Abstract
Particular syngeneic adjuvant-free monoclonal antibodies are immunogenic and elicit antibody responses against the variable region idiotypes (Ids). We here study how heavy-chain constant regions (CH) regulate immune responses to Ids of free, uncomplexed monoclonal antibodies. To this end, we selected two hybridomas, called Id3 and IdA.01, that produce immunogenic IgMλ2 directed toward 2,4,6-trinitrophenyl, and subcloned rare IgG1, IgG3, IgE, or IgA class switch variants. The purified switch variants, which possessed the Ids of their IgM progenitors, were injected repeatedly without added adjuvant into BALB/c mice, and anti-Id IgG responses were determined. These repeated injections revealed that the immunogenicity of Ids was lost by switching to IgG1 and IgG3, restored when the Fc piece of IgG1 was removed, maintained by switching to IgE and monomeric IgA, and lost in polymeric IgA. Loss of immunogenicity was associated with acquisition of Id-specific tolerogenicity, as determined by immunization challenge with Id borne by IgM. An Id borne by IgG induced tolerance when injected at least 90 days before or 3–21 days after immunization with IgM Id was begun. Ids of IgG were also tolerogenic in mice deficient in FcγRIIB or FcγRI + III. The results suggest that Ids that have switched to IgG and pIgA negatively control immune responses to shared Ids, including the Ids of their IgM progenitors.
The variable (V) regions of syngeneic antibodies (Abs), called idiotypes (Ids), can provoke immune responses (1, 2) that are T helper cell (Th)-dependent (3–7). When administered in small amounts as free Abs without added adjuvant, a minor but significant fraction (≈5%) of IgM anti-2,4,6-trinitrophenyl (TNP) monoclonal (m)Abs of the primary repertoire elicited anti-Id Ab responses in BALB/c mice (8), which provided an opportunity to study the influence of the heavy-chain constant region (CH) isotypes on the immunogenicity of Ids. Since free anti-TNP mAb-turned immunogens contain built-in CH regions, the isotype effect can be studied without the confounding variables of immune complex formation such as Ab affinities, the quality and quantity of antigen (Ag)–Ab complexes, rapid clearance or enhanced presentation of the Ag, and masking of antigenic sites.
Apart from a short report showing that injection of a plain IgG myeloma protein into mice led to tolerance to the Id of this IgG (9), and a study from this laboratory indicating that an Id that is immunogenic when borne by IgM, becomes tolerogenic when borne by IgG1, IgG2a, and IgG2b (10), limited information is available on the significance of Ig isotypes for the immunogenicity of Ids. We here study immune responses to switch variants of two mAbs, called Id3 and IdA.01, selected because they induce potent immunity when borne by adjuvant-free IgM (8, 10, 11).
Materials and Methods
Mice.
BALB/c female mice were purchased from Charles River Breeding Laboratories or Harlan Olac (Bichester, U.K.). FcγRII−/− and FcRγ−/− mice on a BALB/c background were purchased from Taconic Farms. The mice were 7–12 weeks old at the start of the experiments. The animals were maintained specific pathogen-free as described (8).
Mono- and Polyclonal Ab.
Hybridoma EM95-producing rat IgG anti-mouse IgE was a gift of Z. Eshar, The Weizmann Institute of Science, Israel. The EM95 mAb was affinity purified on Sepharose-coupled MAR 18.5 (mouse anti-rat kappa, ATCC TIB-216) and used together with biotin-labeled sheep anti-mouse IgE (The Binding Site, Birmingham, U.K.) to detect IgE-producing switch variants of Id3. Affinity-purified unconjugated and biotin-conjugated polyclonal Abs specific for mouse IgA, IgG, and IgG subclasses were purchased from Jackson ImmunoResearch, or Southern Biotechnology Associates, or Zymed. Syngeneic IgG1 anti-Id3 mAb, called TREC1.1 (8), and anti-IdA.01 mAb designated ANUL1, were produced by hybridomas (our local collection) derived from BALB/c mice immunized with the homologous Id borne by IgM.
Isolation of Rare Spontaneous Class Switch Variants.
