Abstract
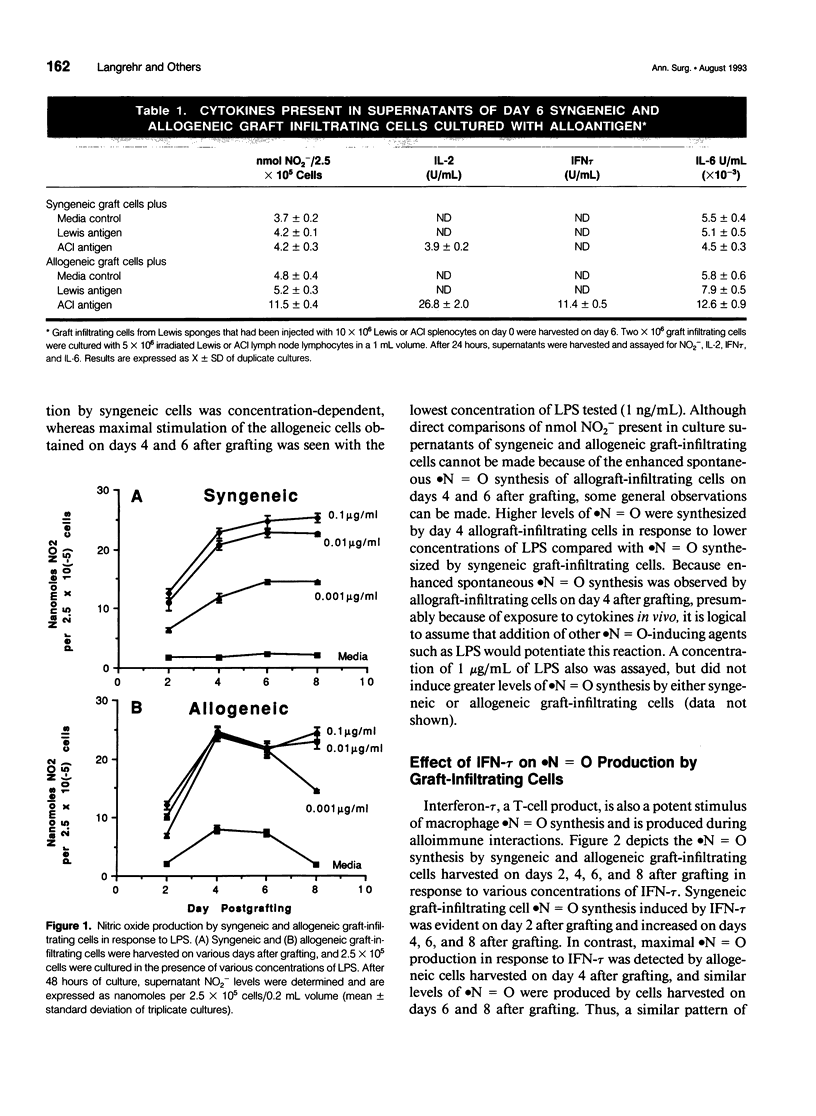
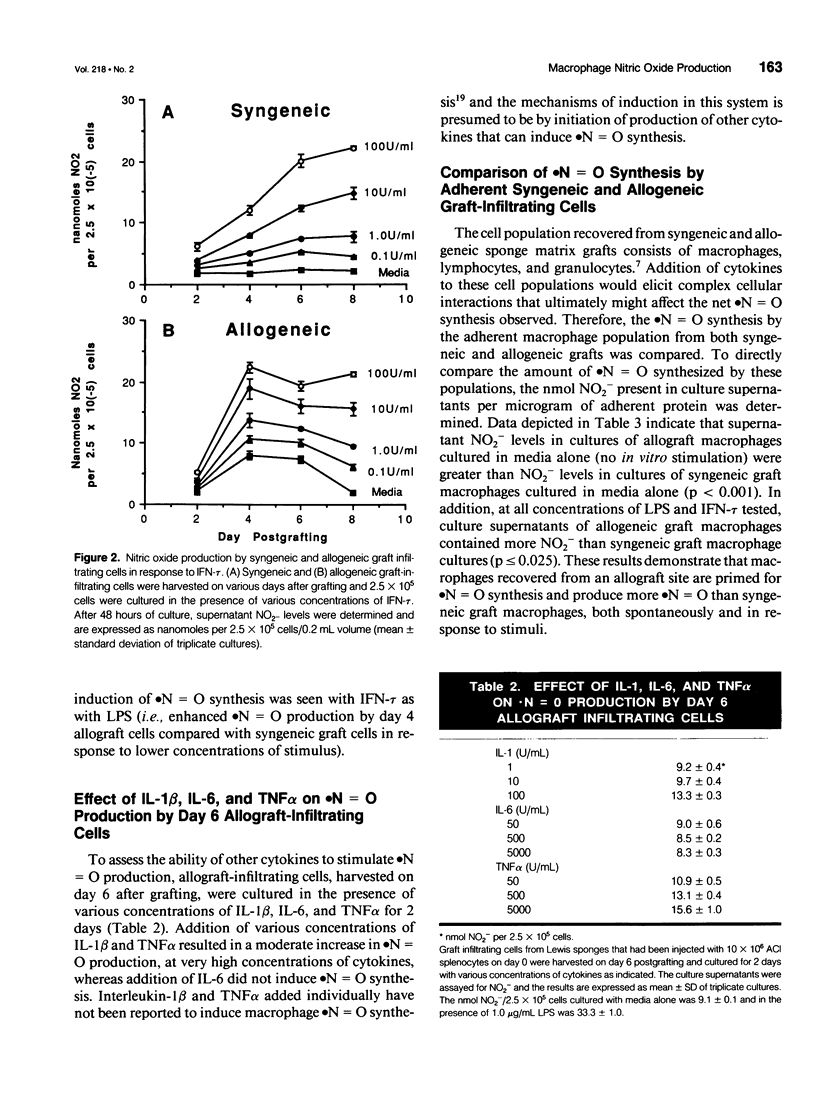
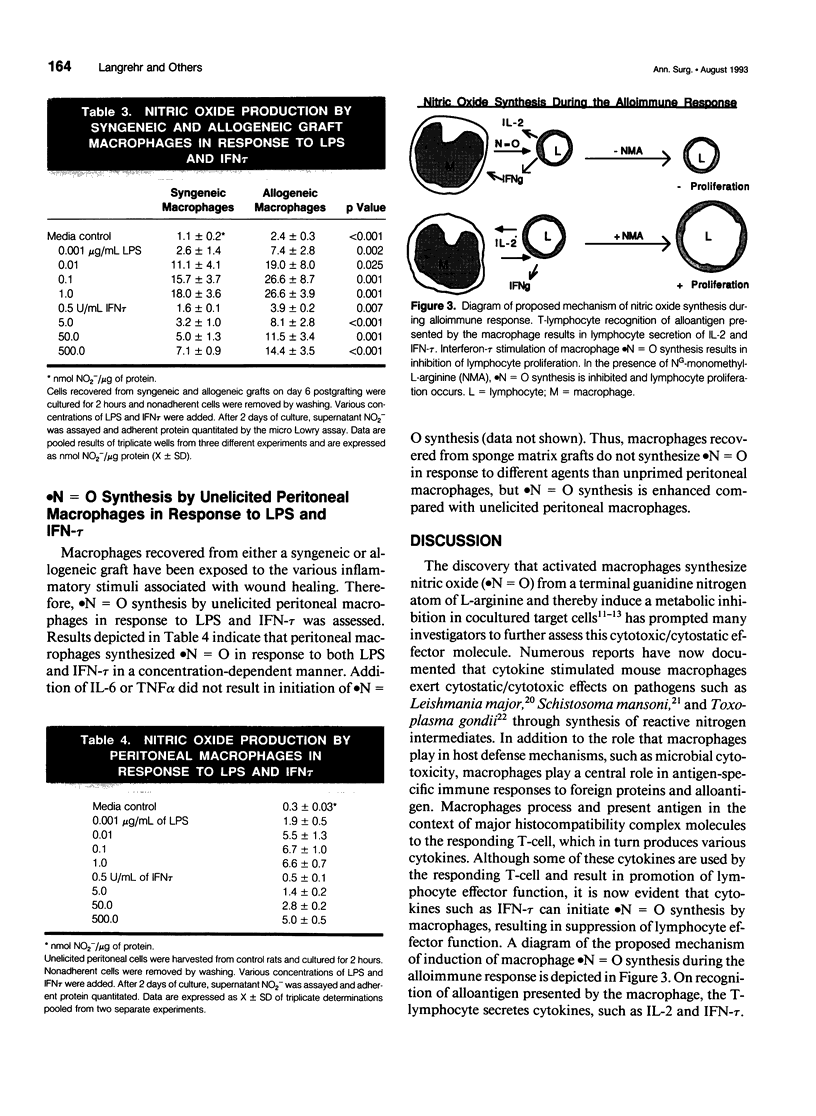
OBJECTIVE: The current study was designed to determine which cytokines produced during an alloimmune response stimulate macrophage nitric oxide (.N = O) production at allograft sites. SUMMARY BACKGROUND DATA: Previous work has demonstrated that rat sponge matrix allograft infiltrating cells produce more .N = O on stimulation with alloantigen than syngeneic graft-infiltrating cells. Addition of NG-monomethyl-L-arginine (NMA), an inhibitor of .N = O synthesis, promotes allospecific cytolytic T-lymphocyte effector function. METHODS: Polyurethane sponges were implanted subcutaneously in recipient Lewis rats and injected with 10 x 10(6) ACl splenocytes. On various days after grafting, graft-infiltrating cells were harvested for in vitro study. Adherent macrophages from the graft infiltrating cell population were obtained by a 2- to 3-hour incubation to plastic dishes with subsequent washing to remove nonadherent cells. RESULTS: Stimulation of unseparated graft-infiltrating cell populations with lipopolysaccharide or interferon-tau resulted in enhanced .N = O synthesis by allograft infiltrating cells compared with syngeneic graft-infiltrating cells, early after grafting. Macrophages recovered from an allograft site spontaneously produce more .N = O than macrophages recovered from syngeneic grafts (p < 0.001). Significantly enhanced levels of .N = O were produced by allograft macrophages compared with syngeneic graft macrophages on stimulation with lipopolysaccharide or interferon-tau (p < or = 0.025). CONCLUSIONS: Nitric oxide appears to be produced in response to the local cytokines secreted by an ongoing rejection reaction. Nitric oxide serves under these circumstances to modulate the alloimmune response.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams L. B., Hibbs J. B., Jr, Taintor R. R., Krahenbuhl J. L. Microbiostatic effect of murine-activated macrophages for Toxoplasma gondii. Role for synthesis of inorganic nitrogen oxides from L-arginine. J Immunol. 1990 Apr 1;144(7):2725–2729. [PubMed] [Google Scholar]
