Abstract
Ancient DNA has been useful in reconciling deep evolutionary relationships and responses to ecological changes in proboscideans. Here, we report the sequencing of a mitochondrial genome from a morphologically distinct Pacific mastodon, as well as from six eastern American mastodons with ages that range through the Middle and Late Pleistocene. We show that Pacific mastodons fall within a deeply divergent mitochondrial clade, extending the range of this species into western Canada and potentially Mexico. We also present evidence for at least three discrete expansion events into northeastern coastal regions and identify two new mastodon clades, which contain temporally distinct but geographically colocalized specimens. We integrate these findings with those of previous work into a comprehensive model of Mammut phylogeography.
Coastal mastodon mitochondrial genomes contextualize species distributions and dispersal patterns near southern glacial limits.
INTRODUCTION
Our understanding of the deep evolutionary relationships within and between extinct taxa and their extant relatives has undergone successive revisions as new paleontological and biomolecular data have become available [see, for example, (1, 2)]. With the growing capability to recover and analyze tiny, ancient, degraded DNA fragments from hundreds of thousands of years old remains, we have begun to answer previously unresolved questions, adding complementary lines of inquiry into paleontological studies, where recovered remains are often too fragmentary for clear morphological identification. Ancient molecular data can help clarify relationships between and estimate divergence times of populations and study their demographics, as well as their responses to various ecological, climatological, and anthropogenic pressures (3–5).
Consequently, North American mastodon taxonomy has also undergone substantial revisions. In the 19th and early 20th centuries, Mammut consisted of many species, which were subsequently synonymized into a single Late Pleistocene species (Mammut americanum) before being split again into two co-occurring Late Pleistocene taxa, the American mastodon (M. americanum) and the Pacific mastodon (Mammut pacificus) (6–8). Previous ancient DNA work on mastodon remains from the American Falls Reservoir has suggested that these two taxa might not be separate species and instead represent discrete morphotypes (9). Instead, it did reveal that Idaho Falls mastodons were part of a predominantly marine isotope stage 5 (MIS 5) clade of mastodons that likely expanded from the contiguous US, up along the Rockies, and into the Arctic in response to interglacial warming (4, 9). Unfortunately, previous work was stymied by the recovery of genetic material from very fragmentary remains, which precluded clear morphological identification (9).
These studies, which highlighted morphological and genetic variability in Late Pleistocene mastodons, suggested a much more complex evolutionary history than previously appreciated. At this time, it is unclear whether and how much of the genetic diversity observed reflects species-level versus population-level divergence. This work begins to disentangle these differences by focusing primarily on mastodons from the peripheries of their core geographic ranges [M. americanum: Great Lakes (10); M. pacificus: California and Idaho (7)] to further our understanding of mastodon phylogeography. Now, we present sequence data from a morphologically assigned Pacific mastodon from Tualatin, OR, as well as six additional American mastodons from Nova Scotia and the eastern seaboard, within which we have identified novel and deep patterns of expansion/extirpation in response to glacial cycling. In tandem, these data fill holes in our understanding of mastodon evolutionary relationships and highlight the acute responsiveness of these species to glacial/interglacial cycles at both coastal margins of the continent.
RESULTS
Recovery of seven mastodon mitochondrial genomes
We managed to reconstruct six complete mitochondrial genomes from mastodon specimens in this study—five from Nova Scotia/the Georges Bank Region and one from Tualatin, OR (fig. S1). We also managed to recover a partial mastodon mitochondrial genome from Northern Ontario (Renison), for which we managed to reconstruct 79.4% of the M. americanum mitochondrial reference (NC_035800) at a mean depth of 5.3× (Table 1). All mastodons analyzed in this study had short mean fragment sizes [34.5 to 52.2 base pairs (bp); Table 1] and characteristic deamination patterns consistent with authentic ancient DNA (fig. S2). The extraction blank processed alongside the samples recovered <20 reads, which mapped to conserved regions of the mitochondrial genome (e.g., ribosomal RNA genes).
Table 1. Coverage statistics.
Mapping and coverage statistics for the mastodon specimens sequenced as part of this study. Coverage values represent percent coverage of the reference at 3× or higher and the mean coverage across resolved positions. The depositional context in which the specimens were found, their associated (A) or direct (D) radiocarbon ages or electron spin resonance dates, and their common names are also provided. B.P., before the present.
| Specimen | Depositional context | Specimen ID | No. of mapped reads | Mean frag. size (bp) | Coverage | Age (B.P.) | References |
|---|---|---|---|---|---|---|---|
| Tualatin | Wetland | UOMNCH F-30282 | 22,955 | 45.2 | 99.3%/63.2× | 11,480 ± 35 (UCIAMS78127; 14C - D) | (17) |
| Renison | Unknown | ROM41948 | 2297 | 42.2 | 79.4%/5.3× | – | (41) |
| Little Narrows | Gypsum sinkhole | NSM015GF007 | 5100 | 34.5 | 86.5%/10.6× | 40,900 ± 2300 (UCIAMS157082; 14C - D) | This study |
| >52,800 (UCIAMS157040; 14C - A) | This study | ||||||
| >50,300 (UCIAMS157044; 14C - A) | This study | ||||||
| Milford | Gypsum sinkhole | NSM019GF009.002 | 260,585 | 52.2 | 100%/827.2× | 74,900 ± 5000 (ESR - A) | (37) |
| Georges Bank | Submerged terrestrial | NSM021GF005.001 | 62,834 | 49.1 | 100%/187.7× | – | – |
| Middle River | Gypsum sinkhole | NSM979GF153.001 | 8558 | 41.8 | 98.2%/21.7× | – | (42) |
| Windsor | Gypsum sinkhole | NSM989GF089.002 | 21,458 | 35.3 | 99.1%/46.1× | – | (42) |
The Tualatin mastodon extends the range of Pacific mastodons
Recent studies highlighting morphological and genetic variability in Late Pleistocene mammutids suggest that M. americanum has a more complex population history than previously appreciated (4, 7, 9). Now, it is unclear whether these differences reflect species-level divergences or whether these differences simply reflect divergent lineages within a continent-wide metapopulation, which we refer to as the M. americanum complex. We tested this morphologically by comparing molar crown length (L):crown width (W) ratios obtained for the Tualatin mastodon against two regionally distinct groups of American mastodons (Great Lakes and Beringia).