Rare subclones in which the VH exon spontaneously rearranged to a new CH element were identified by sandwich ELISA by using isotype- and IgG subclass-specific Abs as described (12). The IgG3-producing variant of Id3 was isolated directly from hybridoma cells producing IgMλ2-Id3. A switched Id3-clone-producing IgG2a + IgM (10), likely because of duplication of the IgH locus with the functional VHDJH rearrangement (13), was the source of a clone, designated 11B-Id3, that made IgG1 + IgG2a, but no IgM. From clone 11B-Id3 we isolated two switch variants: one produced only IgE-Id3 and the other only IgA-Id3. Unlike the other switch variants, the IgE variant of Id3 did not bind to TNP-BSA in ELISA (possibly because IgE lacks a hinge) but it bound to 2,4-dinitrophenyl-lysine-Sepharose. Hybridomas secreting IgM-IdA.01 and IgM-Id07.08 (see ref. 8) served as a source of variants producing the respective IgG1 switch variants (and no other isotype). The switch variants were cloned by limiting dilution. The Id3 and IdA.01 Igs reacted specifically in ELISA with anti-Id3 TREC1.1 and anti-IdA.01 ANUL1, respectively. The authenticity of the serologically defined isotypes and Ids was further confirmed by RNA sequencing (data not shown) according to standard procedures as described (8).
Purification of Monoclonal Class Switch Variants.
All isotypes of Id3 were purified from supernatants of hybridoma cells growing in protein-free medium by their affinity for 2,4-dinitrophenyl-lysine Sepharose (8), and further purified by size-exclusion FPLC on Superdex 200 (Amersham Pharmacia). The Igs were concentrated by ultrafiltration. Natural mIgA and pIgA represented ≈8% and ≈92%, respectively, of IgA-Id3. IgG1-IdA.01 and IgG1-Id07.08 were purified from hybridoma supernatants on protein A Sepharose.
IgM Monomers and F(ab′)2γ.
Secretory IgM-IdA.01 was converted to 8S monomers by reduction with 0.01 M DTT for 1 h at 37°C and alkylation with 0.015 M iodoacetamide, followed by size-exclusion FPLC on Superose 6 HR. Digestion of IgG1-IdA.01 with pepsin (25 μg/ml) was performed in 0.1 M citrate, pH 3.5, at 37°C for 5 h. The digest was subjected to FPLC on Superose 6 HR (Amersham Pharmacia), and fractions corresponding to Mr ≈ 100 kDa were collected. The cleavage of IgG was complete as indicated by SDS/PAGE with 12% acrylamide and Coomassie blue staining.
Immunizations.
All immunogens were stored sterile at 4°C and injected in sterile pyrogen-free saline without added adjuvant. All priming injections were given s.c. at the base of the tail and three to four sites on the back because by this route Ags are delivered to regional draining lymph nodes. Three weeks later biweekly i.p. boosters were begun. The mice were bled from the femoral vein before and 3 weeks after priming and 2 weeks after each booster.
ELISA.
Sera from animals immunized with the various switch variants were assessed for IgG anti-Id Ab activity by ELISA as described (8), by using the homologous Id born by IgM mAb as coat Ag, and alkaline phosphatase-conjugated rabbit anti-mouse IgG (Zymed) as second Ab.
Results
The Influence of Various CH Isotypes on the Immunogenicity of Id.
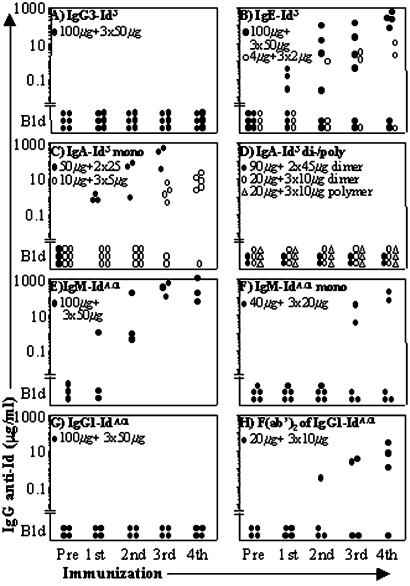
We examined the immunogenicity of Id3 borne by IgG3, IgA, and IgE. The results are shown in Fig. 1. None of six BALB/c mice responded to immunization with IgG3-Id3 (Fig. 1A) which, therefore, is nonimmunogenic in common with Id3 borne by IgG1, IgG2a, and IgG2b (10). Immunization with IgE-Id3 (100 μg + 50 μg × 3) elicited large amounts of IgG anti-Id3 Abs (90–300 μg/ml) in 4 of 6 animals, and weaker but clearly significant responses (2–10 μg/ml) developed in 2 of 3 mice immunized with a 25-fold lower dose (Fig. 1B), and in 1 of 3 animals given 25 μg + 12 μg × 3 (data not shown). With respect to the natural (i.e., not chemically treated) mIgA-Id3, 16 of 17 animals immunized with doses ranging from 10 μg + 5 μg × 3 to 90 μg + 45 μg × 2 produced >1 μg/ml of anti-Id3 IgG (Fig. 1C, and data not shown). In striking contrast, none of 14 animals injected with the same dose range of pIgA-Id3 responded (Fig. 1D, and data not shown).