- Albina J. E., Abate J. A., Henry W. L., Jr Nitric oxide production is required for murine resident peritoneal macrophages to suppress mitogen-stimulated T cell proliferation. Role of IFN-gamma in the induction of the nitric oxide-synthesizing pathway. J Immunol. 1991 Jul 1;147(1):144–148. [PubMed] [Google Scholar]
- Albina J. E., Henry W. L., Jr Suppression of lymphocyte proliferation through the nitric oxide synthesizing pathway. J Surg Res. 1991 Apr;50(4):403–409. doi: 10.1016/0022-4804(91)90210-d. [DOI] [PubMed] [Google Scholar]
- Albina J. E., Mills C. D., Henry W. L., Jr, Caldwell M. D. Regulation of macrophage physiology by L-arginine: role of the oxidative L-arginine deiminase pathway. J Immunol. 1989 Dec 1;143(11):3641–3646. [PubMed] [Google Scholar]
- Albina J. E., Mills C. D., Henry W. L., Jr, Caldwell M. D. Temporal expression of different pathways of 1-arginine metabolism in healing wounds. J Immunol. 1990 May 15;144(10):3877–3880. [PubMed] [Google Scholar]
- Corbin J. L., Reporter M. N-G-methylated arginines; a convenient preparation of N-G-methylarginine. Anal Biochem. 1974 Jan;57(1):310–312. doi: 10.1016/0003-2697(74)90080-3. [DOI] [PubMed] [Google Scholar]
- Curran R. D., Billiar T. R., Stuehr D. J., Ochoa J. B., Harbrecht B. G., Flint S. G., Simmons R. L. Multiple cytokines are required to induce hepatocyte nitric oxide production and inhibit total protein synthesis. Ann Surg. 1990 Oct;212(4):462–471. doi: 10.1097/00000658-199010000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denis M. Tumor necrosis factor and granulocyte macrophage-colony stimulating factor stimulate human macrophages to restrict growth of virulent Mycobacterium avium and to kill avirulent M. avium: killing effector mechanism depends on the generation of reactive nitrogen intermediates. J Leukoc Biol. 1991 Apr;49(4):380–387. doi: 10.1002/jlb.49.4.380. [DOI] [PubMed] [Google Scholar]
