Abstract
Cdc34 is an E2-conjugating enzyme required for catalyzing the polyubiquitination reaction mediated by the Skp1·CUL1·F-box (SCF) protein E3 ubiquitin (Ub) ligase. Here, we show that the activity of human Cdc34 in the Ub–Ub ligation reaction was enhanced dramatically by SCF's core Ub ligase module, composed of a heterodimeric complex formed by the ROC1 RING finger protein and the CUL1 C terminus that contains a Nedd8 moiety covalently conjugated at K720. Unexpectedly, we found that N-terminal fusion of a GST moiety to human Cdc34 generated dimeric GST-Cdc34 that was constitutively active in supporting the assembly of K48-linked polyUb chains independently of SCF. Furthermore, fusion of a FK506-binding protein (FKBP) to the N terminus of human Cdc34 yielded FKBP-Cdc34 that was induced to form a dimer upon treatment with the chemical inducer AP20187. The AP20187-induced dimeric form of FKBP-Cdc34 was substantially more active than the monomer in catalyzing Ub–Ub ligation. Thus, juxtaposition of human Cdc34 activates its catalytic capability, suggesting that the SCF-mediated polyubiquitination reaction may require the conversion of Cdc34 from an inactive monomer to a highly active dimeric form.
Keywords: SCF, ubiquitination
Ubiquitin (Ub)-dependent proteolysis is a remarkably intricate biochemical process in which the 26S proteasome recognizes and subsequently degrades a target protein tagged with lysine-48-linked polyUb chains, generated through a modification reaction known as ubiquitination. Ubiquitination requires activation of monomeric Ub through formation of an initial thiol–ester bond with the E1 activating enzyme followed by transfer of the thiol–ester-linked Ub to an E2 conjugating enzyme (1). The enzymatic generation of signaling Ub chains attached to a substrate is mediated typically by an E3 Ub ligase, which contains either a HECT domain or a RING finger motif and functions by recognizing both the substrate and an E2 (2).
The Skp1·CUL1·F-box protein·ROC1 (SCF) complex is a prototype of cullin (CUL)-based RING E3 ligases that are defined by two signature components: a CUL-family molecule and its RING finger partner ROC1/Rbx1/Hrt1 (reviewed in ref. 3). SCF utilizes the CUL1 scaffold to anchor a substrate-targeting module (comprised of the Skp1·F-box protein heterodimer) and to tether ROC1 (via the CUL domain), respectively (4–6). As a result of this multisubunit interaction, an F-box protein (member of a large family of substrate-targeting molecules) is placed in proximity to ROC1, which functions to recruit an E2 conjugating enzyme (7–9), thereby initiating the ubiquitination of a bound substrate. As revealed by a large body of genetic and biochemical experiments, the SCF and other CUL-based RING E3 ligases are activated by conjugation of Nedd8 to a conserved lysine residue within a CUL protein (a process termed neddylation) (3), which mechanistically parallels ubiquitination.
In budding yeast, the activity of SCF requires Cdc34, a class II E2 Ub-conjugating enzyme that catalyzes the formation of K48-linked polyUb chains (10). It has been shown that SCFCdc4 and Saccharomyces cerevisiae (sc) Cdc34 cooperate to mediate Ub-dependent degradation of the Sic1 CDK inhibitor, an event necessary for the transition from the G1 to S-phase (reviewed in ref. 11). The fact that human Cdc34 cDNA can substitute for scCdc34 underscores a functional conservation between the two orthologues (12). However, despite the accumulation of extensive information concerning the large number of substrates targeted by the SCF/Cdc34 ubiquitination system, much remains to be learned about how this enzymatic process is executed. To this end, in this report, we present evidence suggesting that the dimerization of Cdc34 plays a role in increasing its ability to assemble Ub chains.
Materials and Methods
Induction of FK506-Binding Protein (FKBP)-Cdc34 Dimerization and Purification of Dimerized FKBP-Cdc34. To induce dimerization of FKBP-Cdc34, 106 high five (HF) insect cells were infected (moi = 5), singly or in combination with baculoviruses expressing FKBP-HA-His-Cdc34 or FKBP-Flag-Cdc34 (constructed as described in Supporting Text, which is published as supporting information on the PNAS web site). At 43 h postinfection, cells were treated with or without AP20187 (0.1 or 1 μM; kindly provided by ARIAD Pharmaceuticals) for 4 h. Cells were harvested, washed once with cold PBS, and resuspended in 0.2 ml of buffer B (25 mM Hepes-KOH, pH 7.5/10% glycerol/0.05% Nonidet P-40/0.5 M NaCl/1 mM PMSF). The resuspension was sonicated (three repetitive 20-s treatments) and centrifuged at 10,000 × g for 30 min at 4°C. Soluble extracts (30 μl) were mixed with anti-Flag (M2) or anti-HA (20 μl; Sigma) beads for 4 h at 4°C. The resulting beads were washed three times with buffer B and the bound proteins were eluted with SDS loading buffer followed by SDS/PAGE separation/immunoblot analysis (shown in Fig. 4B).