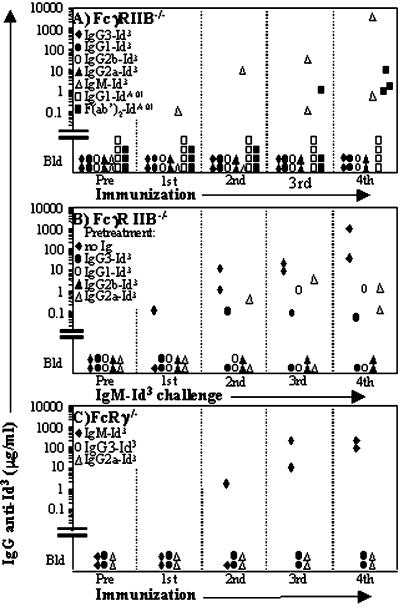
Figure 1.
The immunogenicity of Ig idiotypes (Ids) is profoundly influenced by the isotype, Fcγ piece, and state of IgA polymerization. The y axis shows the serum levels of IgG Ab against plates coated with the homologous Id borne by IgM, determined by ELISA as described in Materials and Methods. The immunogen and the priming and booster doses are indicated at the top left parts of A–H. Each symbol represents an individual mouse. Pre, preimmunization serum; Bld, below level of detection; mono, monomer; di/poly, dimer/polymer.
To examine whether the nonimmunogenicity of Id born by IgG is unique to Id3 or has broader application, we turned to the potently immunogenic Id of a nonmutated IgMλ2 anti-TNP mAb called IdA.01 (8). All animals immunized with IgM-IdA.01 produced anti-IdA.01 IgG (Fig. 1E). Two of five animals injected with the IgM-IdA.01 monomer (reduced/alkylated) also produced large amounts of anti-Id Ab (Fig. 1F), indicating that two copies of IdA.01 per IgM molecule are sufficient for immunogenicity. In contrast, all four animals immunized repeatedly with IgG1-IdA.01 failed to respond (Fig. 1G). Three of four animals responded vigorously to the F(ab′)2 piece of IgG1-IdA.01; after five injections the level of IgG anti-IdA.01 Abs had reached a mean of 105 μg/ml (Fig. 1H), indicating that by removing the Fcγ piece from IgG1, IdA.01 regained immunogenicity. All of the described antisera contained anti-Id IgG1, but not IgG2a, exclusively specific for the Id used for immunization and they did not contain anti-BSA IgG (data not shown), confirming previous results (8).
Ids Borne by IgG3 and IgG1 Induce Long-Lasting Unresponsiveness.
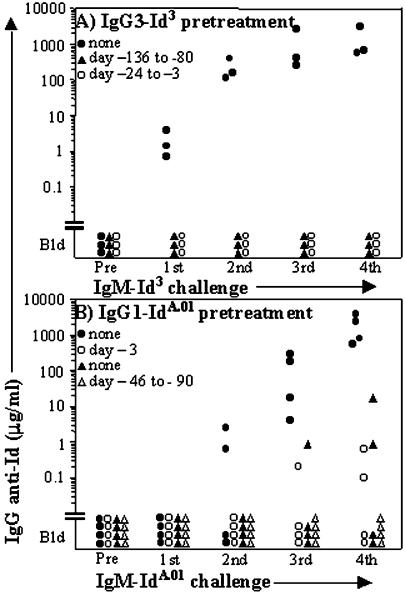
To ascertain whether IgG3 induces unresponsiveness to Id, mice were injected s.c. with IgG3-Id3, 100 μg on day 0 and 50 μg on day 21, and beginning on day 24 they received a series of biweekly immunization-challenges with IgM-Id3 alone. Fig. 2A shows that none of three IgG3-Id3 preinjected animals responded to IgM-Id3. Another group of mice that had been given five biweekly injections (100 μg + 50 μg × 4) of IgG3-Id3 (see Fig. 1A) was rested for 80 days before the IgM-Id3 challenges. These animals were also unresponsive to Id3, whereas 3 of 3 mice that had not received IgG3-Id3 responded to IgM-Id3 (Fig. 2A).
