Abstract
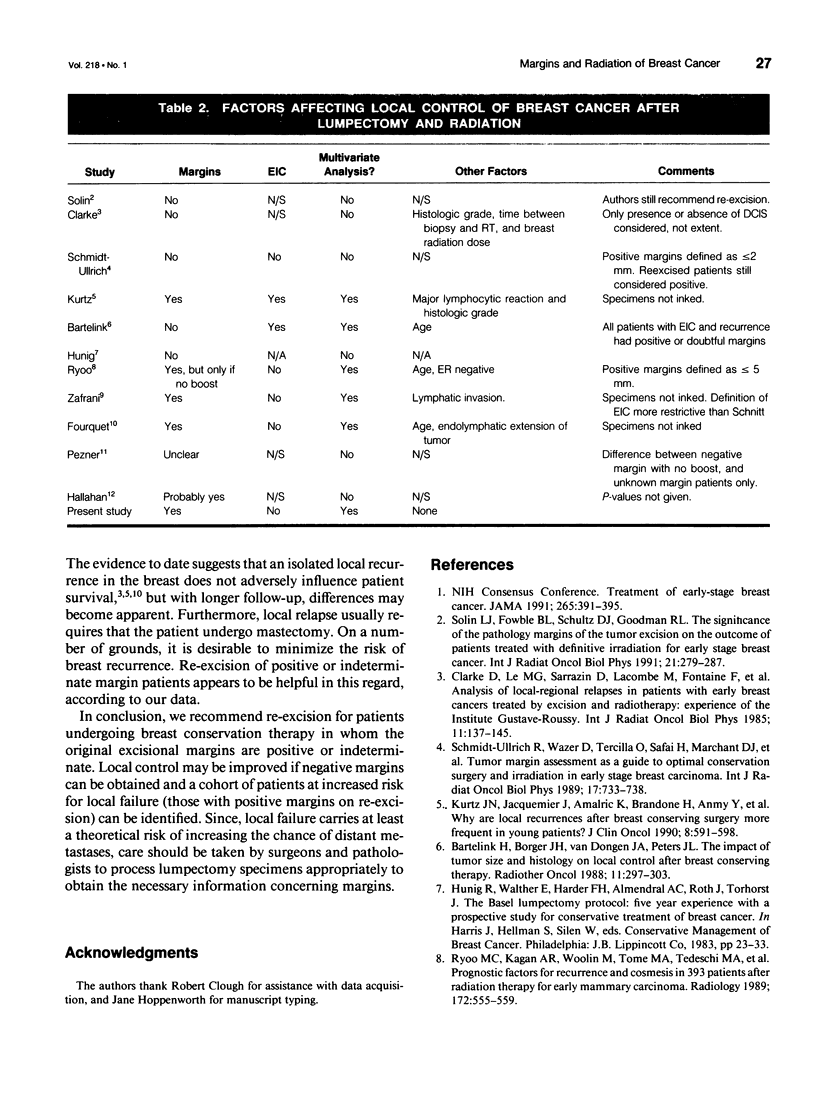
OBJECTIVE: The authors determined whether microscopically positive surgical margins are detrimental to the outcome of early stage breast cancer patients treated with conservation surgery and radiation therapy. SUMMARY BACKGROUND DATA: The optimal extent of breast surgery required for patients treated with conservation surgery and radiation therapy has not been established. To achieve breast preservation with good cosmesis, it is desirable to resect as little normal tissue as possible. However, it is critical that the resection does not leave behind a tumor burden that cannot be adequately managed by moderate doses of radiation. It is not known whether microscopically positive surgical margins are detrimental to patient outcome. METHODS: The records of 259 consecutive women (262 breasts) treated with local excision (complete removal of gross tumor with a margin) and axillary dissection followed by radiation therapy for clinical stage I and II infiltrating ductal breast cancer at Duke University Medical Center and the University of North Carolina between 1983 and 1988 were reviewed. Surgical margins were considered positive if tumor extended to the inked margins; otherwise the margins were considered negative. Margins that could not be determined, either because the original pathology report did not comment on margins, or because the original specimen had not been inked were called indeterminate. RESULTS: Of the 262 tumors, 32 (12%) had positive margins, 132 (50%) had negative margins, and the remaining 98 (38%) had indeterminate margins. There were 11 (4%) local failures; 3/32 (9%) from the positive margin group, 2/132 (1.5%) from the negative margin group, and 6/98 (6%) from the indeterminate group. The actuarial local failure rates at 5 years were 10%, 2%, and 10%, respectively, p = 0.014 positive vs. negative, p = 0.08 positive vs. indeterminate (log rank test). Margin status had no impact on survival or freedom from distant metastasis; 63 patients who originally had positive or indeterminate margins were re-excised. Two of 7 with positive margins after re-excision versus 1/56 rendered margin negative had a local recurrence. CONCLUSIONS: The authors recommend re-excision for patients with positive margins because of improved local control of those rendered margin negative and identification of those patients at high risk for local failure (those who remain positive after re-excision). Because margin status impacts on local control, tumor margins after conservation surgery should be accurately determined in all patients.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amalric R., Santamaria F., Robert F., Seigle J., Altschuler C., Kurtz J. M., Spitalier J. M., Brandone H., Ayme Y., Pollet J. F. Radiation therapy with or without primary limited surgery for operable breast cancer: a 20-year experience at the Marseilles Cancer Institute. Cancer. 1982 Jan 1;49(1):30–34. doi: 10.1002/1097-0142(19820101)49:1<30::aid-cncr2820490107>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Bartelink H., Borger J. H., van Dongen J. A., Peterse J. L. The impact of tumor size and histology on local control after breast-conserving therapy. Radiother Oncol. 1988 Apr;11(4):297–303. doi: 10.1016/0167-8140(88)90200-9. [DOI] [PubMed] [Google Scholar]