Fig. 4.
AP20187-induced dimerization of FKBP-Cdc34. (A) A scheme of the dimerization of FKBP-Cdc34 induced by the dimerizing agent AP20187. (B) An immunoprecipitation/immunoblot experiment is shown, with extracts of insect cells infected with the indicated baculoviruses. (C) Superose 6 gel-filtration experiments are shown with M2-affinity-purified FKBP-Cdc34 (10 μg), derived from cells treated with (rows 1, 2, and 5) or without (rows 3, 4, and 6) AP20187. Aliquots of the indicated fractions were analyzed by immunoblots with anti-Flag (rows 1 and 3) or anti-HA (rows 2 and 4) antibodies and by silver staining (rows 5 and 6). (D) A glycerol gradient experiment is shown, with M2-affinity-purified FKBP-Cdc34 (2 μg), derived from cells treated with (Upper) or without (Lower) AP20187. Aliquots of the indicated fractions were subjected to immunoblot analysis with anti-Flag antibodies. In lane 1, L denotes the materials loaded onto the gradient. In C and D, arrows below the blots mark the migration positions of the indicated molecular size markers. F-F-Cdc34 denotes FKBP-Flag-Cdc34, and F-H-H-Cdc34 represents FKBP-HA-His-Cdc34.
For large-scale purification of dimerized FKBP-Cdc34, 3.25 × 107 HF cells were coinfected (moi = 5) with baculoviruses expressing FKBP-HA-His-Cdc34 and FKBP-Flag-Cdc34. At 43 h postinfection, cells were treated with or without 1 μM AP20187 for 4 h. Cells were harvested as described above and resuspended in buffer B (6 ml). After sonication, the resuspension was cleared by centrifugation at 35,000 × g for 30 min at 4°C. Soluble extracts were mixed with M2 beads (200 μl) for 4 h at 4°C, followed by washing three times with buffer B. Bound proteins were eluted with Flag peptide (1 mg/ml) in buffer B (1.6 ml). The resulting material was then concentrated by using centrifugal filters (Amicon-Millipore). The yield of free FKBP-Cdc34 or FKBP-Cdc34 bound to AP20187 was 40 μg (0.5 mg/ml) or 80 μg (1 mg/ml), respectively.
Other Protein Reagents. GST-Cdc34 and its derivatives were constructed and purified as described in Supporting Text. The preparation of other reagents was as described: 32P-Ub (13), Nedd8 and ROC1–CUL1324-776 (14), and Cdc34 and Ubc12 (15). E1 and APP-BP1/Uba3 were purchased from Boston Biochem (Cambridge, MA).
Assays. Ub ligation–reaction mixture (30 μl) contained 50 mM Tris·HCl, pH 7.4, 5 mM MgCl2, 2 mM NaF, 10 nM okadaic acid, 2 mM ATP, 0.6 mM DTT, 0.1 mg/ml BSA, 32P-Ub (150 pmol), E1 (0.6 pmol), and the indicated amount of Cdc34 (or Cdc34 fusion enzyme). For reactions carried out with the neddylated ROC1–CUL1 complex, purified ROC1–CUL1324-776 complex, in amount as indicated, was preincubated with Nedd8 (75 pmol), APP-BP1/Uba3 (0.15 pmol), and Ubc12 (25 pmol) in the above reaction mixture lacking 32P-Ub, E1, and Cdc34 for 5 min at room temperature. In all cases, ubiquitination was initiated by the addition of Cdc34 (or Cdc34 fusion enzyme), and the reaction was incubated for times as indicated at specified temperatures. Where indicated, DTT was added to a final concentration of 0.1 M before the addition of SDS loading buffer (10 μl). Aliquots were separated by 4–20% SDS/PAGE followed by autoradiography. Quantitation of the ubiquitination products was performed by using the PhosphorImager (Bio-Rad).
Free Ub chain synthesis–reaction mixture (30 μl) contained the same components as described above, with the exception that 32P-Ub was replaced by bovine Ub (150 pmol) and 32P-UbK48R (50 pmol). The reaction was carried out and analyzed as described above.
Sizing chromatography was carried out as described in Supporting Text.
