Abstract
OBJECTIVE: This study evaluates the efficacy of personally inspecting marginal thoracic organ donors to expand the donor pool. SUMMARY BACKGROUND DATA: The present donor criteria for heart and lung transplantation are very strict and result in exclusion of many potential thoracic organ donors. Due to a limited donor pool, 20-30% of patients die waiting for transplantation. METHODS: The authors have performed a prospective study of personally inspecting marginal donor organs that previously would have been rejected by standard donor criteria. RESULTS: Fourteen marginal hearts and eleven marginal lungs were inspected. All 14 marginal hearts and 10 of the marginal lungs were transplanted. All cardiac transplant patients did well. The mean ejection fraction of the donor hearts preoperatively was 39 +/- 11% (range 15-50%). Postoperatively, the ejection fraction of the donor hearts improved significantly to 55 +/- 3% (p < 0.002). Nine of the ten lung transplant patients did well and were operative survivors. Our donor pool expanded by 36% over the study period. CONCLUSIONS: The present donor criteria for heart and lung transplantation are too strict. Personal inspection of marginal thoracic donor organs will help to maximize donor utilization.
Full text
PDF

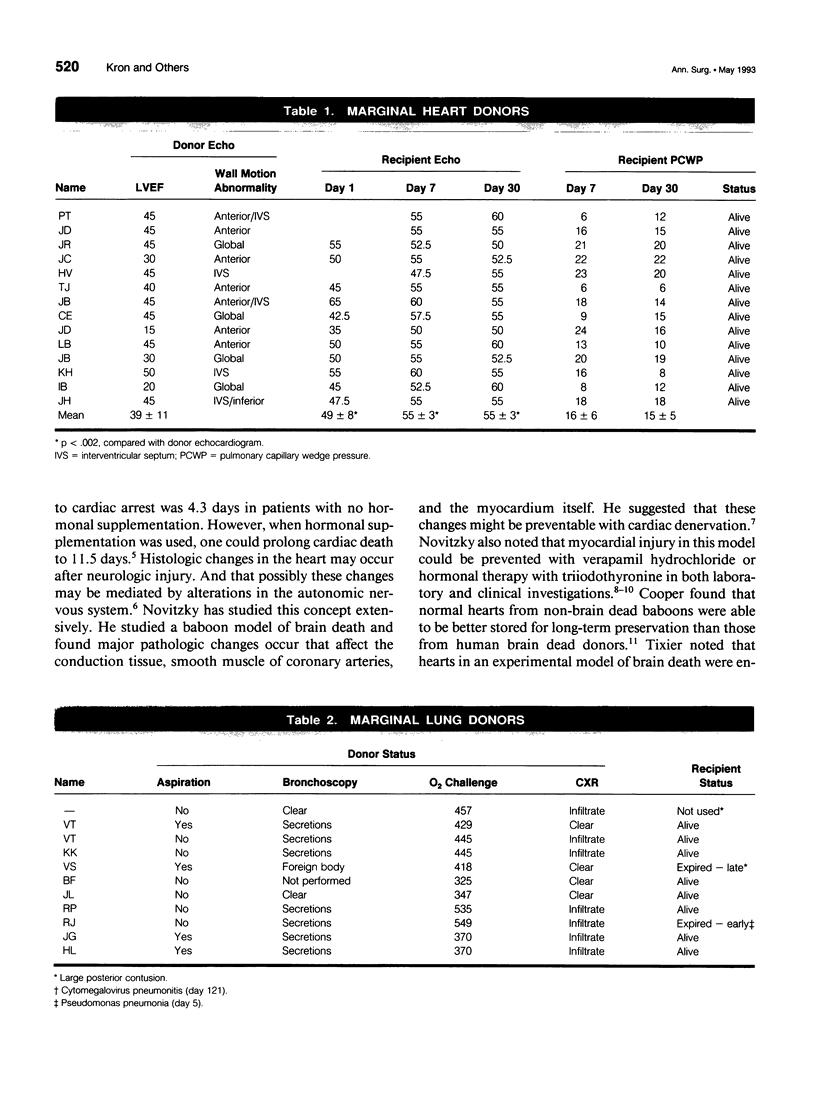
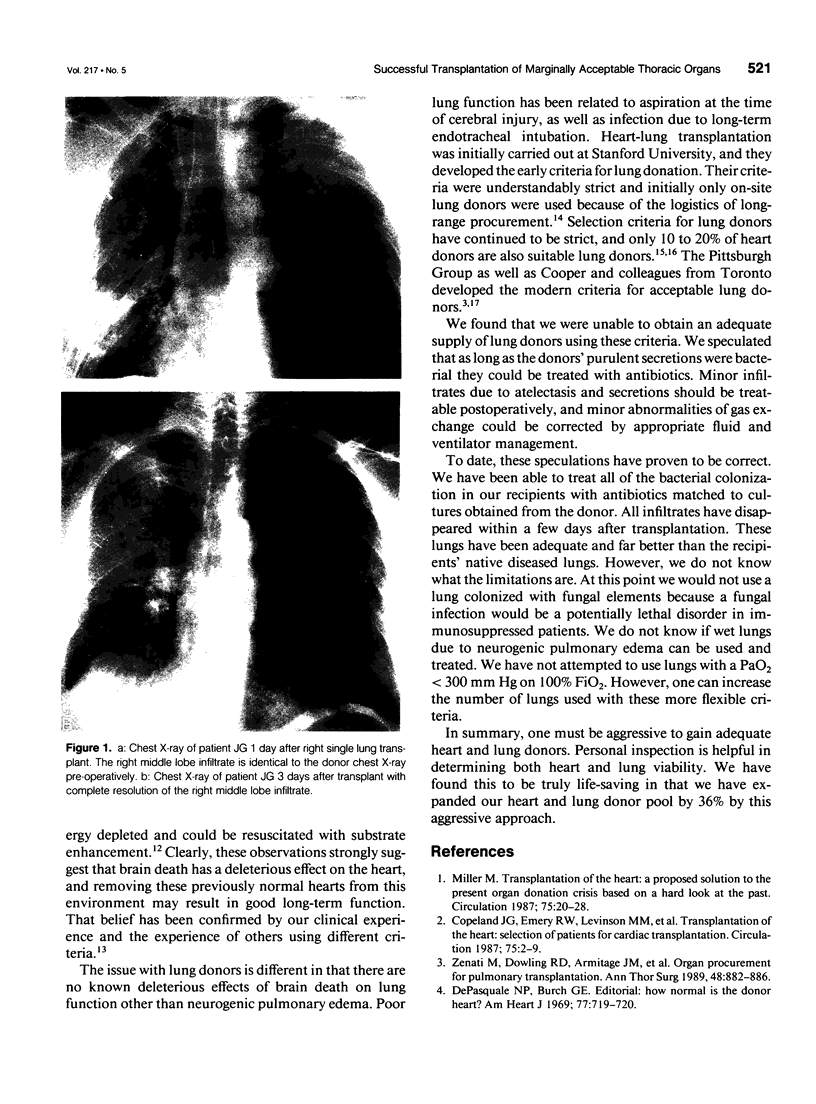

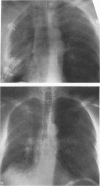
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cooper D. K., Novitzky D., Wicomb W. N. The pathophysiological effects of brain death on potential donor organs, with particular reference to the heart. Ann R Coll Surg Engl. 1989 Jul;71(4):261–266. [PMC free article] [PubMed] [Google Scholar]
- Copeland J. G., Emery R. W., Levinson M. M., Icenogle T. B., Carrier M., Ott R. A., Copeland J. A., McAleer-Rhenman M. J., Nicholson S. M. Selection of patients for cardiac transplantation. Circulation. 1987 Jan;75(1):2–9. doi: 10.1161/01.cir.75.1.2. [DOI] [PubMed] [Google Scholar]