Figure 2.
Two Ids that have switched to IgG3 and IgG 1, respectively, induce long-lasting tolerance to the homologous Id born by IgM. The serum levels of IgG Ab against plates coated with the homologous Id borne by IgM are shown. (A) IgG3-Id3 is tolerogenic. All mice were challenged with IgM-Id3, first 100 μg s.c. followed after 3 weeks by biweekly boosters of 50 μg i.p. (▴) IgG3-Id3 (100 μg) was injected s.c. followed by 4 biweekly injections of 50 μg i.p.; the IgM-Id3 challenge started after a rest period of 80 days. (○) The IgM-Id3 challenge started 3 days after the last of two injections of IgG-Id3 (100 μg s.c. and 50 μg i.p.) spaced 3 weeks apart. (●) The mice received IgM-Id3 only. (B) IgG1-IdA.01 is tolerogenic. (▴) IgG1-IdA.01 (100 μg) was injected s.c. followed by 3 biweekly i.p. boosters of 50 μg. After a rest period of 90 days, the biweekly IgM-IdA.01 challenge (10 μg + 5 μg × 3) was started; this dose is immunogenic (see Fig. 4). (○) IgM-IdA.01 challenge (100 μg + 50 μg × 3) was begun 3 days after a single s.c. injection of 100 μg of IgG1-IdA.01. (●) The mice received biweekly injections of IgM-IdA.01 (100 μg + 50 μg × 3) only.
To find out whether the tolerogenicity of IgG-Id3 has broader relevance, mice were given a single s.c. injection of 100 μg of the IgG1 switch variant of IdA.01 and, beginning 3 days later, challenged repeatedly with IgM-IdA.01. As shown in Fig. 2B, the mean humoral response to IdA.01 of mice that had not been preinjected with IgG1, was ≈10,000 times higher than that of the preinjected animals (e.g., 1,800 μg/ml vs. 0.17 μg/ml). Another group of animals that received a series of four biweekly IgG1-IdA.01 injections failed to respond to immunization challenge with IgM-IdA.01 that was begun 90 days after the last injection of IgG (Fig. 2B). The long duration (at least 80–90 days) of the tolerance induced by Id3 and IdA.01 borne by noncomplexed IgG3 and IgG1, respectively, probably excluded the possibility that nonresponsiveness was due to masking of B cell antigen receptors (BCRs) or Id-recognizing serum Abs by the IgG Id.
Unresponsiveness Induced by Id Borne by IgG Is Id-Specific.
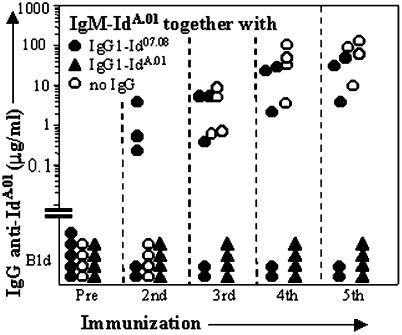
Mice were immunized repeatedly, this time with a mixture consisting of the immunogenic IgM-IdA.01 and its tolerogenic IgG1 class switch variant; the dose of IgG was twice that of IgM. As shown in Fig. 3, all animals failed to make anti-IdA.01 Ab throughout. By contrast, 3 of 5 mice immunized with IgM-IdA.01 mixed with an unrelated Id07.08 (8) borne by IgG1 mounted a vigorous anti-IdA.01 response (Fig. 3). In a parallel experiment, the anti-BSA responses of animals immunized repeatedly with adjuvant-free BSA mixed with IgG1-IdA.01 did not differ appreciably from those of mice given BSA alone (data not shown). In addition, mice preinjected with IgG2a-Id3 and that were unresponsive to challenge with IgM-Id3, responded to IgM-IdA.01 (data not shown). Thus, the unresponsiveness induced by IdA.01 of IgG1 is specific and cannot be prevented by coinjection of IdA.01 borne by IgM.
Figure 3.
The tolerance induced by IgG1 is Id-specific. The mice were immunized biweekly with IgM-IdA.01 (10 μg + 5 μg × 4) either alone (○) or mixed with IgG1-IdA.01 (▴) or the unrelated IgG1-Id07.08 (●). For each injection the dose of IgG1 was twice that of IgM. The serum levels of IgG Ab against IgM-IdA.01 are shown.
Unresponsiveness to Id Can Develop When Id Borne by IgG Is Injected After Priming with IgM.
