Abstract
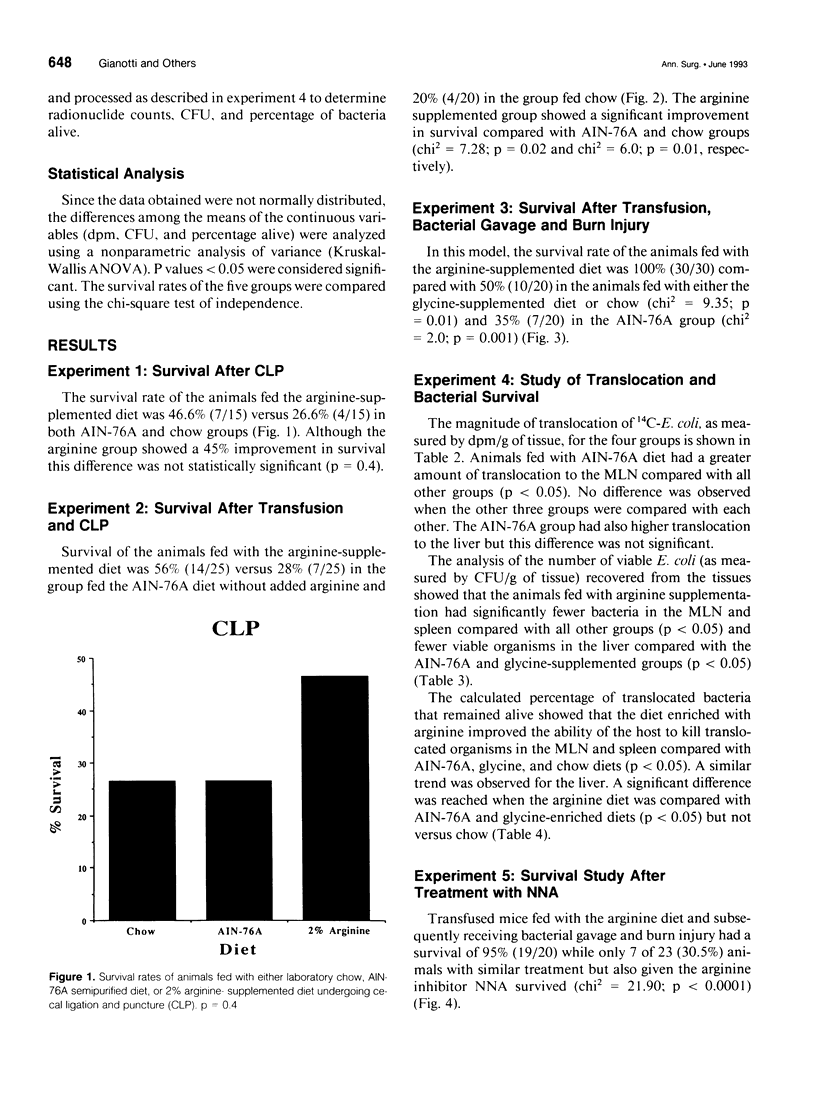
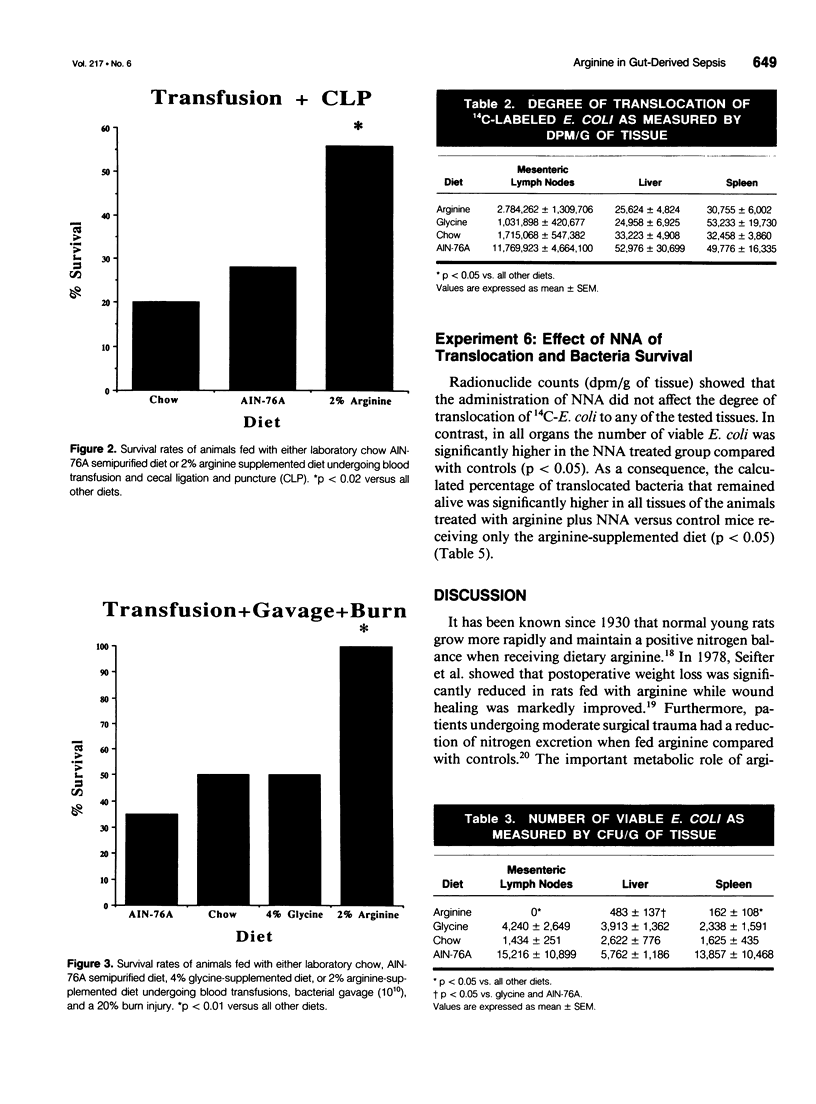
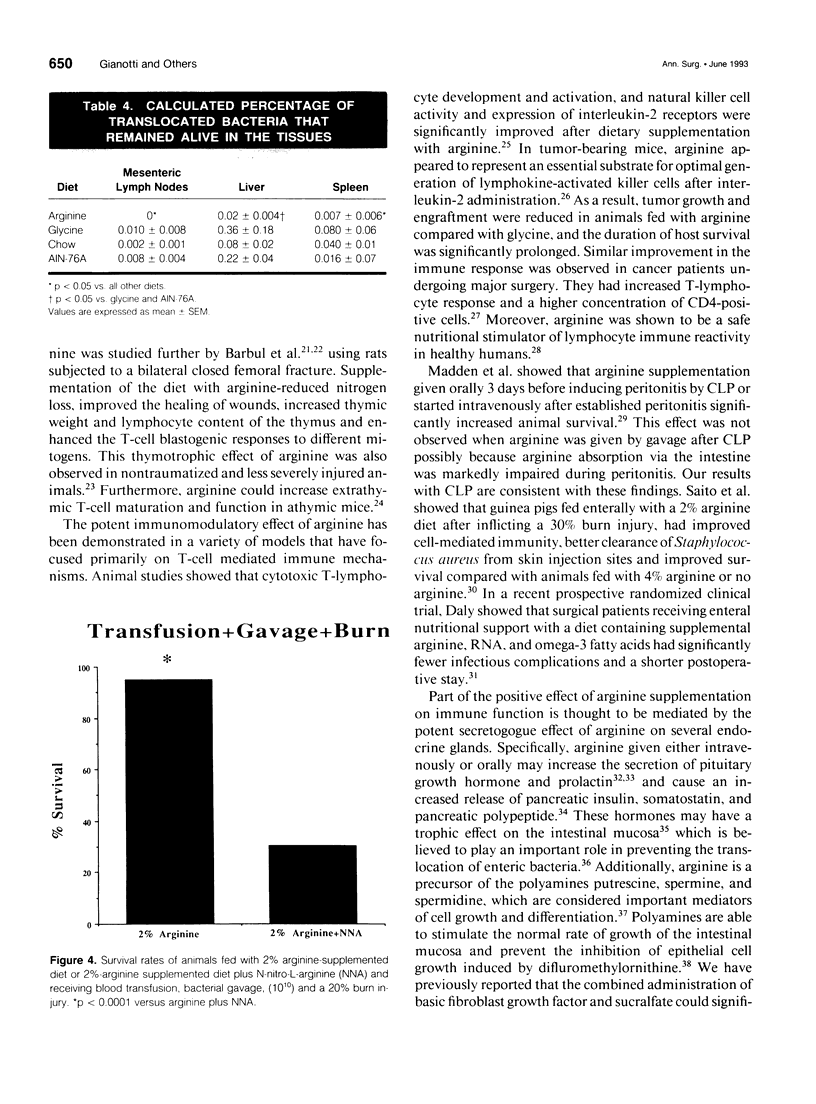
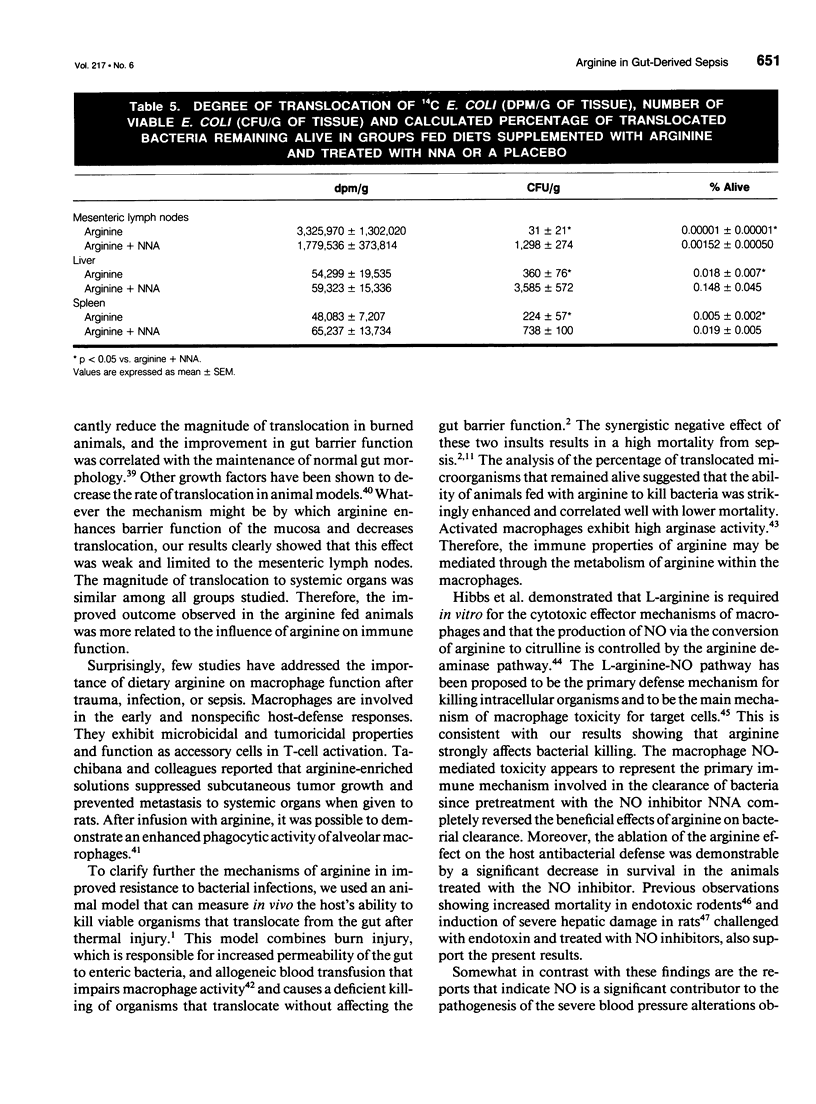
OBJECTIVE: The effect of arginine on survival rates and host defense mechanisms was studied using two clinically relevant models of infection that included transfusion-induced immunosuppression. SUMMARY BACKGROUND DATA: Dietary arginine will improve resistance to infection but its role in transfusion-induced immunosuppression and bacterial translocation (gut-derived sepsis) has not been defined. METHODS: Balb/c mice were fed for 10 days with either a defined AIN-76A diet, an AIN-76A diet supplemented with 2% arginine, an AIN-76A diet supplemented with 4% glycine, or standard laboratory chow. In most experiments, the mice were then transfused with allogeneic blood and allowed to feed for an additional 5 days before undergoing either cecal ligation and puncture (CLP) or gavage with 10(10) Escherichia coli and a 20% burn injury. Additional animals fed with the arginine supplemented diet were treated with the nitric oxide inhibitor N omega-Nitro-L-arginine (NNA) before gavage and burn. The effect of these diets and NNA on the degree of translocation of 14C-radiolabeled E. coli from the intestine and the ability of the host to kill translocated organisms was also investigated. Mice were fed and received transfusion, gavage, and burn as above. Mesenteric lymph nodes (MLN), liver and spleen were harvested 4 hours postburn. RESULTS: Survival after CLP was 56% in the arginine-supplemented group versus 28% in the AIN-76A group and 20% in the chow group (p < 0.02). After gavage and burn, survival was 100% in the arginine-supplemented group versus 50% in both the glycine-supplemented and chow groups and 35% in the AIN-76A group (p < 0.01). In animals receiving the arginine-supplemented diet, treatment with NNA decreased survival from 95% to 30.5% (p < 0.0001). Greater translocation, as measured by radionuclide counts, was observed to the MLN of the AIN-76A group. However, there