- DePasquale N. P., Burch G. E. How normal is the donor heart? Am Heart J. 1969 Jun;77(6):719–720. doi: 10.1016/0002-8703(69)90405-0. [DOI] [PubMed] [Google Scholar]
- Hakim M., Higenbottam T., Bethune D., Cory-Pearce R., English T. A., Kneeshaw J., Wells F. C., Wallwork J. Selection and procurement of combined heart and lung grafts for transplantation. J Thorac Cardiovasc Surg. 1988 Mar;95(3):474–479. [PubMed] [Google Scholar]
- Harjula A., Baldwin J. C., Starnes V. A., Stinson E. B., Oyer P. E., Jamieson S. W., Shumway N. E. Proper donor selection for heart-lung transplantation. The Stanford experience. J Thorac Cardiovasc Surg. 1987 Dec;94(6):874–880. [PubMed] [Google Scholar]
- Hunt D., Gore I. Myocardial lesions following experimental intracranial hemorrhage: prevention with propranolol. Am Heart J. 1972 Feb;83(2):232–236. doi: 10.1016/0002-8703(72)90142-1. [DOI] [PubMed] [Google Scholar]
- Jamieson S. W., Stinson E. B., Oyer P. E., Baldwin J. C., Shumway N. E. Operative technique for heart-lung transplantation. J Thorac Cardiovasc Surg. 1984 Jun;87(6):930–935. [PubMed] [Google Scholar]
- Miller M. A proposed solution to the present organ donation crisis based on a hard look at the past. Circulation. 1987 Jan;75(1):20–28. doi: 10.1161/01.cir.75.1.20. [DOI] [PubMed] [Google Scholar]
- Novitzky D., Cooper D. K., Morrell D., Isaacs S. Change from aerobic to anaerobic metabolism after brain death, and reversal following triiodothyronine therapy. Transplantation. 1988 Jan;45(1):32–36. doi: 10.1097/00007890-198801000-00008. [DOI] [PubMed] [Google Scholar]
- Novitzky D., Cooper D. K., Reichart B. Hemodynamic and metabolic responses to hormonal therapy in brain-dead potential organ donors. Transplantation. 1987 Jun;43(6):852–854. [PubMed] [Google Scholar]