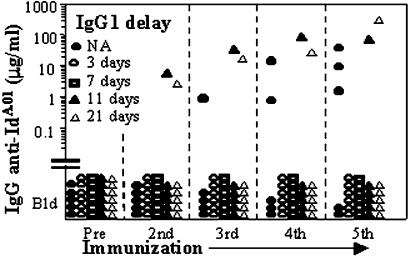
These findings, and the fact that production of IgG Abs is delayed relative to IgM during primary immune responses, led us to speculate that when Ids switch isotype from IgM to IgG they suppress spontaneous in vivo immune responses against shared Ids borne by their IgM progenitors. To test this hypothesis directly, we examined whether unresponsiveness to Id can develop when the first injection of IgG is given after priming with IgM. The experiment is described and the results are shown in Fig. 4. Three of five animals in the control group produced anti-IdA.01 IgG. By contrast, none of the 12 mice that received additional IgG1 3 or 7 days after priming with IgM mounted a detectable Ab response to IdA.01. Moreover, of the 12 mice that received the first injection of IgG1-IdA.01 11 or 21 days after IgM-IdA.01, only two responded, i.e., significantly fewer than the controls (P < 0.02). Thus, Ids that have switched to IgG powerfully suppress humoral responses to immunogenic Id of their IgM forerunners encountered up to 21 days earlier.
Figure 4.
Id borne by IgG1 induces unresponsiveness when injected after priming with the homologous immunogenic Id borne by IgM. The serum levels of IgG Ab against plates coated with IgM-IdA.01 are shown. The mice (n = 29) were primed s.c. with 10 μg of IgM-IdA.01 in saline on day 0 and divided into five groups. Beginning 3 weeks later, all mice received four biweekly boosters, which for one group (●) consisted of IgM-IdA.01 (5 μg) only. The other four groups were boosted with a mixture of 5 μg of IgM- and 10 μg of IgG1-IdA.01. Of these, one group (▵) received no extra IgG, whereas the other three groups received an extra 20-μg dose of IgG1-Id IdA.01 s.c. on days 3 (○), 7 (□), or 11 (▴) after priming. NA, not applicable.
Ids of IgG Fail to Elicit Anti-Id Abs in Mice Deficient in FcγRIIB or FcγRI + FcγRIII (FcγRIIB−/− or FcRγ−/−).
Mice deficient in FcγRIIB (14) responded to IgM-Id3, but not to multiple injections of IgG1, 2a, 2b, and 3 switch variants of Id3 (Fig. 5A). They also failed to respond to IgG1-IdA.01, but they produced significant amounts of anti-IdA.01 IgG when immunized with F(ab′)2 of IgG1-IdA.01 (Fig. 5A). To assess whether the FcγRIIB−/− animals injected with IgG1, 2a, 2b, or 3 switch variants of Id3 had developed tolerance to Id3, the mice were immunization-challenged biweekly with IgM-Id3, beginning 42 days after the fifth injection of its IgG switch variant. As shown in Fig. 5B, 4 of 8 IgG-preimmunized animals failed to respond to the challenge, and the other four animals had very low levels of anti-Id3 Abs (≤1 μg/ml) compared with the controls that had received no IgG-Id3 (mean of 520 μg/ml). FcRγ knockouts, deficient in FcγRI, FcγRIII, and FcɛRI (14), also failed to respond to multiple injections of Id3 borne by IgG2a or IgG3 (IgG1 and IgG2b were not studied), whereas secretory IgM-Id3 elicited anti-Id3 Abs (Fig. 5C). We conclude that the failure to develop immunity to IgG-borne Ids depends neither on FcγRIIB alone nor on FcγRI + FcγRIII. Mice lacking all three FcγR on a BALB/c background were not available.
Figure 5.
The response of FcγRIIB or FcRγ gene knockout mice to Id borne by IgG subclasses and F(ab′)2γ1. The Id immunogens examined are indicated at the top left parts of A–C. The mice were primed s.c. and boosted biweekly i.p. The serum levels of IgG Ab against plates coated with the homologous Id borne by IgM are shown. The symbols represent individual mice. (A) FcγRIIB−/− mice are nonresponders to Id borne by IgG, but respond to IdA.01 borne by IgM and F(ab′)2 of IgG1. The priming and booster doses were 100 μg and 50 μg for Id3, 30 μg and 15 μg for IgG1-IdA.01, and 20 μg and 10 μg for F(ab′)2-IdA.01. (B) Id3 borne by IgG1, 2a, 2b, and 3 is tolerogenic for FcγRIIB−/− mice. The IgG-Id3-injected mice of Fig. 5A as well as noninjected FcγRIIB−/− controls (⧫) were immunization-challenged four times biweekly with IgM-Id3 (100 μg + 50 μg × 3), beginning 6 weeks after the last injection of IgG-Id3. (C) FcRγ−/− mice are nonresponders to Id3 borne by IgG3 and IgG2a. The priming and biweekly booster doses were 100 μg and 50 μg, respectively.