Results
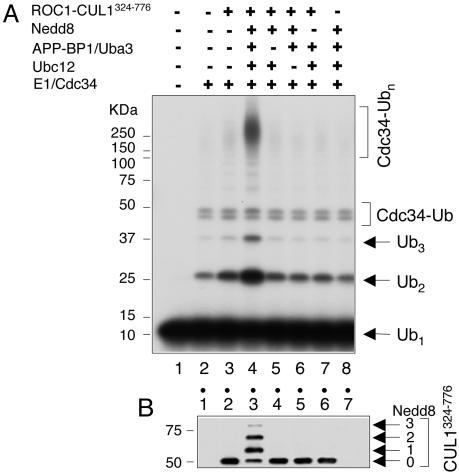
Neddylated ROC1–CUL1 Complex Activates Human Cdc34 to Catalyze Ub Ligation. We examined Ub chain assembly catalyzed by human Cdc34 and influence on the reaction by the SCF core Ub ligase complex (comprised of the ROC1 RING finger protein and the C-terminal half of CUL1 (CUL1324-776), which contains a Nedd8 moiety covalently conjugated at K720, herein referred to as ROC1–CUL1324-776–Nedd8) (14). Incubation of human Cdc34 with E1 and 32P-labeled Ub only weakly supported the synthesis of free Ub chains (Fig. 1A, lane 2). By contrast, the addition of the ROC1–CUL1324-776–Nedd8 complex stimulated the production of unanchored Ub polymers (dimers and trimers) 4-fold and increased the yield of high-molecular-weight Ub conjugates (principally comprised of autoubiquitinated Cdc34) 7-fold (Fig. 1A, lane 4), respectively. Parallel immunoblot analysis detected extensive neddylation of CUL1324-776, as shown by the formation of both mono- and di-Nedd8 moieties (Fig. 1B, lane 3).
Fig. 1.
Stimulation of Cdc34-catalyzed Ub chain assembly by the neddylated ROC1–CUL1 complex. Reactions were performed by using the Ub ligation assay (see Materials and Methods) with ROC1–CUL1324-776 (40 pmol) and Cdc34 (30 pmol) at 37°C for 90 min. Shown are autoradiography (A) and immunoblot analysis (B) with anti-Flag antibody (CUL1324-776 contains an N-terminally inserted Flag epitope).
Next, we used a sensitive free Ub chain synthesis assay to more precisely monitor the Ub ligation by human Cdc34. In this system, we measured the production of a di-Ub species formed by ligation of the 32P-labeled recombinant UbK48R (12 kDa, donor) to the bovine Ub of smaller size (8 kDa, acceptor) and, thus, differing in size from the preexisting Ub dimer (Fig. 2A, lane 1). As shown, ROC1–CUL1324-776–Nedd8 markedly increased the yield of the di-Ub, designated as UbK48R–bUb, by 8-fold (Fig. 2 A, compare lanes 3 and 4 with lanes 8 and 9). Note that a much less pronounced stimulatory effect was observed with ROC1–CUL1324-776 in the absence of neddylation (Fig. 2 A, lanes 5 and 6). Under the conditions used, the predominant product formed was the di-Ub product; low levels of a trimeric Ub species were detected (Fig. 2 A, lane 9), and they were most likely generated through the ligation of 32P-UbK48R to dimeric bovine Ub (UbK48R–bUb2). In the absence of bovine Ub, the di-Ub species disappeared with the concomitant accumulation of conjugates containing Cdc34 and 32P-UbK48R (Fig. 2 A, lane 10). As revealed by subsequent kinetic analysis, the di-Ub product formed as early as 1 min of incubation and accumulated with time (Fig. 2B). Cdc34∼S∼Ub was observed (Fig. 2B, compare lanes 3 and 4 with lanes 8 and 9) but failed to accumulate after 3 min, suggesting that this thiol–ester was highly labile. Importantly, comparative rate analysis showed that ROC1–CUL1324-776–Nedd8 or ROC1–CUL1324-776 increased the synthesis of di-Ub by 20- or 4-fold (Fig. 2 C and D), respectively. As noted, ROC1–CUL1324-776–Nedd8 enabled Cdc34 to convert >10% of the monomeric Ub substrate to the di-Ub species after 60 min of incubation. We conclude from the above results that the neddylated ROC1–CUL1 complex enhances the ability of Cdc34 to catalyze Ub–Ub ligation.
Fig. 2.
Stimulation of Cdc34-catalyzed Ub ligation by the neddylated ROC1–CUL1 complex. Reactions were carried out by using the free Ub chain synthesis assay (see Materials and Methods). (A) A titration experiment in which increasing levels of Cdc34 were incubated with or without ROC1–CUL1324-776 (40 pmol) in either the presence or absence of neddylation for 60 min at 37°C. (B) A time-course experiment is shown. Reactions were carried out as in A with Cdc34 (20 pmol) and ROC1–CUL1324-776–Nedd8 and were incubated for the indicated periods at 16°C. Note that at 0 min, E1∼S∼Ub had already formed, and the ubiquitination reaction was initiated by the addition of Cdc34. Upper, containing proteins migrating slower than the 37-kDa marker, was exposed for a time interval three times longer than was Lower.(C) The kinetic analysis was performed as in B with Cdc34 alone, the ROC1–CUL1324-776 complex, or ROC1–CUL1324-776–Nedd8. No DTT was added in these reactions. (D) The di-Ub formed is graphically represented.