was no difference in translocation to the liver and spleen related to dietary group. Quantitative colony counts and the calculated percentage of remaining viable bacteria showed that the ability to kill translocated organisms was significantly enhanced in animals receiving arginine. Treatment with NNA reversed the beneficial effects of arginine on immune defense. CONCLUSIONS: The benefit of arginine appears to be mediated by improved bactericidal mechanisms via the arginine-nitric oxide pathway.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander J. W., Boyce S. T., Babcock G. F., Gianotti L., Peck M. D., Dunn D. L., Pyles T., Childress C. P., Ash S. K. The process of microbial translocation. Ann Surg. 1990 Oct;212(4):496–512. doi: 10.1097/00000658-199010000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander J. W., Gianotti L., Pyles T., Carey M. A., Babcock G. F. Distribution and survival of Escherichia coli translocating from the intestine after thermal injury. Ann Surg. 1991 Jun;213(6):558–567. doi: 10.1097/00000658-199106000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbul A., Sisto D. A., Wasserkrug H. L., Efron G. Arginine stimulates lymphocyte immune response in healthy human beings. Surgery. 1981 Aug;90(2):244–251. [PubMed] [Google Scholar]
- Barbul A., Sisto D. A., Wasserkrug H. L., Yoshimura N. N., Efron G. Metabolic and immune effects of arginine in postinjury hyperalimentation. J Trauma. 1981 Nov;21(11):970–974. doi: 10.1097/00005373-198111000-00011. [DOI] [PubMed] [Google Scholar]
- Barbul A., Wasserkrug H. L., Seifter E., Rettura G., Levenson S. M., Efron G. Immunostimulatory effects of arginine in normal and injured rats. J Surg Res. 1980 Sep;29(3):228–235. doi: 10.1016/0022-4804(80)90165-1. [DOI] [PubMed] [Google Scholar]
- Barbul A., Wasserkrug H. L., Yoshimura N., Tao R., Efron G. High arginine levels in intravenous hyperalimentation abrogate post-traumatic immune suppression. J Surg Res. 1984 Jun;36(6):620–624. doi: 10.1016/0022-4804(84)90149-5. [DOI] [PubMed] [Google Scholar]