- Ding A. H., Nathan C. F., Stuehr D. J. Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages. Comparison of activating cytokines and evidence for independent production. J Immunol. 1988 Oct 1;141(7):2407–2412. [PubMed] [Google Scholar]
- Fu Y., Blankenhorn E. P. Nitric oxide-induced anti-mitogenic effects in high and low responder rat strains. J Immunol. 1992 Apr 1;148(7):2217–2222. [PubMed] [Google Scholar]
- Green L. C., Wagner D. A., Glogowski J., Skipper P. L., Wishnok J. S., Tannenbaum S. R. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem. 1982 Oct;126(1):131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- Green S. J., Meltzer M. S., Hibbs J. B., Jr, Nacy C. A. Activated macrophages destroy intracellular Leishmania major amastigotes by an L-arginine-dependent killing mechanism. J Immunol. 1990 Jan 1;144(1):278–283. [PubMed] [Google Scholar]
- Hibbs J. B., Jr, Taintor R. R., Vavrin Z., Rachlin E. M. Nitric oxide: a cytotoxic activated macrophage effector molecule. Biochem Biophys Res Commun. 1988 Nov 30;157(1):87–94. doi: 10.1016/s0006-291x(88)80015-9. [DOI] [PubMed] [Google Scholar]
- Hibbs J. B., Jr, Westenfelder C., Taintor R., Vavrin Z., Kablitz C., Baranowski R. L., Ward J. H., Menlove R. L., McMurry M. P., Kushner J. P. Evidence for cytokine-inducible nitric oxide synthesis from L-arginine in patients receiving interleukin-2 therapy. J Clin Invest. 1992 Mar;89(3):867–877. doi: 10.1172/JCI115666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman R. A., Langrehr J. M., Billiar T. R., Curran R. D., Simmons R. L. Alloantigen-induced activation of rat splenocytes is regulated by the oxidative metabolism of L-arginine. J Immunol. 1990 Oct 1;145(7):2220–2226. [PubMed] [Google Scholar]
- Hoffman R. A., Langrehr J. M., Dull K. E., Simmons R. L. Nitric oxide production by mouse sponge matrix allograft-infiltrating cells. Comparison with the rat species. Transplantation. 1993 Mar;55(3):591–596. doi: 10.1097/00007890-199303000-00024. [DOI] [PubMed] [Google Scholar]
- James S. L., Glaven J. Macrophage cytotoxicity against schistosomula of Schistosoma mansoni involves arginine-dependent production of reactive nitrogen intermediates. J Immunol. 1989 Dec 15;143(12):4208–4212. [PubMed] [Google Scholar]