The Tualatin mastodon M3 has an L of 149.89 mm and a maximum W of 78.61 mm, producing an L:W ratio of 1.91. This molar is small but plots comfortably within the range for M. pacificus (Fig. 1). This L:W ratio is within 1 standard deviation of the mean for M. pacificus, although it sits slightly outside the lower boundary of the 95% confidence interval (1.94 to 2.02) but sits well above the upper boundary of the M. americanum 95% confidence interval (1.73 to 1.77). On the basis of its position within the morphospace, here defined by the M. americanum complex and M. pacificus, the Tualatin mastodon can be confidently identified as M. pacificus (7), making it the first Pacific mastodon reported in Oregon and expanding the known geographic range into the Pacific Northwest.
Fig. 1. Tualatin mastodon.
(A) Relationship between molar length and maximum width in the upper third molar (M3) of the Tualatin mastodon in comparison to M. pacificus and M. americanum M3s from the Great Lakes region and Beringia. The 95% confidence interval around each regression line is indicated in the shaded color. (B) Upper occlusal view of the Tualatin mastodon’s M3. Photo credit: A. R. Boehm, UOMNCH.
Genetically, the Tualatin mastodon clusters most closely with a single divergent mastodon from central Alberta (RAM P97.7.1), and we have reassigned them into their own Clade P (originally part of Clade M) (Fig. 2). We estimate that these lineages shared a common ancestor ~221 thousand years ago (ka), although, given the uncertain age estimate on RAM P97.7.1, the 95% highest posterior density (HPD) interval on the node remains broad (139 to 328 ka). However, this age estimate falls after the paleontological appearance of M. pacificus (11), suggesting that RAM P97.7.1, originally identified as an American mastodon, may be better reclassified as a Pacific mastodon, extending the range of M. pacificus deep into western Canada, far from its previous western limit of Montana (11).
Fig. 2. Mastodon phylogeography.
(A) Map of North American mastodon localities with available mitochondrial genomes (including the partial genome from Renison) colored as per their assigned clade/lineage. (B) Maximum clade credibility tree generated using all undated/nonfinite mastodons pre-estimated individually and then combined (Individual model). Specimens generated as part of this study are shown in bold. Nodes with posterior probability values greater than or equal to 0.95 are indicated by green circles. Support for other key nodes is indicated in the text next to the relevant node.
The inclusion of the well-dated Tualatin specimen within our phylogeny has also helped constrain the age of RAM P97.7.1, which had previously produced very wide 95% HPD intervals (4, 9). Our individual dating analysis produced a median age of ~175 ka (95% HPD: 96 to 283 ka). This interval overlaps two interglacial periods and thus precludes the concrete assignment of the specimen to one MIS. However, we do observe complete overlap in the 95% HPD of RAM P 97.7.1 and the other two Alberta mastodons—RAM P94.16.1B (Clade Y; median, 123 ka; 95% HPD: 97 to 151 ka) and RAM P94.5.7 (Clade L; median, ~199 ka; 95% HPD: 143 to 261 ka), both of which fall within established M. americanum complex diversity. This likely co-occurrence of both American and Pacific mastodons highlights midwestern Alberta as a highly dynamic environment with possible hybridization and competition for resources.
Before the Tualatin genome and the reassignment of Pacific mastodons to Clade P, Clade M was composed of RAM P97.7.1 and a Late Pleistocene mastodon from central Mexico (DP1296). This Mexican specimen is deeply divergent from all other mastodons and is occasionally paraphyletic with respect to mastodons in Clade P (Fig. 2; see figs. S5 to S11 for comparison). Resolving the exact relationship between DP1296 and well-identified Pacific and American mastodons will require more mastodons from southern locales and/or nuclear data. However, reassignment of these two members of this clade as M. pacificus and placement of them into Clade P, coupled with the high divergence of DP1296, suggests at least two possible scenarios: (i) The Mexican mastodon DP1296 should also be considered a Pacific mastodon, and the range of this species extended outside of the contiguous United States (i.e., Alberta to Mexico); or (ii) this specimen represents another as-of-yet unidentified “species-level” mitochondrial lineage of mastodons relegated to the southern regions of the continent. As “Clade” M contains a single specimen, we have reclassified it as Lineage M.
East coast mastodon mitochondrial genomes suggest three dispersal events
The phylogenetic placement of the five east coast mastodon mitochondrial genomes and a partial mitochondrial genome from a Northern Ontario mastodon has uncovered an unexpected amount of diversity relative to their geographic proximity. Only two of the new mastodon mitochondrial genomes fall within Clade N—the Milford (NSM019GF009.002) and Middle River (NSM979GF153.001) mastodons—which contained a single, previously sequenced specimen from Nova Scotia (Fig. 2). The Georges Bank mastodon (NSM021GF005.001) clustered within Clade G, a clade composed almost entirely of mastodons from the Great Lakes region. Two of the remaining three mastodons cluster within a newly established Clade S. The Little Narrows mastodon (NSM015GF007) is the sole representative of another deeply divergent group of mastodons from Nova Scotia, which we have denoted as Lineage E to facilitate discussion.
We used Bayesian age estimation to tip date the samples and recovered signals of at least three temporally distinct groups in the eastern mastodon clades/lineages (i.e., G, N, S, and E) (Fig. 3). The Georges Bank mastodon produces a young age estimate (median, ~32 ka; 95% HPD: 21 to 47 ka), consistent with its position in Clade G among other young mastodons that colonized the Great Lakes region and northeastern United States after the Last Glacial Maximum. The estimated median age and posterior probability distribution of the Middle River mastodon (median: ~91 ka; 95% HPD: 69 to 117 ka) are similar to those of other MIS 5 mastodons (~71 to 130 ka), and it is close in age to other mastodons in Clade N, which are dated to 74,900 +/− 5000 thousand years (kyr). The Windsor (NSM989GF089.002) and Renison (ROM41948) mastodons (median ages: ~178 and ~172 ka, respectively) produce diffuse distributions spanning MIS 5 to MIS 7 (Fig. 3). Although the bulk of the Renison’s posterior probability is located at ages older than the 95% HPD range of Middle River, suggesting that these mastodons are likely temporally distinct, there is ~3.7 kyr of overlap between the upper limit of Middle River’s 95% HPD interval and the lower bound of Windsor’s. Little Narrows also produces a diffuse age estimate ranging between MIS 8 and MIS 12, with a median age of 358 ka (95% HPD: 259 to 471 ka). The 95% HPD of Little Narrows does not overlap with other east coast mastodons, suggesting that it is temporally unique and the sole representative of an early expansion into the region (Lineage E). Notably, the median age of Little Narrows is greater than those of UAMES 11095 and UAMES 30197 (~286 and 275 ka, respectively; Clade Y, Alaska), and ~40% of its HPD range falls above the upper limit of the 95% HPD of UAMES 11095, suggesting that it may be the oldest mastodon in our analysis (and the oldest mastodon sequenced to date). Unlike the Clade Y mastodons, this specimen was recovered from a nonpermafrost context and suggested that gypsum sinkholes may have high preservation potential in temperate environments.