- Calle R., Pilleron J. P., Schlienger P., Vilcoq J. R. Conservative management of operable breast cancer: ten years experience at the Foundation Curie. Cancer. 1978 Oct;42(4):2045–2053. doi: 10.1002/1097-0142(197810)42:4<2045::aid-cncr2820420455>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Calle R., Vilcoq J. R., Zafrani B., Vielh P., Fourquet A. Local control and survival of breast cancer treated by limited surgery followed by irradiation. Int J Radiat Oncol Biol Phys. 1986 Jun;12(6):873–878. doi: 10.1016/0360-3016(86)90379-2. [DOI] [PubMed] [Google Scholar]
- Clarke D. H., Lê M. G., Sarrazin D., Lacombe M. J., Fontaine F., Travagli J. P., May-Levin F., Contesso G., Arriagada R. Analysis of local-regional relapses in patients with early breast cancers treated by excision and radiotherapy: experience of the Institut Gustave-Roussy. Int J Radiat Oncol Biol Phys. 1985 Jan;11(1):137–145. doi: 10.1016/0360-3016(85)90372-4. [DOI] [PubMed] [Google Scholar]
- Delouche G., Bachelot F., Premont M., Kurtz J. M. Conservation treatment of early breast cancer: long term results and complications. Int J Radiat Oncol Biol Phys. 1987 Jan;13(1):29–34. doi: 10.1016/0360-3016(87)90256-2. [DOI] [PubMed] [Google Scholar]
- Fisher B., Redmond C., Poisson R., Margolese R., Wolmark N., Wickerham L., Fisher E., Deutsch M., Caplan R., Pilch Y. Eight-year results of a randomized clinical trial comparing total mastectomy and lumpectomy with or without irradiation in the treatment of breast cancer. N Engl J Med. 1989 Mar 30;320(13):822–828. doi: 10.1056/NEJM198903303201302. [DOI] [PubMed] [Google Scholar]
- Fisher E. R., Sass R., Fisher B., Gregorio R., Brown R., Wickerham L. Pathologic findings from the National Surgical Adjuvant Breast Project (protocol 6). II. Relation of local breast recurrence to multicentricity. Cancer. 1986 May 1;57(9):1717–1724. doi: 10.1002/1097-0142(19860501)57:9<1717::aid-cncr2820570902>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Fourquet A., Campana F., Zafrani B., Mosseri V., Vielh P., Durand J. C., Vilcoq J. R. Prognostic factors of breast recurrence in the conservative management of early breast cancer: a 25-year follow-up. Int J Radiat Oncol Biol Phys. 1989 Oct;17(4):719–725. doi: 10.1016/0360-3016(89)90057-6. [DOI] [PubMed] [Google Scholar]
- Fowble B. L., Solin L. J., Schultz D. J., Goodman R. L. Ten year results of conservative surgery and irradiation for stage I and II breast cancer. Int J Radiat Oncol Biol Phys. 1991 Jul;21(2):269–277. doi: 10.1016/0360-3016(91)90771-u. [DOI] [PubMed] [Google Scholar]