Activation of Cdc34 by GST Fusion. Unexpectedly, we found that the dependency on the neddylated ROC1–CUL1 complex for human Cdc34-catalyzed polyubiquitination could be obviated by N-terminal in-frame fusion of a GST moiety to the E2. The results derived from both titration and kinetics experiments showed that, unlike Cdc34, GST-Cdc34 alone supported extensive self-polyubiquitination in a dose- and time-dependent manner [Fig. 3 (compare A, lanes 3–6 and 7–10, and B, lanes 1–6)]. Moreover, GST-Cdc34 stimulated synthesis of dimeric and trimeric free Ub chains by 3- and 8-fold (Fig. 3A, compare lanes 3–6 and 7–10). Furthermore, GST-Cdc34 yielded predominantly K48-linked Ub polymeric species, because substitution of the wild-type Ub with UbK48R inhibited the reaction (Fig. 3B, compare lanes 1–6 and lanes 7–12). Notably, at the highest concentration tested, GST-Cdc34 predominantly underwent monoubiquitination (compare Fig. 3A, lanes 9 and 10), suggesting that E2 concentration may influence its “commitment” for mono- or polyubiquitination by an unknown mechanism. Additionally, the neddylated ROC1–CUL1 complex had no effect on polyubiquitination by GST-Cdc34 (data not shown). Taken together, these data suggest that GST fusion bypasses the ROC1–CUL1324-776–Nedd8 requirement for Ub chain assembly by human Cdc34.
Fig. 3.
In-frame fusion of GST to Cdc34 activates Ub chain assembly. Reactions were carried out as described in Fig. 1, with the exception that ROC1–CUL1 and the neddylation agents were omitted. (A) A titration experiment is shown with increasing amounts of E2 (2.3, 7, 20, or 60 pmol). (B) A time-course experiment is shown with GST-Cdc34 (20 pmol) carried out in the presence of the wild-type Ub (lanes 1–6) or UbK48R (lanes 7–12).
We then determined and compared the hydrodynamic properties of Cdc34 and GST-Cdc34 (Table 1). Based on measured molecular weights of Cdc34 and GST-Cdc34 (36.3 and 118.1 kDa) and the results of analytic equilibrium sedimentation (Table 1), we conclude that Cdc34 and GST-Cdc34 exist as monomer and dimer, respectively. In addition, Cdc34 has a measured frictional coefficient ratio (f/f0) of 1.66 (Table 1), indicative of an elongated polypeptide, which is further increased by GST fusion (to 2.45; Table 1). Taken together, these results demonstrate that GST-Cdc34 is an extensively elongated dimer. On the basis of the three-dimensional structure of the Schistosoma japonicum GST, the C-terminal ends of each subunit protrude in the same direction (16), suggesting that the Cdc34-moeities are placed in close proximity in a GST-Cdc34 dimeric complex.
Table 1. The hydrodynamic properties of human Cdc34 and GST-Cdc34.
| Enzymes | Stokes radius,* Å | S value* (10-13 sec) | Ma,* kDa | Mp, kDa | f/f0* | Equilibrium ultracentrifugation* |
|---|---|---|---|---|---|---|
| Cdc34 | 33.8 | 2.6 | 36.3 | 29.5 | 1.66 | Monomer |
| GST-Cdc34 | 62.2 | 4.6 | 118.1 | 56.6 | 2.45 | Dimer |
Ma, apparent molecular weight, Mp, predicted molecular weight (calculated based on the amino acid content of human Cdc34).
Determined as described in Supporting Text.
Induced Dimerization of FKBP-Cdc34. The above results suggest a model of proximity-induced Cdc34 activation by GST fusion. To test this hypothesis further, we developed the FKBP-based Cdc34 dimerization system that depends on a synthetic dimerizing agent (AP20187; Fig. 4A), which cross-links two molecules of the FKBP domain, owing to its subnanomolar binding affinity (17). To examine dimerization of FKBP-fused Cdc34, we coinfected HF insect cells with baculoviruses constructed to express FKBP-Flag-Cdc34 or FKBP-HA-His-Cdc34 and subsequently treated the infected cells with AP20187 to induce dimerization. As shown in Fig. 4B, both FKBP-Flag-Cdc34 (lanes 3–6) and FKBP-HA-His-Cdc34 (lanes 8 and 10–12) were expressed in either singly infected or coinfected cells. When treated with 1 μM AP20187, FKBP-Flag-Cdc34 and FKBP-HA-His-Cdc34 were found to coimmunoprecipitate (Fig. 4B, lanes 6 and 12). This interaction occurred in an AP20187-concentration-dependent manner, because it was reduced substantially at lower concentrations of AP20187 (Fig. 4B, lanes 5 and 11) and was abolished in the absence of this agent (lanes 4 and 10).
