. [DOI] [PubMed] [Google Scholar]
- Sha W. C., Nelson C. A., Newberry R. D., Kranz D. M., Russell J. H., Loh D. Y. Positive and negative selection of an antigen receptor on T cells in transgenic mice. Nature. 1988 Nov 3;336(6194):73–76. doi: 10.1038/336073a0. [DOI] [PubMed] [Google Scholar]
- Sheng-Tanner X., Miller R. G. Correlation between lymphocyte-induced donor-specific tolerance and donor cell recirculation. J Exp Med. 1992 Aug 1;176(2):407–413. doi: 10.1084/jem.176.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimonkevitz R. P., Bevan M. J. Split tolerance induced by the intrathymic adoptive transfer of thymocyte stem cells. J Exp Med. 1988 Jul 1;168(1):143–156. doi: 10.1084/jem.168.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teh H. S., Kisielow P., Scott B., Kishi H., Uematsu Y., Blüthmann H., von Boehmer H. Thymic major histocompatibility complex antigens and the alpha beta T-cell receptor determine the CD4/CD8 phenotype of T cells. Nature. 1988 Sep 15;335(6187):229–233. doi: 10.1038/335229a0. [DOI] [PubMed] [Google Scholar]
- Todo S., Tzakis A. G., Abu-Elmagd K., Reyes J., Nakamura K., Casavilla A., Selby R., Nour B. M., Wright H., Fung J. J. Intestinal transplantation in composite visceral grafts or alone. Ann Surg. 1992 Sep;216(3):223–234. doi: 10.1097/00000658-199209000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todo S., Tzakis A. G., Abu-Elmagd K., Reyes J., Nakamura K., Casavilla A., Selby R., Nour B. M., Wright H., Fung J. J. Intestinal transplantation in composite visceral grafts or alone. Ann Surg. 1992 Sep;216(3):223–234. doi: 10.1097/00000658-199209000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson D. B., Nowell P. C. Quantitative studies on the mixed lymphocyte interaction in rats. V. Tempo and specificity of the proliferative response and the number of reactive cells from immunized donors. J Exp Med. 1971 Mar 1;133(3):442–453. doi: 10.1084/jem.133.3.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood K. J., Evins J., Morris P. J. Suppression of renal allograft rejection in the rat by class I antigens on purified erythrocytes. Transplantation. 1985 Jan;39(1):56–62. doi: 10.1097/00007890-198501000-00006. [DOI] [PubMed] [Google Scholar]