Fig. 3. Mastodon age estimates.
Marginal posterior densities of specimen ages estimated using a Joint model (all unknown/nonfinite mastodons estimated simultaneously; dashed lines) or an Individual model (all unknown/nonfinite mastodons pre-estimated individually and then combined; solid lines). Each violin represents the 95% HPD interval estimated for each specimen. Specimens are colored on the basis of their clade/lineage assignments. Specimens generated as part of this study are shown in bold. The δ18O record for the past 800 kyr (39) is overlaid below the plot, and the approximate extent of the MIS 5 interglacial period is highlighted in gray. The dashed line represents the lower bound of the analysis, 13,087 ka, the age of the youngest specimen (UWZM 19580).
Following Karpinski et al. (4), we also estimated the ages of unknown or nonfinite mastodons within a single analysis (Joint); however, a recent study in mammoths has shown that Joint analyses exhibit a bias toward older dates and individually dated ages appear to be more in line with externally validated climatic events (12). We recover congruent estimates for mastodons dated as far back as MIS 5 (~130 ka) (Clades Y, G, and N), although often with older estimated medians (Fig. 3 and table S3). However, for specimens that predate MIS 5, we recover progressively older median age estimates and 95% HPD intervals, most exemplified by Little Narrows, which produces a median age of ~650 ka (95% HPD: 464 to 800 ka). Part of this trend toward younger ages is likely due to the estimated rate, which is approximately twice as fast in the Individual analysis as in the Joint (tables S3 and S4). However, irrespective of the method used, we observe the same relative trend between samples and identify at least three separate expansions into the eastern regions of the continent.
To examine whether the degree to which the amount of missing data in our analysis influenced our age estimates, we reran the Joint BEAST analysis but supplied the median estimated age of specimens generated in previous studies as a point prior. We note that this is not a correct way of estimating sample ages, but it serves as a useful proxy for how additional calibration points might affect our age estimates. We observed similar estimated median ages for each of our newly generated mastodon genomes and the same general patterns in the overlap of 95% HPD intervals as we do in our Joint analyses (table S4 and fig. S13). This is highly expected as the fixed ages were also estimated under a Joint model. In general, we almost always recover smaller 95% HPD intervals, although this effect was most pronounced for our oldest specimen (Little Narrows; NSM015GF007), which saw a ~30% reduction in the dated value range. Nevertheless, the date ranges for these HPDs remained always larger in the Joint analyses than under the Individual ones, ranging from about 2.6× greater for Georges Bank to 1.06× greater for Little Narrows.
DISCUSSION
This work continues to expand our limited understanding of Mammut taxonomy and biogeography throughout the Middle and Late Pleistocene in two substantive ways: (i) better contextualization of an individual exhibiting M. pacificus morphology by placing it within the broader diversity of the North American mastodon complex and (ii) producing a refined model of the range dynamics of mastodons on the east coast of the continent. Figure 4 summarizes the phylogeography of Pleistocene Mammut species and lineages.
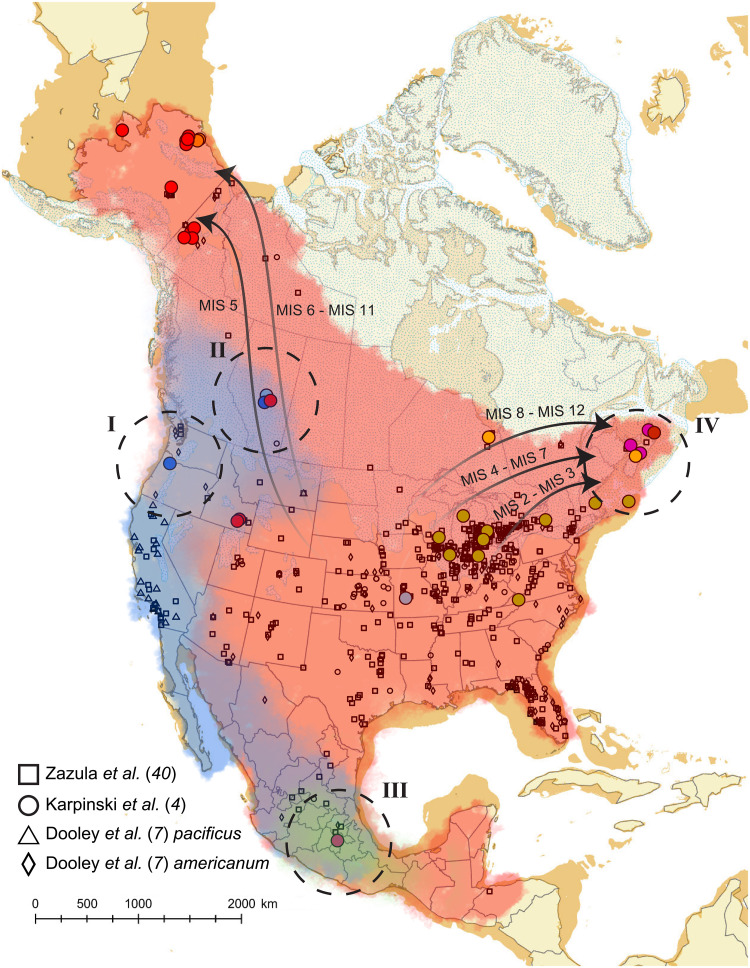
Fig. 4. Pleistocene Mammut biogeography.
Geographic model of our understanding of mastodon distributions and relationships throughout the Middle to Late Pleistocene. The approximate inferred distributions of the American mastodon morphological complex are shaded in red, and those for the Pacific mastodon are shaded in blue. The locations of mastodon remains from previous large studies (4, 7, 40) are also provided (data S2). The single M. pacificus specimen from McDonald et al. (11) was combined with other Pacific mastodons from Dooley et al. (7). Arrows indicate minimum confirmed expansions in response to glacial/interglacial cycles, with their timing ranges as estimated by the Individual model indicated in the text. Arrows fade as they enter the contiguous United States because of the lack of a known source population. Key contributions from this study are indicated by dashed circles and roman numerals. (I) First mitochondrial data from a clearly identified Pacific mastodon from Tualatin, OR. (II) Reclassification of an Alberta mastodon (RAM P97.7.1) as M. pacificus, extending the range of Pacific mastodons into western Canada and into regions where it might have been concurrent with mastodons showing characteristics of the M. americanum morphological complex. (III) Possible extension of M. pacificus into Mexico or the identification of another Mammut “species” (shown in green). (IV) Increased genetic resolution in eastern North America and the identification of at least three expansions into the eastern seaboard.