Next, we affinity-purified FKBP-Flag-Cdc34 and bound proteins via anti-Flag (M2)-matrix from cells that coexpressed FKBP-Flag-Cdc34 and FKBP-HA-His-Cdc34, in either the presence or absence of AP20187. Each purified preparation was gel filtered through a Superose 6 column (Amersham Biosciences), and the resulting fractions were analyzed by immunoblot and silver stain analyses (Fig. 4C). Immunoblot experiments revealed that the M2-immunoprecipitates from the AP20187-treated cells contained a single peak of FKBP-Flag-Cdc34 and of FKBP-HA-His-Cdc34, both eluting precisely coincidentally in fractions 22–24 (Fig. 4C, rows 1 and 2, lanes 2 and 3). As revealed by silver stain analysis, FKBP-Flag-Cdc34 was significantly more abundant than FKBP-HA-His-Cdc34 (Fig. 4C, row 5, lanes 2 and 3), consistent with the use of the M2-purification procedure that yielded both FKBP-Flag-Cdc34·FKBP-Flag-Cdc34 and FKBP-Flag-Cdc34·FKBP-HA-His-Cdc34 dimeric complexes. By comparison, sizing column separation of the M2 immunoprecipitates derived from cells that were not treated with AP20187 resulted in the elution of FKBP-Flag-Cdc34 in fractions 26–28 (Fig. 4C, rows 3 and 6, lanes 4 and 5), whereas FKBP-HA-His-Cdc34 was not detected (Fig. 4C, row 4). These results thus confirmed a stable interaction between FKBP-Flag-Cdc34 and FKBP-HA-His-Cdc34 in response to AP20187. Furthermore, these data suggest that nearly all FKBP-Flag-Cdc34 molecules were bound to AP20187 and existed in dimeric forms, as evidenced by a substantial AP20187-induced shift of the FKBP-Flag-Cdc34 protein peak from fractions 26–28 to fractions 22–24.
Finally, we performed a glycerol gradient analysis. As revealed by immunoblot analysis, whereas the AP20187-bound FKBP-Cdc34 peaked at fractions 20–24 (Fig. 4D Upper, lanes 8–10), the AP20187-free fusion enzymes were at fractions 26–30 (Fig. 4D Lower, lanes 11–13). These results led to determination of the apparent molecular weight for AP20187-free FKBP-Cdc34 as 37 kDa (based on its Stokes radius of 27.1 Å and sedimentation value of 3.3 S; see Supporting Text for a detailed description). Because the predicted molecular mass of the monomeric FKBP-Cdc34 is 43 kDa, we conclude that, in the absence of AP20187, FKBP-Cdc34 exists as a monomer. By contrast, based on a Stokes radius of 39.8 Å and sedimentation value of 5.0 S, the AP20187-bound FKBP-Cdc34 was calculated to have an apparent molecular mass of 82 kDa and, therefore, must exist as a dimer. Taken together, these findings demonstrate that AP20187 induces dimerization of FKBP-Cdc34.
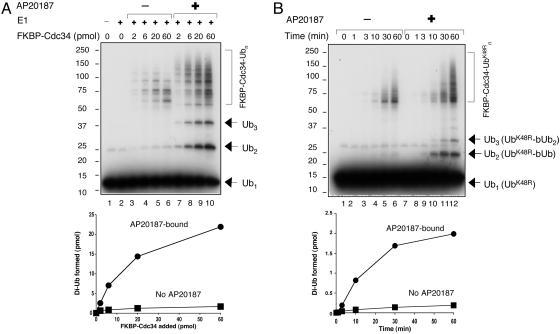
Dimerization of FKBP-Cdc34 Activates Ub Ligation. Having determined that in vivo treatment with AP20187 yielded dimeric FKBP-Cdc34, we next assessed whether dimerization of FKBP-Cdc34 increased the Ub ligation activity. It was expected that fusion of a relatively bulky domain (FKBP or GST) would introduce significant alterations in the interactions between SCF and Cdc34. Therefore, the Ub chain assembly assay, but not substrate ubiquitination, was used to determine whether Cdc34 could be activated by heterologous dimerization. As shown by titration experiments, the AP20187-bound dimeric FKBP-Flag-Cdc34 was more active than the monomeric enzyme in catalyzing both free Ub chain synthesis and E2 autoubiquitination (Fig. 5A, compare lanes 3–6 and 7–10). Quantitation of these results revealed that the dimerized FKBP-Cdc34 yielded 10 times more of the di-Ub product than the monomers (Fig. 5A Lower). Furthermore, kinetic analysis showed that the dimeric FKBP-Flag-Cdc34 produced di-Ub at a rate 10-fold higher than that observed with the monomer (Fig. 5B Upper, compare lanes 2–6 and 8–12, and Lower). To rule out the possibility that the AP20187-mediated stimulatory effects were due to the presence of both FKBP-Flag-Cdc34 and FKBP-HA-His-Cdc34 in the purified fusion enzyme preparation, we isolated FKBP-Flag-Cdc34 alone from singly infected insect cells treated with or without AP20187. In agreement with the results obtained with preparations that contained both FKBP-Flag-Cdc34 and FKBP-HA-His-Cdc34, purified FKBP-Flag-Cdc34 alone existed as a dimer only when formed in the presence of AP20187. This dimeric form of FKBP-Flag-Cdc34 was significantly more active than the monomer (data not shown). In addition, the neddylated ROC1–CUL1 complex was found to activate the monomeric FKBP-Cdc34, whereas it had little effect on the dimeric FKBP-Cdc34 (data not shown). Thus, we conclude that the dimerization of FKBP-Cdc34 activates Ub ligation.