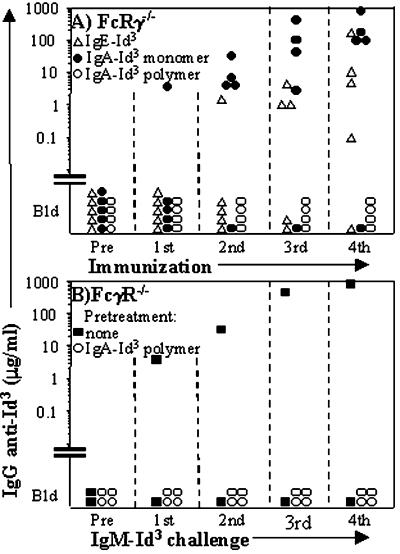
Responsiveness to Id3 Borne by IgE and IgA Is Intact in Mice Lacking FcγRI + FcγRIII + FcɛRI (FcRγ−/−).
Because FcRγ−/− mice lack expression of the high-affinity FcɛRI, we immunized five mice with Id3 borne by IgE. Three mice produced large amounts of IgG anti-Id3 Ab (Fig. 6A). We also considered the possibility that mouse IgA-receptors (FcαR) might exist which, like the human FcαR CD89, use the common activation subunit, the γ-chain. We therefore immunized FcRγ−/− mice with Id3 borne by pIgA and by mIgA derived from pIgA by reduction/alkylation. Confirming the results with natural mIgA and pIgA in wild-type mice (Fig. 1 C and D), FcRγ−/− animals mounted strong responses to mIgA-Id3 and were completely nonresponsive to Id3 of pIgA (Fig. 6A). The pIgA-Id3-injected animals also failed to respond to a subsequent immunization-challenge with secretory IgM-Id3 (Fig. 6B). The results suggest that (i) FcɛRI is not required for responses to Id3 of IgE, (ii) if Fcα receptors that depend on the γ-chain exist in the mouse, they are not required for the striking differential response to Id3 borne by mIgA versus pIgA, and (iii) pIgA can be tolerogenic.
Figure 6.
The response of FcRγ−/− mice to Id3 borne by IgE and by mIgA or pIgA. The serum levels of IgG Ab against IgM-Id3 are shown. (A) FcRγ−/− mice are responders to IgE and mIgA, but nonresponders to pIgA. The mice were primed with 50 μg s.c. and boosted biweekly i.p. with 25 μg of Id3 borne by the indicated isotypes. For this experiment, mIgA was derived from pIgA by reduction/alkylation (see Materials and Methods). (B) FcRγ−/− mice injected with pIgA-Id3 have become tolerant to Id3 borne by IgM. Beginning 20 days after the last injection with pIgA-Id3 (see A), the mice were immunization-challenged with IgM-Id3, first 100 μg s.c. followed after 21 days by biweekly injections of 50 μg i.p. Nonpreinjected mice (■) served as controls.
Discussion
The present and previous (10) results indicate that immunogenic Ids borne by adjuvant-free IgM anti-TNP mAbs acquire tolerogenicity by switching to IgG1, 2a, 2b, 3, or pIgA, regain immunogenicity when the Fc piece of IgG1 is removed, and maintain immunogenicity by switching to IgE and natural IgA monomers. Because Th-dependent Ags usually induce naive B cells of the systemic and mucosal immune systems to undergo class switching to IgG and pIgA, respectively, the Ig isotype-based negative regulation of Id immunity may operate in both compartments, which may be advantageous. However, because the therapeutic IgG mAb anti-CD52 (Campath) is immunogenic (15), Ids of IgG Abs with autoreactivity need further study.