- Billiar T. R., Curran R. D., Harbrecht B. G., Stuehr D. J., Demetris A. J., Simmons R. L. Modulation of nitrogen oxide synthesis in vivo: NG-monomethyl-L-arginine inhibits endotoxin-induced nitrate/nitrate biosynthesis while promoting hepatic damage. J Leukoc Biol. 1990 Dec;48(6):565–569. doi: 10.1002/jlb.48.6.565. [DOI] [PubMed] [Google Scholar]
- Border J. R., Hassett J., LaDuca J., Seibel R., Steinberg S., Mills B., Losi P., Border D. The gut origin septic states in blunt multiple trauma (ISS = 40) in the ICU. Ann Surg. 1987 Oct;206(4):427–448. doi: 10.1097/00000658-198710000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrico C. J., Meakins J. L., Marshall J. C., Fry D., Maier R. V. Multiple-organ-failure syndrome. Arch Surg. 1986 Feb;121(2):196–208. doi: 10.1001/archsurg.1986.01400020082010. [DOI] [PubMed] [Google Scholar]
- Currie G. A., Gyure L., Cifuentes L. Microenvironmental arginine depletion by macrophages in vivo. Br J Cancer. 1979 Jun;39(6):613–620. doi: 10.1038/bjc.1979.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly J. M., Lieberman M. D., Goldfine J., Shou J., Weintraub F., Rosato E. F., Lavin P. Enteral nutrition with supplemental arginine, RNA, and omega-3 fatty acids in patients after operation: immunologic, metabolic, and clinical outcome. Surgery. 1992 Jul;112(1):56–67. [PubMed] [Google Scholar]
- Daly J. M., Reynolds J., Thom A., Kinsley L., Dietrick-Gallagher M., Shou J., Ruggieri B. Immune and metabolic effects of arginine in the surgical patient. Ann Surg. 1988 Oct;208(4):512–523. doi: 10.1097/00000658-198810000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deitch E. A. Simple intestinal obstruction causes bacterial translocation in man. Arch Surg. 1989 Jun;124(6):699–701. doi: 10.1001/archsurg.1989.01410060065013. [DOI] [PubMed] [Google Scholar]