- Jiang H., Stewart C. A., Fast D. J., Leu R. W. Tumor target-derived soluble factor synergizes with IFN-gamma and IL-2 to activate macrophages for tumor necrosis factor and nitric oxide production to mediate cytotoxicity of the same target. J Immunol. 1992 Sep 15;149(6):2137–2146. [PubMed] [Google Scholar]
- Kilbourn R. G., Belloni P. Endothelial cell production of nitrogen oxides in response to interferon gamma in combination with tumor necrosis factor, interleukin-1, or endotoxin. J Natl Cancer Inst. 1990 May 2;82(9):772–776. doi: 10.1093/jnci/82.9.772. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lancaster J. R., Jr, Langrehr J. M., Bergonia H. A., Murase N., Simmons R. L., Hoffman R. A. EPR detection of heme and nonheme iron-containing protein nitrosylation by nitric oxide during rejection of rat heart allograft. J Biol Chem. 1992 Jun 5;267(16):10994–10998. [PubMed] [Google Scholar]
- Langrehr J. M., Dull K. E., Ochoa J. B., Billiar T. R., Ildstad S. T., Schraut W. H., Simmons R. L., Hoffman R. A. Evidence that nitric oxide production by in vivo allosensitized cells inhibits the development of allospecific CTL. Transplantation. 1992 Mar;53(3):632–640. doi: 10.1097/00007890-199203000-00027. [DOI] [PubMed] [Google Scholar]
- Langrehr J. M., Hoffman R. A., Billiar T. R., Lee K. K., Schraut W. H., Simmons R. L. Nitric oxide synthesis in the in vivo allograft response: a possible regulatory mechanism. Surgery. 1991 Aug;110(2):335–342. [PubMed] [Google Scholar]
- Langrehr J. M., Murase N., Markus P. M., Cai X., Neuhaus P., Schraut W., Simmons R. L., Hoffman R. A. Nitric oxide production in host-versus-graft and graft-versus-host reactions in the rat. J Clin Invest. 1992 Aug;90(2):679–683. doi: 10.1172/JCI115911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langrehr J. M., Müller A. R., Bergonia H. A., Jacob T. D., Lee T. K., Schraut W. H., Lancaster J. R., Jr, Hoffman R. A., Simmons R. L. Detection of nitric oxide by electron paramagnetic resonance spectroscopy during rejection and graft-versus-host disease after small-bowel transplantation in the rat. Surgery. 1992 Aug;112(2):395–402. [PubMed] [Google Scholar]