The mechanism underlying the differential immunogenicity of Id borne by the various isotypes remains unclear. Masking of serum anti-Id Abs by soluble IgG Id is very unlikely given that tolerance was still intact 90 days after the last injection of IgG. Because immunogenic forms such as IgM, IgE, and F(ab′)2γ, have shorter half-lives than tolerogenic IgG, brief presence of Id might be immunogenic, whereas continued exposure could be tolerogenic. However, the fact that nonimmunogenic pIgA is cleared more rapidly than mIgA by the poly(Ig) receptor of mouse hepatocytes (16, 17), is not in accord with this hypothesis. Nonresponsiveness to IgG Id cannot be caused by weak BCR cross-linking because (i) other bivalent forms of Id such as IgE-Id3, mIgA-Id3, F(ab′)2 of IgG1-IdA.01, and 8S IgM subunits of IdA.01 were immunogenic, and (ii) pIgA, which should be a good cross-linker, was nonimmunogenic. One possible scenario is based on the ability of CD19 to amplify BCR-mediated signaling and augment humoral immune responses when it is cross-linked to BCR of naive B cells (18). Thus, when free IgM binds to surface Ig of anti-Id B cells, it might coligate BCR and the CR2/CD19 coreceptor either by means of attached split products of C4 and C3, or independently of complement (19). However, CD19-dependent mechanisms cannot be required for Id immunogenicity because Id3 borne by mIgA and IdA.01 of F(ab′)2γ, which do not bind CD19 (19) and are weak complement activators, and IgE that does not activate complement at all (20), were also immunogenic. We cannot rule out that IgE-Id3 bridged surface Ig and FcɛRII on anti-Id3 B cells, allowing for augmented BCR signaling (21), but this finding would only be relevant for IgE Id.
The finding that IdA.01 regained immunogenicity when IgG1 was converted to F(ab′)2 indicated that engagement of Fcγ receptors is required for the negative response to Id. Two principal ways exist by which FcγRI, IIB, and III can inhibit anti-Id responses. The first is cocrosslinking of BCR with FcγRIIB on B cells, leading to inhibition of B cell activation (22, 23) and B cell antigen presentation to T cells (24, 25). However, mice lacking FcγRIIB were also nonresponders to Id borne by all IgG subclasses, including IgG3, demonstrating that FcγRIIB alone is not responsible. Furthermore, it is unlikely that significant coligation of FcγRIIB and anti-Id BCR remained 80–90 days after the last injection of IgG, yet tolerance to Id of IgM was still intact. The second way is that anti-Id B cells that had bound Id of IgG Ab were killed by Ab-dependent cell-mediated cytotoxicity (ADCC), as exemplified by the efficient in vivo elimination of aggressive B cell tumors mediated by IgG Abs (26, 27). ADCC could account for the negative response to Id of mice given IgG1 IdA.01 up to 80–90 days before or 3–21 days after challenge with IgM. In this regard, we found that FcRγ−/− mice that are deficient in FcRI and III still failed to respond to both IgG2a- and IgG3-switch variants of Id3. Because FcγRIII is the only known FcγR of NK cells (28), a principal cell type involved in ADCC, the result suggested that NK cell-mediated cytotoxicity was not substantially involved. ADCC mediated by FcγRIIB expressed on monocytes/macrophages of FcRγ−/− mice also seems unlikely, because FcγRIIB inhibits calcium mobilization and the calcium-dependent ADCC function (27, 29). Our finding that IgG3-Id3 was nonimmunogenic and induced tolerance to immunization challenge with IgM-Id3 opens the possibility that alternative Fcγ receptors, as yet uncharacterized, were involved (30, 31). On this note, a family of human Fc receptor homologs with preferential B cell expression has recently been described (32).
The present observations on IgA-Id3, together with a previous study of adjuvant-free myeloma protein IgA (315) (7), indicate that, in the absence of added adjuvant, Ids are dramatically more immunogenic when borne by mIgA (and IgM) than by pIgA. This finding is surprising in view of the similarity in higher order structure and transport of pIgA and IgM (20), and it is not due to chemical treatment (reduction/alkylation), because natural mIgA Id3 too was immunogenic (Fig. 1C). In the human, the transferrin receptor has recently been shown to bind mIgA1 better than pIgA1 (33), but it is not known whether the murine transferrin receptor binds IgA. Possible involvement of the receptor for both IgM and IgA (Fcα/μR) (34, 35) cannot explain the differential immunogenicity of Id borne by pIgA and IgM. Finally, effector cells bearing an alternative, as yet unknown, IgA Fc receptor might attack anti-Id B cells much more efficiently by way of bound pIgA than mIgA. When mixed with complete Freund's adjuvant, however, polymeric forms of an IgA myeloma protein (S117) also elicited anti-Id Ab responses (36), perhaps because complete Freund's adjuvant lowers the threshold for Th cell activation and decreases Th cell apoptosis (37).