- Fukushima R., Gianotti L., Alexander J. W., Pyles T. The degree of bacterial translocation is a determinant factor for mortality after burn injury and is improved by prostaglandin analogs. Ann Surg. 1992 Oct;216(4):438–445. doi: 10.1097/00000658-199210000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galandiuk S., George C. D., Pietsch J. D., Byck D. C., DeWeese R. C., Polk H. C., Jr An experimental assessment of the effect of blood transfusion on susceptibility to bacterial infection. Surgery. 1990 Sep;108(3):567–571. [PubMed] [Google Scholar]
- Gianotti L., Alexander J. W., Fukushima R., Pyles T. Reduction of bacterial translocation with oral fibroblast growth factor and sucralfate. Am J Surg. 1993 Jan;165(1):195–201. doi: 10.1016/s0002-9610(05)80425-8. [DOI] [PubMed] [Google Scholar]
- Gianotti L., Pyles T., Alexander J. W., Babcock G. F., Carey M. A. Impact of blood transfusion and burn injury on microbial translocation and bacterial survival. Transfusion. 1992 May;32(4):312–317. doi: 10.1046/j.1537-2995.1992.32492263443.x. [DOI] [PubMed] [Google Scholar]
- Gonce S. J., Peck M. D., Alexander J. W., Miskell P. W. Arginine supplementation and its effect on established peritonitis in guinea pigs. JPEN J Parenter Enteral Nutr. 1990 May-Jun;14(3):237–244. doi: 10.1177/0148607190014003237. [DOI] [PubMed] [Google Scholar]
- Hibbs J. B., Jr, Taintor R. R., Vavrin Z. Macrophage cytotoxicity: role for L-arginine deiminase and imino nitrogen oxidation to nitrite. Science. 1987 Jan 23;235(4787):473–476. doi: 10.1126/science.2432665. [DOI] [PubMed] [Google Scholar]
- Hibbs J. B., Jr, Vavrin Z., Taintor R. R. L-arginine is required for expression of the activated macrophage effector mechanism causing selective metabolic inhibition in target cells. J Immunol. 1987 Jan 15;138(2):550–565. [PubMed] [Google Scholar]
- Hibbs J. B., Jr, Westenfelder C., Taintor R., Vavrin Z., Kablitz C., Baranowski R. L., Ward J. H., Menlove R. L., McMurry M. P., Kushner J. P. Evidence for cytokine-inducible nitric oxide synthesis from L-arginine in patients receiving interleukin-2 therapy. J Clin Invest. 1992 Mar;89(3):867–877. doi: 10.1172/JCI115666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isidori A., Lo Monaco A., Cappa M. A study of growth hormone release in man after oral administration of amino acids. Curr Med Res Opin. 1981;7(7):475–481. doi: 10.1185/03007998109114287. [DOI] [PubMed] [Google Scholar]