- Marletta M. A., Yoon P. S., Iyengar R., Leaf C. D., Wishnok J. S. Macrophage oxidation of L-arginine to nitrite and nitrate: nitric oxide is an intermediate. Biochemistry. 1988 Nov 29;27(24):8706–8711. doi: 10.1021/bi00424a003. [DOI] [PubMed] [Google Scholar]
- Mills C. D. Molecular basis of "suppressor" macrophages. Arginine metabolism via the nitric oxide synthetase pathway. J Immunol. 1991 Apr 15;146(8):2719–2723. [PubMed] [Google Scholar]
- Murray H. W., Teitelbaum R. F. L-arginine-dependent reactive nitrogen intermediates and the antimicrobial effect of activated human mononuclear phagocytes. J Infect Dis. 1992 Mar;165(3):513–517. doi: 10.1093/infdis/165.3.513. [DOI] [PubMed] [Google Scholar]
- Nussler A. K., Di Silvio M., Billiar T. R., Hoffman R. A., Geller D. A., Selby R., Madariaga J., Simmons R. L. Stimulation of the nitric oxide synthase pathway in human hepatocytes by cytokines and endotoxin. J Exp Med. 1992 Jul 1;176(1):261–264. doi: 10.1084/jem.176.1.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochoa J. B., Curti B., Peitzman A. B., Simmons R. L., Billiar T. R., Hoffman R., Rault R., Longo D. L., Urba W. J., Ochoa A. C. Increased circulating nitrogen oxides after human tumor immunotherapy: correlation with toxic hemodynamic changes. J Natl Cancer Inst. 1992 Jun 3;84(11):864–867. doi: 10.1093/jnci/84.11.864. [DOI] [PubMed] [Google Scholar]
- Ochoa J. B., Udekwu A. O., Billiar T. R., Curran R. D., Cerra F. B., Simmons R. L., Peitzman A. B. Nitrogen oxide levels in patients after trauma and during sepsis. Ann Surg. 1991 Nov;214(5):621–626. doi: 10.1097/00000658-199111000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuehr D. J., Nathan C. F. Nitric oxide. A macrophage product responsible for cytostasis and respiratory inhibition in tumor target cells. J Exp Med. 1989 May 1;169(5):1543–1555. doi: 10.1084/jem.169.5.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]