Previous work has described conflicts between the paleontological and genetic classifications of American and Pacific mastodons (9) and suggested that the two species are not distinct at the mitochondrial level but rather that they may exhibit similar patterns as those seen between woolly and Columbian mammoths or bison (Bison bison), steppe bison (Bison priscus), and giant long-horned bison (Bison latifrons) (13, 14). However, those previous studies were complicated by the fragmentary nature of the samples, from which we recovered DNA, precluding proper morphological assignment to M. pacificus (9). Here, the completeness of the Tualatin mastodon, which enabled unambiguous morphological characterization as M. pacificus, combined with its mitochondrial genome, has allowed us to resolve a key question vis-a-vis mastodon taxonomy (Fig. 4, I). Its phylogenetic position (and possibly other Pacific mastodons) within a newly assigned Clade P is partially explained by the deep divergence of this clade relative to others, extending the geographic range of Pacific mastodons into Alberta, Canada (Fig. 4, II). In addition, morphologically and genetically well-characterized American mastodons are known from the same geographic area as RAM P97.7.1 and with similar temporal estimates. This suggests that these species likely interacted in Alberta and opens the possibility that they interbred, likely during northward expansions, as has been reported for black bears (2).
Our work raises additional questions surrounding the relationship of DP1296 (Mexico) to other mastodons. The occasionally paraphyletic nature of this lineage, combined with its deep divergence, makes it difficult to ascertain if Lineage M represents a highly divergent lineage within M. pacificus or M. americanum or rather a third, potentially cryptic species within North America (Fig. 4, III). Previous genetic studies have shown that the diversity within Clade M (including Lineage P) is greater than within or between any of the other mastodon clades [see figure S24 in (4)], also recapitulated in our phylogenies. While a reinterrogation of Mammut taxonomy and morphological variation is needed (9), our results suggest that if M. pacificus continues to be considered a separate Mammut species, DP1296, and other associated Mexican mastodons, might best be considered a yet undescribed third species in Pleistocene North America on the basis of mitochondrial data.
Our east coast mastodon mitochondrial genomes reveal a great amount of diversity not captured in previous studies, expanding the phylogeography of this region. We observe at least three, but likely four, pulses into the region spanning the Middle and Late Pleistocene (Fig. 4, IV). While Clade N and Lineage E currently only include mastodons from Nova Scotia, given the geographic spread observed in clades S (Nova Scotia to Northern Ontario) and G (the American Midwest to Northeast), it is likely that these animals were part of much larger and highly diverse populations that expanded tracking shifting climates. However, except for the Middle River (Clade N) and Georges Bank mastodons (Clade G), we are unable to link these clades to specific glacial/interglacial periods during which these expansions might have occurred, as the posterior probability estimates are too diffuse and span multiple MIS stages. Despite this lack of deep-time precision, this study contains the second such evidence of northward expansion and extirpation patterns in North American Pleistocene browsers and illustrates that these dispersal patterns were likely a regular occurrence on both sides of the continent in all transiently glaciated regions, not just Beringia.
This work highlights the importance of remains from outside the core, high-density ranges of mastodon species (10). These border regions in transiently glaciated or coastal environments were highly dynamic, both environmentally and ecologically, and are prime locations to directly observe macroscale species responses and interactions to Pleistocene climates. We have shown that even a few specimens from these areas can be immensely informative to key phylogeographic insights. This will likely be especially true for mastodons from more southern locales where biomolecular preservation is poor, but where glacial refugia likely persisted (e.g., Florida), or help shed light on the deep diversity of mastodon lineages, specifically the relationship between northern M. pacificus samples and their potential southern relatives in Mexico.
There are important limitations to our work. First, more genetic data from well-dated mastodons would better calibrate phylogenetic analyses and reduce the uncertainty of our current temporal estimates. This is especially true for mastodons from outside their Late Pleistocene core range and from deeper time periods, allowing for more accurate linking between dispersals and climatic events. For example, we show here that the inclusion of a single-dated specimen phylogenetically proximate to an unknown (Tualatin and RAM P97.7.1) had large effects on the estimated age distribution [see prior age estimates in (4) for comparison]. Second, mitochondrial phylogenies have inherent limitations because of their nonrecombining nature and tracking maternal lineages alone and can produce deeper divergence dates and occasionally obscure species relationships (13, 15, 16). The identification of well-preserved mastodons and the recovery of complete nuclear genomes would provide considerably more information and much needed details as to the relationships between American, Pacific, and potentially cryptic mastodon species and identify the degree to which hybridization may have occurred between migrating populations responding to the dynamic shifting glacial/interglacial cycles of the Pleistocene.
MATERIALS AND METHODS
Sample acquisition and subsampling
Subsamples from each mastodon were taken at their respective institutions and sent to the McMaster University Ancient DNA Centre. Subsamples were opened and processed in dedicated ancient DNA clean rooms thereafter.
Taxonomic identification of the Tualatin mastodon
The Tualatin mastodon is designated F-30282 in the University of Oregon Museum of Natural and Cultural History (UOMNCH), but much of the specimen is on display in the Tualatin Public Library and the Tualatin Historical Society. The skeleton is partially complete, with most of the left side of the animal present. Portions of the crania were recovered but were either discarded or lost over time. However, a fragment of the left maxillary tooth row containing M2 and M3 was saved. It was recovered in 1962 along the Tualatin River, a tributary of the Columbia River, OR. Previous research on the specimen reports an age of 13,114 to 13,706 years before the present (Table 1) (17), which postdates the Missoula flood events and human colonization of the Pacific Northwest (18, 19). Following published methods [i.e., (7, 20)], the molars were measured using digital calipers. These measurements are compared to a large dataset of Mammut upper M3s, including M. pacificus [n = 39; (7)], interglacial M. americanum from Alaska and the Yukon (n = 7; Clades A and Y), and late glacial M. americanum from the Great Lakes (n = 46) (data S1).
A 3D model of the Tualatin specimen was also created with structure-from-motion photogrammetry (Morphosource ID: 000683205). The specimen was photographed with a Nikon d5000 12.3 MP DX Digital SLR camera with a Nikkor 18-55mm f/3.5-5.6G VR II lens. The camera was mounted on a tripod positioned 40 cm from the specimen. The specimen was placed on a turntable and rotated 10° for each photo while the camera was operated with a remote shutter release to reduce image blur. The specimen was photographed at three different angles (15°, 30°, and 45°) to capture the entire surface. A total of 235 images was used to create a digital 3D model, generated using Agisoft Metashape Standard Version 1.7.1 software following established methods [e.g., (21–23)].