- Hallahan D. E., Michel A. G., Halpern H. J., Awan A. M., Desser R., Bitran J., Recant W., Wyman B., Spelbring D. R., Weichselbaum R. R. Breast conserving surgery and definitive irradiation for early stage breast cancer. Int J Radiat Oncol Biol Phys. 1989 Dec;17(6):1211–1216. doi: 10.1016/0360-3016(89)90528-2. [DOI] [PubMed] [Google Scholar]
- Høst H., Brennhovd I. O., Loeb M. Postoperative radiotherapy in breast cancer--long-term results from the Oslo study. Int J Radiat Oncol Biol Phys. 1986 May;12(5):727–732. doi: 10.1016/0360-3016(86)90029-5. [DOI] [PubMed] [Google Scholar]
- Kurtz J. M., Jacquemier J., Amalric R., Brandone H., Ayme Y., Hans D., Bressac C., Spitalier J. M. Why are local recurrences after breast-conserving therapy more frequent in younger patients? J Clin Oncol. 1990 Apr;8(4):591–598. doi: 10.1200/JCO.1990.8.4.591. [DOI] [PubMed] [Google Scholar]
- Leung S., Otmezguine Y., Calitchi E., Mazeron J. J., Le Bourgeois J. P., Pierquin B. Locoregional recurrences following radical external beam irradiation and interstitial implantation for operable breast cancer--a twenty three year experience. Radiother Oncol. 1986 Jan;5(1):1–10. doi: 10.1016/s0167-8140(86)80002-0. [DOI] [PubMed] [Google Scholar]
- Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep. 1966 Mar;50(3):163–170. [PubMed] [Google Scholar]
- Montague E. D. Conservation surgery and radiation therapy in the treatment of operable breast cancer. Cancer. 1984 Feb 1;53(3 Suppl):700–704. doi: 10.1002/1097-0142(19840201)53:3+<700::aid-cncr2820531318>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Mustakallio S. Conservative treatment of breast carcinoma--review of 25 years follow up. Clin Radiol. 1972 Jan;23(1):110–116. doi: 10.1016/s0009-9260(72)80151-x. [DOI] [PubMed] [Google Scholar]
- Olivotto I. A., Rose M. A., Osteen R. T., Love S., Cady B., Silver B., Recht A., Harris J. R. Late cosmetic outcome after conservative surgery and radiotherapy: analysis of causes of cosmetic failure. Int J Radiat Oncol Biol Phys. 1989 Oct;17(4):747–753. doi: 10.1016/0360-3016(89)90061-8. [DOI] [PubMed] [Google Scholar]
- Pezner R. D., Lipsett J. A., Desai K., Vora N., Terz J., Hill L. R., Luk K. H. To boost or not to boost: decreasing radiation therapy in conservative breast cancer treatment when "inked" tumor resection margins are pathologically free of cancer. Int J Radiat Oncol Biol Phys. 1988 May;14(5):873–877. doi: 10.1016/0360-3016(88)90008-9. [DOI] [PubMed] [Google Scholar]
- Recht A., Silver B., Schnitt S., Connolly J., Hellman S., Harris J. R. Breast relapse following primary radiation therapy for early breast cancer. I. Classification, frequency and salvage. Int J Radiat Oncol Biol Phys. 1985 Jul;11(7):1271–1276. doi: 10.1016/0360-3016(85)90241-x. [DOI] [PubMed] [Google Scholar]