- Kay J. E., Lindsay V. J. Polyamine synthesis during lymphocyte activation. Induction of ornithine decarboxylase and S-adenosyl methionine decarboxylase. Exp Cell Res. 1973 Mar 15;77(1):428–436. doi: 10.1016/0014-4827(73)90597-1. [DOI] [PubMed] [Google Scholar]
- Kilbourn R. G., Belloni P. Endothelial cell production of nitrogen oxides in response to interferon gamma in combination with tumor necrosis factor, interleukin-1, or endotoxin. J Natl Cancer Inst. 1990 May 2;82(9):772–776. doi: 10.1093/jnci/82.9.772. [DOI] [PubMed] [Google Scholar]
- Kilbourn R. G., Griffith O. W. Overproduction of nitric oxide in cytokine-mediated and septic shock. J Natl Cancer Inst. 1992 Jun 3;84(11):827–831. doi: 10.1093/jnci/84.11.827. [DOI] [PubMed] [Google Scholar]
- Kirk S. J., Regan M. C., Wasserkrug H. L., Sodeyama M., Barbul A. Arginine enhances T-cell responses in athymic nude mice. JPEN J Parenter Enteral Nutr. 1992 Sep-Oct;16(5):429–432. doi: 10.1177/0148607192016005429. [DOI] [PubMed] [Google Scholar]
- Lieberman M. D., Nishioka K., Redmond H. P., Daly J. M. Enhancement of interleukin-2 immunotherapy with L-arginine. Ann Surg. 1992 Feb;215(2):157–165. doi: 10.1097/00000658-199202000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L., Ma J. W., Deitch E. A., Specian R. D., Berg R. D. Genetic susceptibility to mucosal damage leads to bacterial translocation in a murine burn model. J Trauma. 1989 Sep;29(9):1245–1251. doi: 10.1097/00005373-198909000-00010. [DOI] [PubMed] [Google Scholar]
- Madden H. P., Breslin R. J., Wasserkrug H. L., Efron G., Barbul A. Stimulation of T cell immunity by arginine enhances survival in peritonitis. J Surg Res. 1988 Jun;44(6):658–663. doi: 10.1016/0022-4804(88)90097-2. [DOI] [PubMed] [Google Scholar]
- Marletta M. A., Yoon P. S., Iyengar R., Leaf C. D., Wishnok J. S. Macrophage oxidation of L-arginine to nitrite and nitrate: nitric oxide is an intermediate. Biochemistry. 1988 Nov 29;27(24):8706–8711. doi: 10.1021/bi00424a003. [DOI] [PubMed] [Google Scholar]