New radiocarbon ages
A bone sample from specimen Little Narrows (NSM015GF007) was sent for radiocarbon analysis to the Keck-CCAMS facility at the University of California, Irvine. The sample was decalcified using 0.5 M HCl, rinsed with Milli-Q water, and hydrolyzed overnight at 60°C with 0.01 M HCl, and the high-molecular-weight fraction was isolated.
Two wood samples associated with the collection site of Little Narrows were sent for radiocarbon analysis to the Keck-CCAMS facility at the University of California, Irvine. The samples were treated in 1 M HCl at 70°C for 30 min to dissolve any contaminating carbonate from dust or soil, then washed with 1 M NaOH at 70°C for 30 min to remove soil humics, repeating until clear, and lastly washed with 1 M HCl at 70°C for 30 min to remove atmospheric CO2 absorbed during the alkaline washes. Samples were washed with Milli-Q water to remove chloride and dried on a heating block or in a vacuum oven.
DNA extraction and sequencing
Samples were initially processed as previously described (4, 9) with a few modifications. Briefly, ~150 to 350 mg of material was subsampled from each specimen and manually pulverized. Subsamples were prewashed with 300 μl of 0.5 M EDTA for 20 min at 1000 rpm to remove dust, and the wash was discarded. Subsamples were then demineralized using 0.75 to 1 ml of 0.5 M EDTA (pH 8.0; room temperature; 2 to 5 days with shaking at 1000 to 2000 rpm) and digested using 0.75 to 1 ml of a proteinase K digestion buffer [0.01 M tris-Cl (pH 9), 0.20% sarcosyl, proteinase K (0.25 mg/ml), and 0.01 M CaCl2; 45°C for ~3 days with rotation] in two successive rounds. Demineralization and digestion supernatants were pooled for extraction. All processing steps were accompanied by an extraction blank, which was treated identically to the subsamples but contained no material.
DNA was extracted as described by Dabney et al. (24) with the following modifications: Roche High Pure Viral Nucleic Acid columns were used instead of MinElute columns, 1 ml of supernatant was mixed with 13 ml of binding buffer and successively passed through the column until all supernatant was processed, and DNA was eluted twice with 25 μl of EBT (buffer EB and 0.05% Tween 20).
Extracted DNA was converted into non–uracil-DNA glycosylase–treated libraries using a double-stranded library protocol. Double-stranded libraries used ~20 μl of input in 40-μl reactions using previously described methods (25, 26) with the following modifications: NEBuffer 2.1 replaced NEBuffer 2, MinElute cleanups were done with two washes of 750 μl of PE buffer and an additional 60-s dry spin, and libraries were heat deactivated at 80°C following adapter fill-in instead of being column purified. A total of 12.5 μl of each library was used for indexing polymerase chain reaction (PCR) with unique P5 and P7 index adapters. Indexing PCR was performed in 40-μl reactions (1X KAPA SYBR Fast qPCR Master Mix; 750 nM each P5/P7 indexing primer) for a maximum of 20 cycles (denaturing at 95°C for 30 s; annealing at 60°C for 45 s), although libraries were pulled earlier if they were observed to be undergoing exponential amplification. Indexing PCR reactions were then purified over MinElute columns as before, except eluted in 13 μl of EBT.
To increase endogenous DNA, we also performed one round of in-solution enrichment using a previously published proboscidean mitochondrial genome bait set (27). Double-stranded libraries were processed as described previously (9) using 9.05 μl of indexed library.
Enriched libraries were pooled to approximately equimolar concentrations and size selected for fragments between ∼150 and 500 bp (3% Nusieve GTG Agarose Gel; 100 V for 35 min). DNA was purified from gel plugs using the QIAquick Gel Extraction Kit with the following modifications: an additional wash with 700 μl of PE buffer, an additional dry spin at maximum speed, and elution in 20 μl. Libraries were sequenced on an Illumina HiSeq 1500 using 2 × 90–bp chemistry.
We subsequently extracted more material for the Renison and Tualatin mastodons as our initial sequencing did not generate complete mitochondrial genomes (>80% coverage of the reference genome at a minimum depth of 3×). Six additional extracts each were generated from the Renison molar and the Tualatin pelvis (table S1) with ~25 to 50 mg of input. The material was demineralized as described by Dabney et al. (24) (for libraries ending in A or D in table S1) or as above with a modified proteinase K digestion buffer [0.02 M tris-Cl (pH 9), 0.5% sarcosyl, proteinase K (0.25 mg/ml), 0.005 M CaCl2, 1% polyvinyl pyrrolidone, 50 mM dithiothreitol, and 2.5 mM N-phenacylthiazolium bromide] for 24 hours (for libraries ending in B, C, E, or F in table S1). DNA was extracted as described above, except eluted in 2× 20 μl of TET buffer (0.01 M tris-HCl, 0.001 M EDTA, and 0.05% Tween 20).
All subsequent extracts were converted into single-stranded libraries using an established protocol (28). A total of 12.5 μl of each library was indexed as described before and subject to two rounds of in-solution enrichment as described above. Enriched libraries were pooled in equimolar concentrations and sequenced on a NextSeq 2000 using 2 × 50–bp chemistry.
Sequence processing and curation
Reads were mapped to the M. americanum mitochondrial reference genome (NC_035800) extended by ~20 bp on either end as previously described (9). Briefly, demultiplexed reads were trimmed and merged using the ancient DNA settings in leeHom (29). Reads were mapped to the padded reference with a network-aware version of BWA (30) (https://github.com/mpieva/network-aware-bwa) with established ancient DNA settings: maximum edit distance of 0.01 (-n 0.01), a maximum of two gap openings (-o 2), and seeding effectively disabled (-l 16500). Mapped reads that were merged or properly paired were extracted using the retrieveMapped_single_and_ProperlyPair program of libbam (https://github.com/grenaud/libbam) and collapsed on the basis of unique 5′ and 3′ positions to remove PCR duplicates (https://bitbucket.org/ustenzel/biohazard/src/master/). Reads were then filtered to a minimum fragment size of 24 bp and a minimum mapping quality of 30 using SAMtools (31). Specimens for which multiple libraries were sequenced were then combined using the merge function of SAMtools. To ensure authenticity, we also examined the cytosine deamination signal in our final alignments using MapDamage2.0 (32).
Alignments were then imported into Geneious (version 2021.2.2) and manually curated to remove sequencing artifacts and insertions not supported by a majority of the aligned reads. A majority consensus was called requiring at least 50% of reads at a given position to support the majority base and any position with less than 3× coverage masked with N’s. We further masked a portion of the 16S region in the Renison mastodon alignment as it contained many short, stacked reads, which have previously been shown to likely be of bacterial origin (9). The variable number tandem repeat region of each new consensus was also masked as in the NC_035800 reference, and the final consensus sequences were truncated from either end to account for the padded reference.