Fig. 5.
Activation of Ub ligation by dimerization of FKBP-Cdc34. (A) Reactions, carried out as described in Fig. 3, were incubated for 60 min at 37°C. (B) The free Ub chain assembly assay was carried out as described in Fig. 2B, with the exception that ROC1–CUL1 and the neddylation agents were omitted. Purified FKBP-Cdc34 (20 pmol), derived from cells that were treated with (lanes 7–12) or without (lanes 1–6) AP20187, was incubated for the indicated periods at 37°C. No DTT was added. A Lower and B Lower are quantitative representations of the amount of the di-Ub formed.
Discussion
Role for E2–E2 Juxtaposition in Cdc34 Activation. Human Cdc34 weakly supports the assembly of polyUb chains and, therefore, must undergo activation during ubiquitination mediated by the SCF, which dramatically increases the Ub ligation activity of Cdc34, resulting in 20-fold stimulation in the formation of di-Ub (Fig. 2). In this study, we have explored the nature of the Cdc34 activation and suggest that the activation may involve bringing E2 molecules into proximity, based on the findings that Cdc34 can be converted to a catalytically “activated” state by forced juxtaposition of the E2 enzyme through GST- or FKBP-based heterologous dimerization (Figs. 3, 4, 5 and Table 1).
Previous studies have shown that dimerization of the E2–25K core (amino acids 1–153) by GST fusion markedly enhanced the catalytic synthesis of polyUb chains by the truncated enzyme (18). However, this activation apparently proceeded through a mechanism involving formation of thiol-linked Ub chains that was not observed with GST-fused full-length E2–25K (18). For this reason, it is unclear whether GST-Cdc34 and GST-E2–25K (1–153) use the same mechanism to mediate their increased ubiquitination activity.
Seol et al. (8) have previously reported that polylysine activates the autoubiquitination of yeast Cdc34 independently of a RING finger protein. In agreement with this finding, we found that the formation of K48-linked polyUb chains by human Cdc34 was stimulated by the addition of either l- or d-type polylysine but not by polyglutamic or polyaspartic acid. In addition, human Cdc34 efficiently bound to polylysine-coupled agarose beads, and the polylysine-bound human Cdc34 formed large aggregates that pelleted upon sedimentation in glycerol gradients (data not shown). These results appear consistent with a role played by dimerization/multimerization of Cdc34, which activates its ability to assemble Ub chains.
Ub ligation by Cdc34 requires the alignment of two Ub molecules in a manner that permits Lys-48 of one molecule (the acceptor) to react with Gly-76 of the other (the donor) molecule bound to the catalytic Cys-93 of Cdc34, as shown by the ability of Cdc34 to form an Ub dimer composed of the UbK48R donor and the Ub+1 acceptor [deficient in thiol–ester formation (19); data not shown]. How might juxtaposed Cdc34 molecules work to promote Ub ligation? Conceivably, once placed into proximity by GST- or FKBP-mediated fusion, juxtaposed Cdc34 molecules may engage in homodimeric interactions. Such a structure could resemble the Ubc13-Mms2 heterodimer (20) to create/stabilize a docking site for the loading and aligning of the acceptor Ub with the donor Ub for ligation.
Models for Transient Juxtaposition/Dimerization of Cdc34 for Activated Ubiquitination. Although it remains unclear whether the observed activation of Cdc34 containing heterologous dimerization domains has physiological relevance, previous studies suggest a role for scCdc34 dimerization (21–24). Self-association among ScCdc34 molecules can be detected by coimmunoprecipitation and this activity appears to require the integrity of the Cdc34-Ub thiol–ester (24). In addition, mutagenesis studies demonstrated that the ScCdc34 S97 residue is required for its self-association, ability to assemble multiUb chains, and cell viability (23, 24), further underscoring a role for E2 self-association in the assembly of polyUb chains.