- Merimee T. J., Rabinowitz D., Riggs L., Burgess J. A., Rimoin D. L., McKusick V. A. Plasma growth hormone after arginine infusion. Clinical experiences. N Engl J Med. 1967 Feb 23;276(8):434–439. doi: 10.1056/NEJM196702232760803. [DOI] [PubMed] [Google Scholar]
- Mochizuki H., Trocki O., Dominioni L., Brackett K. A., Joffe S. N., Alexander J. W. Mechanism of prevention of postburn hypermetabolism and catabolism by early enteral feeding. Ann Surg. 1984 Sep;200(3):297–310. doi: 10.1097/00000658-198409000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moncada S., Higgs E. A. Endogenous nitric oxide: physiology, pathology and clinical relevance. Eur J Clin Invest. 1991 Aug;21(4):361–374. doi: 10.1111/j.1365-2362.1991.tb01383.x. [DOI] [PubMed] [Google Scholar]
- Nava E., Palmer R. M., Moncada S. Inhibition of nitric oxide synthesis in septic shock: how much is beneficial? Lancet. 1991 Dec 21;338(8782-8783):1555–1557. doi: 10.1016/0140-6736(91)92375-c. [DOI] [PubMed] [Google Scholar]
- Offenbartl K., Bengmark S. Intraabdominal infections and gut origin sepsis. World J Surg. 1990 Mar-Apr;14(2):191–195. doi: 10.1007/BF01664872. [DOI] [PubMed] [Google Scholar]
- Petros A., Bennett D., Vallance P. Effect of nitric oxide synthase inhibitors on hypotension in patients with septic shock. Lancet. 1991 Dec 21;338(8782-8783):1557–1558. doi: 10.1016/0140-6736(91)92376-d. [DOI] [PubMed] [Google Scholar]
- Reynolds J. V., Daly J. M., Zhang S., Evantash E., Shou J., Sigal R., Ziegler M. M. Immunomodulatory mechanisms of arginine. Surgery. 1988 Aug;104(2):142–151. [PubMed] [Google Scholar]
- Rush B. F., Jr, Redan J. A., Flanagan J. J., Jr, Heneghan J. B., Hsieh J., Murphy T. F., Smith S., Machiedo G. W. Does the bacteremia observed in hemorrhagic shock have clinical significance? A study in germ-free animals. Ann Surg. 1989 Sep;210(3):342–347. doi: 10.1097/00000658-198909000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito H., Trocki O., Wang S. L., Gonce S. J., Joffe S. N., Alexander J. W. Metabolic and immune effects of dietary arginine supplementation after burn. Arch Surg. 1987 Jul;122(7):784–789. doi: 10.1001/archsurg.1987.01400190050010. [DOI] [PubMed] [Google Scholar]