Phylogenetic analysis
Consensus sequences for the new mastodon specimens were aligned to all available complete mastodon mitochondrial genomes (n = 37), both with and without the partial Renison mitochondrial consensus, using Muscle version 5.1 (33). Separately, we also aligned the full dataset using two mammoth mitochondrial genomes (NC_007596 and NC_015529) as outgroups in rooted phylogenies. Model selection and maximum likelihood phylogenies were conducted using IQ-TREE version 1.6.12 (34, 35), choosing the model that minimized the small-sample corrected Akaike information criterion estimate in each case. Maximum likelihood trees were generated with 1000 bootstraps and the chosen model: all mastodons (TPM3u + F + I + G4); all mastodons without Renison (TPM3u + F + I + G4); all mastodons with mammoth outgroups (TIM3 + F + I + G4). We subsequently also constructed phylogenies with each of the datasets above using an HKY + G4 + F model (used in subsequent analyses with BEAST) to verify that the substitution model should not affect our observed topology.
BEAST analyses were conducted as per the Joint and Individual analyses described previously (4, 9) in BEAST version 1.15 (36). In short, the median calibrated age for each specimen with a finite radiocarbon date was supplied to calibrate the model. Under the Joint model, all specimens with unknown ages were fit with diffuse gamma distributions (shape, 1; scale, 200,000) and a uniform prior restricting their age using priors between 800 and 50 ka (for specimens that were/deemed to be nonfinite) or 0 ka (for specimens that may potentially yield finite ages) (table S2). The Milford mastodon (NSM019GF009.002) is a juvenile mastodon associated with the adult East Milford mastodon, N.S., and as such is also expected have a date of 74.9 ± 5.0 ka (37), and a previously obtained radiocarbon estimate for the Tualatin mastodon (17) was calibrated in Calib version 8.2 (38) to produce a median estimate of 13,360 years before the present. Three newly obtained radiocarbon ages related to the Little Narrows mastodons (NSM015GF007) were consistent with this specimen being outside the limits of radiocarbon dating (Table 1 and tables S5 and S6), and as such, it was treated as another nonfinite mastodon. The uniform bounds on the Georges Bank mastodon (NSM021GF005.001) were set between 0 and 800 ka because of this specimen’s position in Clade G, which is composed entirely of mastodons dated to <50 kyr. Under the Individual model, each unknown specimen was pre-estimated as above but independently alongside all mastodons with known temporal information. These pre-estimates were then used as priors in a combined analysis including all unknown/nonfinite and dated mastodons (table S2). To test the degree to which the amount of unknown mastodons was affecting the ranges of our age predictions, we subsequently ran an additional model wherein the ages of each mastodon, not generated as part of this study, were supplied as point estimates equal to their median calibrated radiocarbon age or the median age that we previously estimated (9). Three independent chains were run for 500 million generations (sampling every 10,000) to assess convergence in each model. Chains were combined for the final analysis.
Acknowledgments
We thank the Tualatin Ice Age Foundation, Tualatin Heritage Cernt, Tualatin Public Library, Y. Addington, C. Leigh, J. Thompson, M. Full, and the University of Oregon Museum of Natural and Cultural History for access and permission to sample the Tualatin mastodon, as well as the Nova Scotia Museum for access to the east coast mastodons, and the Royal Ontario Museum for permission to sample the mastodon from Renison, ON. We also acknowledge that the Little Narrows specimen (NSM015GF007) was found by quarry worker S. MacLeod and collected for the Nova Scotia Museum by J. Calder and K. Ogden under permit P2014NS03. Likewise, the Windsor mastodon NSM989GF089.00 was found by quarry worker A. Wilcox and collected by R. Grantham, Curator of Geology of the Nova Scotia Museum. We also thank B. Golding as well as HMS Research Computing for access to computational resources used in this work. We also thank B. Golding, B. Evans, and all members of the Poinar, Golding, and Evans lab groups for support and feedback during this project. We thank the reviewers for constructively critical reviews. H.P. thanks the DeGroote family for an endowed research chair.
Funding: This work was supported by an NSERC Discovery Grant (grant no. 4184-15) and CRC to H.P.
Author contributions: Conceptualization: E.K., H.N.P., A.R.B., and C.W. Methodology: E.K., H.N.P., and A.R.B. Investigation: E.K., S.B., and A.R.B. Visualization: E.K., A.R.B., and C.W. Data curation: E.K., T.F., A.R.B., and H.N.P. Resources: A.R.B., T.F., C.W., and H.N.P. Validation: E.K. and H.N.P. Formal analysis: E.K., A.R.B., C.W., and H.N.P. Software: E.K. Funding acquisition: H.N.P. Project administration: E.K. and H.N.P. Supervision: A.R.B. and H.N.P. Writing—original draft: E.K., C.W., A.R.B., and H.N.P. Writing—review and editing: E.K., S.B., C.W., A.R.B., T.F., and H.N.P.
Competing interests: The authors declare that they have no competing interests.
Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Bulk sequencing data were deposited to NCBI’s SRA under bioproject PRJNA1190488. Mastodon mitochondrial genomes generated during this study were deposited to GenBank (PQ672301-PQ672307).