However, human Cdc34 exists primarily in a monomeric form in solution (Table 1). Moreover, incubation of human Cdc34 with the neddylated ROC1–CUL1 complex did not promote its transition into a dimer that could be detected by gel filtration experiments (data not shown), an observation inconsistent with the notion that activation of human Cdc34 by SCF involves formation of a stable E2 homodimer. These findings prompted us to propose a SCF-mediated transient conversion of human Cdc34 from the inactive monomeric form to the highly active dimeric state. This dynamic monomer–dimer transition may dictate an inactivation–activation cycle for human Cdc34. The transient nature may be advantageous, considering that constitutive, stable Cdc34 dimers would result in the synthesis of unproductive free Ub chains and self-ubiquitinated E2 species. A recent study by Deffenbaugh et al. (25) has shown that the release of Ub-charged Cdc34-S∼Ub from the RING domain is essential for ubiquitination of Sic1 by SCFCdc4, indicating a dynamic interaction between SCF and Cdc34-S∼Ub. Because the C terminus of human Cdc34 is required for interaction with the ROC1–CUL1 complex (15) but is largely dispensable for Ub chain assembly with GST-fusion (see Fig. 6, which is published as supporting information on the PNAS web site), it may be that the transient juxtaposition of Cdc34 molecules is mediated by an interaction between its C terminus and the neddylated ROC1–CUL1 complex. Hopefully, future development of techniques for monitoring transient homomeric interactions in living cells will permit an assessment of whether SCF mediates a dynamic conversion of Cdc34 from a monomer to a dimeric form during the ubiquitination reaction.
We envision two models that may account for the role played by the neddylated ROC1–CUL1 in promoting the transient juxtaposition/dimerization of Cdc34. One involves a possible ROC1-mediated E3 homodimerization, based on an intrinsic homo- or heterodimerization property shared by the RAG1 and BRCA1/BARD1 RING finger proteins (26, 27). However, this hypothesis is inconsistent with the observed monomeric structure of ROC1/Rbx1 in reconstituted SCF, as determined by crystallographic analysis (6). On the other hand, it cannot be ruled out that posttranslational modifications might promote dimerization of ROC1, thus providing two adjacent binding surfaces for the interactions of two Cdc34 molecules to stimulate Ub ligation. Alternatively, whereas ROC1 serves as a platform for anchoring one Cdc34 molecule, the neighboring CUL1K720-linked Nedd8 may have a role in recruiting the second E2 molecule. Intriguingly, Nedd8 contains a Leu-8/Ile-44/Val-70 hydrophobic patch conserved in Ub (28). This Ub Leu-8/Ile-44/Val-70 patch has been shown to bind to Ub E2 variant, Ub interacting motif, and CUE domain (29–31).
Supplementary Material
Acknowledgments
We thank Victor M. Rivera (ARIAD Pharmaceuticals, Cambridge, MA) for providing the pC4-Fv1E plasmid and the AP20187 chemical dimerizer. This work was supported by Public Health Service Grants CA94006 (to A.K.A.), GM38559 (to J.H.), and GM61051 and CA095634 (to Z.-Q.P.).
Author contributions: S.G., K.W., A.K.A., J.H., and Z.-Q.P. designed research; S.G., K.Y., C.R.E., I.T., V.B., and Z.-Q.P. performed research; C.R.E., I.T., V.P.B., and A.K.A. contributed new reagents/analytic tools; S.G., A.K.A., J.H., and Z.-Q.P. analyzed data; and S.G. and Z.-Q.P. wrote the paper.
Abbreviations: CUL, cullin; FKBP, FK506-binding protein; HF, high five; sc, Saccharomyces cerevisiae; SCF, Skp1·CUL1·F-box; Ub, ubiquitin.
References
- 1.Hershko, A. & Ciechanover, A. (1998) Annu. Rev. Biochem. 67, 425–479. [DOI] [PubMed] [Google Scholar]
- 2.Pickart, C. M. (2001) Annu. Rev. Biochem. 70, 503–533. [DOI] [PubMed] [Google Scholar]
- 3.Pan, Z.-Q., Kentsis, A., Dias, D. C., Yamoah, K. & Wu, K. (2004) Oncogene 23, 1985–1997. [DOI] [PubMed] [Google Scholar]