- Novitzky D., Cooper D. K., Rose A. G., Reichart B. Prevention of myocardial injury by pretreatment with verapamil hydrochloride prior to experimental brain death: efficacy in a baboon model. Am J Emerg Med. 1987 Jan;5(1):11–18. doi: 10.1016/0735-6757(87)90282-8. [DOI] [PubMed] [Google Scholar]
- Novitzky D., Rose A. G., Cooper D. K. Injury of myocardial conduction tissue and coronary artery smooth muscle following brain death in the baboon. Transplantation. 1988 May;45(5):964–966. doi: 10.1097/00007890-198805000-00025. [DOI] [PubMed] [Google Scholar]
- Patterson G. A., Cooper J. D. Status of lung transplantation. Surg Clin North Am. 1988 Jun;68(3):545–558. doi: 10.1016/s0039-6109(16)44533-0. [DOI] [PubMed] [Google Scholar]
- Sweeney M. S., Lammermeier D. E., Frazier O. H., Burnett C. M., Haupt H. M., Duncan J. M. Extension of donor criteria in cardiac transplantation: surgical risk versus supply-side economics. Ann Thorac Surg. 1990 Jul;50(1):7–11. doi: 10.1016/0003-4975(90)90071-d. [DOI] [PubMed] [Google Scholar]
- Taniguchi S., Kitamura S., Kawachi K., Doi Y., Aoyama N. Effects of hormonal supplements on the maintenance of cardiac function in potential donor patients after cerebral death. Eur J Cardiothorac Surg. 1992;6(2):96–102. doi: 10.1016/1010-7940(92)90082-9. [DOI] [PubMed] [Google Scholar]
- Tixier D., Matheis G., Buckberg G. D., Young H. H. Donor hearts with impaired hemodynamics. Benefit of warm substrate-enriched blood cardioplegic solution for induction of cardioplegia during cardiac harvesting. J Thorac Cardiovasc Surg. 1991 Aug;102(2):207–214. [PubMed] [Google Scholar]
- Zenati M., Dowling R. D., Armitage J. M., Kormos R. L., Dummer J. S., Hardesty R. L., Griffith B. P. Organ procurement for pulmonary transplantation. Ann Thorac Surg. 1989 Dec;48(6):882–886. doi: 10.1016/0003-4975(89)90696-6. [DOI] [PubMed] [Google Scholar]