Supplementary Materials
The PDF file includes:
Specimen Provenance
Figs. S1 to S16
Tables S1 to S7
Legends for data S1 and S2
References
Other Supplementary Material for this manuscript includes the following:
Data S1 and S2
REFERENCES AND NOTES
- 1.Welker F., Collins M. J., Thomas J. A., Wadsley M., Brace S., Cappellini E., Turvey S. T., Reguero M., Gelfo J. N., Kramarz A., Burger J., Thomas-oates J., Ashford D. A., Ashton P. D., Rowsell K., Porter D. M., Kessler B., Fischer R., Baessmann C., Kaspar S., Olsen J. V., Kiley P., Elliott J. A., Kelstrup C. D., Mullin V., Hofreiter M., Willerslev E., Hublin J., Orlando L., Barnes I., Macphee R. D. E., Ancient proteins resolve the evolutionary history of Darwin’s South American ungulates. Nature 522, 81–84 (2015). [DOI] [PubMed] [Google Scholar]
- 2.Pedersen M. W., De Sanctis B., Saremi N. F., Sikora M., Puckett E. E., Gu Z., Moon K. L., Kapp J. D., Vinner L., Vardanyan Z., Ardelean C. F., Arroyo-Cabrales J., Cahill J. A., Heintzman P. D., Zazula G., MacPhee R. D. E., Shapiro B., Durbin R., Willerslev E., Environmental genomics of Late Pleistocene black bears and giant short-faced bears. Curr. Biol. 31, 12 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kjær K. H., Winther Pedersen M., De Sanctis B., De Cahsan B., Korneliussen T. S., Michelsen C. S., Sand K. K., Jelavić S., Ruter A. H., Schmidt A. M. A., Kjeldsen K. K., Tesakov A. S., Snowball I., Gosse J. C., Alsos I. G., Wang Y., Dockter C., Rasmussen M., Jørgensen M. E., Skadhauge B., Prohaska A., Kristensen J. Å., Bjerager M., Allentoft M. E., Coissac E., Alsos I. G., Coissac E., Rouillard A., Simakova A., Fernandez-Guerra A., Bowler C., Macias-Fauria M., Vinner L., Welch J. J., Hidy A. J., Sikora M., Collins M. J., Durbin R., Larsen N. K., Willerslev E., A 2-million-year-old ecosystem in Greenland uncovered by environmental DNA. Nature 612, 283–291 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karpinski E., Hackenberger D., Zazula G., Widga C., Duggan A. T., Golding G. B., Kuch M., Klunk J., Jass C. N., Groves P., Druckenmiller P., Schubert B. W., Arroyo-Cabrales J., Simpson W. F., Hoganson J. W., Fisher D. C., Ho S. Y. W., MacPhee R. D. E., Poinar H. N., American mastodon mitochondrial genomes suggest multiple dispersal events in response to Pleistocene climate oscillations. Nat. Commun. 11, 4048 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van der Valk T., Pečnerová P., Díez-del-Molino D., Bergström A., Oppenheimer J., Hartmann S., Xenikoudakis G., Thomas J. A., Dehasque M., Sağlıcan E., Fidan F. R., Barnes I., Liu S., Somel M., Heintzman P. D., Nikolskiy P., Shapiro B., Skoglund P., Hofreiter M., Lister A. M., Götherström A., Dalén L., Million-year-old DNA sheds light on the genomic history of mammoths. Nature 591, 265–269 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Osborn H. F., Evolution and geographic distribution of the proboscidea: Moeritheres, deinotheres and mastodonts. J. Mammal. 15, 177–184 (1934). [Google Scholar]
- 7.Dooley A. C., Scott E., Green J., Springer K. B., Dooley B. S., Smith G. J., Mammut pacificus sp. nov., a newly recognized species of mastodon from the Pleistocene of western North America. PeerJ 7, e6614 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.J. J. Saunders, “The Proboscidea - Evolution and palaeoecology of elephants and their relatives” in The Proboscidea: Evolution and Palaeoecology of Elephants and Their Relatives, J. Shoshani, P. Tassy, Eds. (Oxford Univ. Press, 1996), pp. 271–279. [Google Scholar]
- 9.Karpinski E., Widga C., Boehm A. R., Peecook B. R., Kuch M., Murchie T. J., Poinar H. N., Mastodon mitochondrial genomes from American falls, Idaho. Quat. Int. 668, 1–6 (2023). [Google Scholar]
- 10.Dreimanis A., Extinction of mastodons in eastern North America: Testing a new climatic-environmental hypothesis. Ohio J. Sci. 68, 257–272 (1968). [Google Scholar]
- 11.McDonald A. T., Atwater A. L., Dooley A. C. Jr., Hohman C. J. H., The easternmost occurrence of Mammut pacificus (Proboscidea: Mammutidae), based on a partial skull from eastern Montana, USA. PeerJ 8, e10030 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chacón-Duque J. C., Thomas Thorpe J. A., Li W., Dehasque M., Pečnerová P., Barlow A., Díez-del-Molino D., Henneberger K., Jin C., Moreland K. N., Paijmans J. L. A., van der Valk T., Westbury M. V., Wijnands F., Barnes I., Germonpré M., Hall E., Hewitson S., Mol D., Nikolskiy P., Sablin M., Vartanyan S., Zazula G. D., Götherström A., Lister A. M., Hofreiter M., Heintzman P. D., Dalén L., A million years of mammoth mitogenome evolution. Mol. Biol. Evol. 42, 4 (2025). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Enk J., Devault A., Debruyne R., King C. E., Treangen T., O’Rourke D., Salzberg S. L., Fisher D., MacPhee R., Poinar H., Complete Columbian mammoth mitogenome suggests interbreeding with woolly mammoths. Genome Biol. 12, R51 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Froese D., Stiller M., Heintzman P. D., Reyes A. V., Zazula G. D., Soares A. E. R., Meyer M., Hall E., Jensen B. J. L., Arnold L. J., Macphee R. D. E., Shapiro B., Grayson D. K., Fossil and genomic evidence constrains the timing of bison arrival in North America. Proc. Natl. Acad. Sci. U.S.A. 114, 3457–3462 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roca A. L., Georgiadis N., O’Brien S. J., Cytonuclear genomic dissociation in African elephant species. Nat. Genet. 37, 96–100 (2005). [DOI] [PubMed] [Google Scholar]
- 16.Palkopoulou E., Lipson M., Mallick S., Nielsen S., Rohland N., Baleka S., Karpinski E., Ivancevic A. M., To T.-H., Daniel Kortschak R., Raison J. M., Qu Z., Chin T.-J., Alt K. W., Claesson S., Dalén L., MacPhee R. D. E., Meller H., Roca A. L., Ryder O. A., Heiman D., Young S., Breen M., Williams C., Aken B. L., Ruffier M., Karlsson E., Johnson J., Palma F. D., Alfoldi J., Adelson D. L., Mailund T., Munch K., Lindblad-Toh K., Hofreiter M., Poinar H., Reich D., A comprehensive genomic history of extinct and living elephants. Proc. Natl. Acad. Sci. U.S.A. 115, E2566–E2574 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gilmour D. M., Butler V. L., O’Connor J. E., Davis E. B., Culleton B. J., Kennett D. J., Hodgins G., Chronology and ecology of Late Pleistocene megafauna in the Northern Willamette Valley, Oregon. Quat. Res. 83, 127–136 (2015). [Google Scholar]