- 4.Patton, E. E., Willems, A., Sa, D., Kuras, L., Thomas, D., Craig, K. L. & Tyers, M. (1998) Genes Dev. 12, 692–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu, K., Fuchs, S. Y., Chen, A., Tan, P., Gomez, C., Ronai, Z. & Pan, Z.-Q. (2000) Mol. Cell. Biol. 20, 1382–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zheng, N., Schulman, B. A., Song, L., Miller, J. J., Jeffrey, P. D., Wang, P., Chu, C., Koepp, D. M., Elledge, S. J., Pagano, M., et al. (2002) Nature 416, 703–709. [DOI] [PubMed] [Google Scholar]
- 7.Skowyra, D., Koepp, D. M., Kamura, T., Conrad, M. N., Conaway, R. C., Conaway, J. W., Elledge, S. J. & Harper, J. W. (1999) Science 284, 662–665. [DOI] [PubMed] [Google Scholar]
- 8.Seol, J. H., Feldman, R. M., Zachariae, W., Shevchenko, A., Correll, C. C., Lyapina, S., Chi, Y., Galova, M., Claypool, J., Sandmeyer, S., et al. (1999) Genes Dev. 13, 1614–1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen, A., Wu, K., Fuchs, S. Y., Tan, P., Gomez, C. & Pan, Z.-Q. (2000) J. Biol. Chem. 275, 15432–15439. [DOI] [PubMed] [Google Scholar]
- 10.Banerjee, A., Gregori, L., Xu, Y. & Chau, V. (1993) J. Biol. Chem. 268, 5668–5675. [PubMed] [Google Scholar]
- 11.Koepp, D. M., Harper, J. W. & Elledge, S. J. (1999) Cell 97, 431–434. [DOI] [PubMed] [Google Scholar]
- 12.Plon, S. E., Leppig, K. A., Do, H. N. & Groudine, M. (1993) Proc. Natl. Acad. Sci. USA 90, 10484–10488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tan, P., Fuchs, S. Y., Chen, A., Wu, K., Gomez, C., Ronai, Z. & Pan, Z.-Q. (1999) Mol. Cell 3, 527–533. [DOI] [PubMed] [Google Scholar]
- 14.Wu, K., Chen, A. & Pan, Z.-Q. (2000) J. Biol. Chem. 275, 32317–32324. [DOI] [PubMed] [Google Scholar]
- 15.Wu, K., Chen, A., Tan, P. & Pan, Z.-Q. (2002) J. Biol. Chem. 277, 516–527. [DOI] [PubMed] [Google Scholar]
- 16.Lim, K., Xo, J. X., Keeling, K., Gilliland, G. L., Ji, X., Ruker, F. & Carter, D. C. (1994) Protein Sci. 3, 2233–2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clackson, T., Yang, W., Rozamus, L. W., Hatada, M., Amara, J. F., Rollins, C. T., Stevenson, L. F., Magari, S. R., Wood, S. A., Courage, N. L., et al. (1998) Proc. Natl. Acad. Sci. USA 95, 10437–10442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haldeman, M. T., Xia, G., Kasperek, E. M. & Pickart, C. M. (1997) Biochemistry 36, 10526–10537. [DOI] [PubMed] [Google Scholar]
- 19.Lam, Y. A, Pickart, C. M., Alban, A., Landon, M., Jamieson, C., Ramage, R., Mayer, R. J. & Layfield, R. (2000) Proc. Natl. Acad. Sci. USA 97, 9902–9906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.VanDemark, A. P., Hofmann, R. M., Tsui, C., Pickart, C. M. & Wolberger, C. (2001) Cell 105, 711–720. [DOI] [PubMed] [Google Scholar]
- 21.Silver, E. T., Gwozd, T. J., Ptak, C., Goebl, M. & Ellison, M. J. (1992) EMBO J. 11, 3091–3098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ptak, C., Prendergast, J. A., Hodgins, R., Kay, C. M., Chau, V. & Ellison, M. J. (1994) J. Biol. Chem. 269, 26539–26545. [PubMed] [Google Scholar]
- 23.Liu, Y., Mathias, N., Steussy, C. N. & Goebl, M. G. (1995) Mol. Cell. Biol. 15, 5635–5644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Varelas, X., Ptak, C. & Ellison, M. J. (2003) Mol. Cell. Biol. 23, 5388–5400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deffenbaugh, A. E., Scaglione, K. M., Zhang, L., Moore, J. M., Buranda, T., Sklar, L. A. & Skowyra, D. (2003) Cell 114, 611–622. [DOI] [PubMed] [Google Scholar]
- 26.Bellon, S. F., Rodgers, K. K., Schatz, D. G., Coleman, J. E. & Steitz, T. A. (1997) Nat. Struct. Biol. 4, 586–591. [DOI] [PubMed] [Google Scholar]
- 27.Brzovic, P. S., Rajagopal, P., Hoyt, D. W., King, M. C. & Klevit, R. E. (2001) Nat. Struct. Biol. 8, 833–837. [DOI] [PubMed] [Google Scholar]
- 28.Whitby, F. G., Xia, G., Pickart, C. M. & Hill, C. P. (1998) J. Biol. Chem. 273, 34983–34991. [DOI] [PubMed] [Google Scholar]
- 29.Kang, R. S., Daniels, C. M., Francis, S. A., Shih, S. C., Salerno, W. J., Hicke, L. & Radhakrishnan, I. (2003) Cell 113, 621–630. [DOI] [PubMed] [Google Scholar]
- 30.Swanson, K. A., Kang, R. S., Stamenova, S. D., Hicke, L. & Radhakrishnan, I. (2003) EMBO J. 22, 4597–4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sundquist, W. I., Schubert. H. L., Kelly, B. N., Hill, G. C., Holton, J. M. & Hill, C. P. (2004) Mol. Cell 13, 783–789. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.