- Schoeffel U., Windfuhr M., Freudenberg N., Treutner K. H., Jacobs E., Galanos C. The role of intestinal endotoxin in experimental peritonitis. Circ Shock. 1989 Jan;27(1):83–91. [PubMed] [Google Scholar]
- Seifter E., Rettura G., Barbul A., Levenson S. M. Arginine: an essential amino acid for injured rats. Surgery. 1978 Aug;84(2):224–230. [PubMed] [Google Scholar]
- Stieritz D. D., Holder I. A. Experimental studies of the pathogenesis of infections due to Pseudomonas aeruginosa: description of a burned mouse model. J Infect Dis. 1975 Jun;131(6):688–691. doi: 10.1093/infdis/131.6.688. [DOI] [PubMed] [Google Scholar]
- Tachibana K., Mukai K., Hiraoka I., Moriguchi S., Takama S., Kishino Y. Evaluation of the effect of arginine-enriched amino acid solution on tumor growth. JPEN J Parenter Enteral Nutr. 1985 Jul-Aug;9(4):428–434. doi: 10.1177/0148607185009004428. [DOI] [PubMed] [Google Scholar]
- Utsumi M., Makimura H., Ishihara K., Morita S., Baba S. Determination of immunoreactive somatostatin in rat plasma and responses to arginine, glucose and glucagon infusion. Diabetologia. 1979 Nov;17(5):319–323. doi: 10.1007/BF01235888. [DOI] [PubMed] [Google Scholar]
- Wang J. Y., McCormack S. A., Viar M. J., Johnson L. R. Stimulation of proximal small intestinal mucosal growth by luminal polyamines. Am J Physiol. 1991 Sep;261(3 Pt 1):G504–G511. doi: 10.1152/ajpgi.1991.261.3.G504. [DOI] [PubMed] [Google Scholar]
- Waymack J. P., Rapien J., Garnett D., Tweddell J. S., Alexander J. W. Effect of transfusion on immune function in a traumatized animal model. Arch Surg. 1986 Jan;121(1):50–55. doi: 10.1001/archsurg.1986.01400010056007. [DOI] [PubMed] [Google Scholar]
- Williamson R. C., Bauer F. L., Ross J. S., Malt R. A. Contributions of bile and pancreatic juice to cell proliferation in ileal mucosa. Surgery. 1978 May;83(5):570–576. [PubMed] [Google Scholar]