- 18.Davis L. G., Madsen D. B., Becerra-Valdivia L., Higham T., Sisson D. A., Skinner S. M., Stueber D., Nyers A. J., Keen-Zebert A., Neudorf C., Cheyney M., Izuho M., Iizuka F., Burns S. R., Epps C. W., Willis S. C., Buvit I., Late Upper Paleolithic occupation at Cooper’s Ferry, Idaho, USA, ~16,000 years ago. Science 365, 891–897 (2019). [DOI] [PubMed] [Google Scholar]
- 19.O’Connor J. E., Baker V. R., Waitt R. B., Smith L. N., Cannon C. M., George D. L., Denlinger R. P., The Missoula and Bonneville floods—A review of ice-age megafloods in the Columbia River basin. Earth Sci. Rev. 208, 103181 (2020). [Google Scholar]
- 20.H. F. Osborn, M. R. Percy, Proboscidea: A Monograph of the Discovery, Evolution, Migration and Extinction of the Mastodonts and Elephants of the World (American Museum Press, 1936). [Google Scholar]
- 21.Lauria G., Sineo L., Ficarra S., A detailed method for creating digital 3D models of human crania: An example of close-range photogrammetry based on the use of Structure-from-Motion (SfM) in virtual anthropology. Archaeol. Anthropol. Sci. 14, 42 (2022). [Google Scholar]
- 22.Medina J. J., Maley J. M., Sannapareddy S., Medina N. N., Gilman C. M., McCormack J. E., A rapid and cost-effective pipeline for digitization of museum specimens with 3D photogrammetry. PLOS ONE 15, e0236417 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Otero A., Pérez Moreno A., Falkingham P., Cassini G., Ruella A., Militello M., Toledo N., Three-dimensional image surface acquisition in vertebrate paleontology: A review of principal techniques. Publ. Electrón. Asoc. Paleontol. Argent. 20, 1–14 (2020). [Google Scholar]
- 24.Dabney J., Knapp M., Glocke I., Gansauge M.-T., Weihmann A., Nickel B., Valdiosera C., García N., Pääbo S., Arsuaga J.-L., Meyer M., Complete mitochondrial genome sequence of a Middle Pleistocene cave bear reconstructed from ultrashort DNA fragments. Proc. Natl. Acad. Sci. U.S.A. 110, 15758–15763 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meyer M., Kircher M., Illumina sequencing library preparation for highly multiplexed target capture and sequencing. Cold Spring Harb. Protoc. 2010, pdb.prot5448 (2010). [DOI] [PubMed] [Google Scholar]
- 26.Kircher M., Sawyer S., Meyer M., Double indexing overcomes inaccuracies in multiplex sequencing on the Illumina platform. Nucleic Acids Res. 40, e3 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Enk J., Devault A., Widga C., Saunders J., Szpak P., Southon J., Rouillard J.-M., Shapiro B., Golding G. B., Zazula G., Froese D., Fisher D. C., Macphee R. D. E., Poinar H., Mammuthus population dynamics in Late Pleistocene North America: Divergence, phylogeogrpaphy and introgression. Front. Ecol. Evol. 4, 42 (2016). [Google Scholar]
- 28.Kapp J. D., Green R. E., Shapiro B., A fast and efficient single-stranded genomic library preparation method optimized for ancient DNA. J. Hered. 112, 241–249 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Renaud G., Stenzel U., Kelso J., leeHom: Adaptor trimming and merging for Illumina sequencing reads. Nucleic Acids Res. 42, –e141 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li H., Durbin R., Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25, 1754–1760 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li H., Handsaker B., Wysoker A., Fennell T., Ruan J., Homer N., Marth G., Abecasis G., Durbin R., 1000 Genome Project Data Processing Subgroup , The sequence alignment/map format and SAMtools. Bioinformatics 25, 2078–2079 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jónsson H., Ginolhac A., Schubert M., Johnson P. L. F., Orlando L., MapDamage2.0: Fast approximate Bayesian estimates of ancient DNA damage parameters. Bioinformatics 29, 1682–1684 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Edgar R. C., Muscle5: High-accuracy alignment ensembles enable unbiased assessments of sequence homology and phylogeny. Nat. Commun. 13, 6968 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kalyaanamoorthy S., Minh B. Q., Wong T. K. F., Von Haeseler A., Jermiin L. S., ModelFinder: Fast model selection for accurate phylogenetic estimates. Nat. Methods 14, 587–589 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nguyen L. T., Schmidt H. A., Von Haeseler A., Minh B. Q., IQ-TREE: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 32, 268–274 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Suchard M. A., Lemey P., Baele G., Ayres D. L., Drummond A. J., Rambaut A., Bayesian phylogenetic and phylodynamic data integration using BEAST 1.10. Virus Evol. 4, vey016 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Godfrey-Smith D. I., Grist A. M., Stea R. R., Dosimetric and radiocarbon chronology of a pre-Wisconsinan mastodon fossil locality at East Milford, Nova Scotia, Canada. Quat. Sci. Rev. 22, 1353–1360 (2003). [Google Scholar]
- 38.M. Stuiver, P. J. Reimer, R. W. Reimer, CALIB 8.2 [WWW program] (2021); http://calib.org/calib/calib.html.
- 39.Lisiecki L. E., Raymo M. E., A Pliocene-Pleistocene stack of 57 globally distributed benthic δ18O records. Paleoceanography 20, PA1003 (2005). [Google Scholar]
- 40.Zazula G. D., MacPhee R. D. E., Metcalfe J. Z., Reyes A. V., Brock F., Druckenmiller P. S., Groves P., Harington C. R., Hodgins G. W. L., Kunz M. L., Longstaffe F. J., Mann D. H., McDonald H. G., Nalawade-Chavan S., Southon J. R., American mastodon extirpation in the Arctic and Subarctic predates human colonization and terminal Pleistocene climate change. Proc. Natl. Acad. Sci. U.S.A. 111, 18460–18465 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bell R., On the occurrence of mammoth and mastodon remains around Hudson bay. Geol. Soc. Am. Bull. 9, 369–390 (1897). [Google Scholar]
- 42.Harington C. R., Grant D. R., Mott R. J., The Hillsborough, New Brunswick, mastodon and comments on other Pleistocene mastodon fossils from Nova Scotia. Can. J. Earth Sci. 30, 1242–1253 (1993). [Google Scholar]
- 43.D. M. Gilmour, Chronology and Ecology of Late Pleistocene Megafauna in the Northern Willamette Valley, Oregon. Unpublished Master of Arts thesis. Department of Anthropology, Portland State University, Portland (2011). [Google Scholar]
- 44.Dooley A. C., Widga C., Stoneburg B. E., Jass C., Bravo-Cuevas V. M., Boehm A., Scott E., McDonald A. T., Volmut M., Re-evaluation of mastodon material from Oregon and Washington, USA, Alberta, Canada, and Hidalgo and Jalisco, Mexico. PeerJ 13, e18848 (2025). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Specimen Provenance
Figs. S1 to S16
Tables S1 to S7
Legends for data S1 and S2
References
Data S1 and S2