- Rosenman J., Bernard S., Kober C., Leland W., Varia M., Newsome J. Local recurrences in patients with breast cancer at the North Carolina Memorial Hospital (1970-1982). Cancer. 1986 Apr 1;57(7):1421–1425. doi: 10.1002/1097-0142(19860401)57:7<1421::aid-cncr2820570730>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Ryoo M. C., Kagan A. R., Wollin M., Tomé M. A., Tedeschi M. A., Rao A. R., Hintz B. L., Kuruvilla A. M., Nussbaum H., Streeter O. E., Jr Prognostic factors for recurrence and cosmesis in 393 patients after radiation therapy for early mammary carcinoma. Radiology. 1989 Aug;172(2):555–559. doi: 10.1148/radiology.172.2.2546175. [DOI] [PubMed] [Google Scholar]
- Sarrazin D., Lê M., Rouëssé J., Contesso G., Petit J. Y., Lacour J., Viguier J., Hill C. Conservative treatment versus mastectomy in breast cancer tumors with macroscopic diameter of 20 millimeters or less. The experience of the Institut Gustave-Roussy. Cancer. 1984 Mar 1;53(5):1209–1213. doi: 10.1002/1097-0142(19840301)53:5<1209::aid-cncr2820530531>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Schmidt-Ullrich R., Wazer D. E., Tercilla O., Safaii H., Marchant D. J., Smith T. J., Homer M. A., Robert N. J. Tumor margin assessment as a guide to optimal conservation surgery and irradiation in early stage breast carcinoma. Int J Radiat Oncol Biol Phys. 1989 Oct;17(4):733–738. doi: 10.1016/0360-3016(89)90059-x. [DOI] [PubMed] [Google Scholar]
- Schnitt S. J., Connolly J. L., Harris J. R., Hellman S., Cohen R. B. Pathologic predictors of early local recurrence in Stage I and II breast cancer treated by primary radiation therapy. Cancer. 1984 Mar 1;53(5):1049–1057. doi: 10.1002/1097-0142(19840301)53:5<1049::aid-cncr2820530506>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Solin L. J., Fowble B. L., Schultz D. J., Goodman R. L. The significance of the pathology margins of the tumor excision on the outcome of patients treated with definitive irradiation for early stage breast cancer. Int J Radiat Oncol Biol Phys. 1991 Jul;21(2):279–287. doi: 10.1016/0360-3016(91)90772-v. [DOI] [PubMed] [Google Scholar]
- Van Limbergen E., Rijnders A., van der Schueren E., Lerut T., Christiaens R. Cosmetic evaluation of breast conserving treatment for mammary cancer. 2. A quantitative analysis of the influence of radiation dose, fractionation schedules and surgical treatment techniques on cosmetic results. Radiother Oncol. 1989 Dec;16(4):253–267. doi: 10.1016/0167-8140(89)90037-6. [DOI] [PubMed] [Google Scholar]
- Veronesi U., Salvadori B., Luini A., Banfi A., Zucali R., Del Vecchio M., Saccozzi R., Beretta E., Boracchi P., Farante G. Conservative treatment of early breast cancer. Long-term results of 1232 cases treated with quadrantectomy, axillary dissection, and radiotherapy. Ann Surg. 1990 Mar;211(3):250–259. [PMC free article] [PubMed] [Google Scholar]
- Zafrani B., Vielh P., Fourquet A., Mosseri V., Durand J. C., Salmon R. J., Vilcoq J. R. Conservative treatment of early breast cancer: prognostic value of the ductal in situ component and other pathological variables on local control and survival. Long-term results. Eur J Cancer Clin Oncol. 1989 Nov;25(11):1645–1650. doi: 10.1016/0277-5379(89)90311-8. [DOI] [PubMed] [Google Scholar]