Because humoral responses to Ids of particular adjuvant-free mAbs depend on Th cells (3–7), probably Th2 (8), our study leaves open the possibility that APCs such as B cells (38, 39) or immature dendritic cells (DCs) (40) presented Ids borne by IgG and pIgA in a nonimmunogenic or tolerogenic form to Th cells. In that case, B cells would receive signal 1 from their anti-Id BCR in the absence of T cell help (signal 2). According to the two-signal model of acquired immunity (41; reviewed in ref. 42), this reception of signal 1 should lead to B cell tolerance. The observation that mice injected with hapten-conjugated polyclonal mouse IgG became tolerant to the hapten (43, 44) is compatible with this scenario, although ADCC was not ruled out. However, this model fails to explain why supposed absence of signal 2 was limited to IgG and pIgA. Perhaps pepsin treatment of IgG and subtle denaturation of IgM, mIgA, and IgE during purification led to “altered self” with acquisition of immunogenicity. Although a significant role for “altered self” cannot be excluded, we think it is unlikely because (i) the Ids of the present and previous (8) studies were purified under gentle conditions, (ii) only ≈5% of 73 purified IgM anti-TNP mAbs were immunogenic (8), (iii) we are not aware that natural mIgA has been shown to be more susceptible to denaturation than pIgA, and (iv) denatured Igs are also produced during in vivo catabolism. The two-signal model also does not easily explain why the mice were nonresponders to IdA.01 injected as a mixture of IgG and IgM species, because in this situation IdA.01 borne by IgM should activate IdA.01-specific Th that provided signal 2 for the B cells. On this note, an emerging notion is that proteins captured and processed by DCs in the steady state, i.e., immature DCs, are tolerogenic because they delete T cells or induce suppressor T cells (40). Applied to our data this hypothesis, in common with the two-signal model, does not easily account for the differential effect of CH isotypes on Id immunogenicity. Furthermore, on the basis of previous data, we have argued that IdA.01 IgM can elicit Th2 immunity without an adjuvant (8), and others have observed that MyD88-deficient mice with defective Toll function can make ovalbumin-specific IgG1 after immunization with ovalbumin in complete Freund's adjuvant (45). Thus, immature or resting DCs may indeed support responses of Th type 2 cells. If that is so, it is of obvious importance for physiological Id immunity.
It is relevant to consider our results in light of Jerne's Id network theory of the immune system (46). We found a positive response when anti-Id3 paratopes (i.e., antigen-binding sites) on BCRs recognized free IgE Id3. Others have reported that when a public Id on BCRs was recognized by the paratopes of free antipublic Id IgE, the Id was suppressed (47). These opposite results agree with Jerne's tentative proposal that stimulation occurs when paratopes on receptor molecules recognize Ids on free Abs, and repression ensues in the reverse situation when Ids of receptor molecules are recognized by paratopes of free Abs (46). However, chances are that the IgE antipublic Id mAb of Muller and Rajewsky was nonimmunogenic. If so, it differed radically from IgE-Id3, which might account for its repression of Id. We propose that a positive or negative outcome of Id-based interactions between BCRs and free Abs is determined by a combination of the isotype of the free mAbs and the immunogenicity of their Ids. Accordingly, free Abs that have (i) immunogenic Ids, (ii) a permissive isotype (IgM, mIgA, and IgE), and (iii) paratopes that recognize a given Id will augment expression of that Id. On the basis of our previous studies (4–6, 8, 48), we hypothesize that in this situation the immunogenic anti-Id set augments expression of their target Id by inducing collaboration between target Id-bearing B cells and Th specific for V-region peptides derived from the anti-Id set. On the other hand, treatment of neonatal or adult mice with free anti-Id Abs bearing a dominant nonpermissive isotype (IgG, pIgA) should lead to long-lasting loss of expression of that Id, in agreement with published data for IgG (reviewed in ref. 49).
Although the role of Ids as endogenous immunogens still remains to be established, they are significant for therapeutic application of Abs. The present work suggests that class switching to IgG and pIgA may serve to dampen Id immunity, which may be advantageous.
Acknowledgments
We thank R. H. Schwartz for comments on the manuscript. The work was supported by the Norwegian Cancer Society and Aakre's Fund.
Abbreviations
- Ag
antigen
- BCR
B cell Ag receptor
- Id
idiotype
- mIgA
monomeric IgA
- pIgA
polymeric IgA
- TNP
2,4,6-trinitrophenyl
- Th
T helper
- ADCC
Ab-dependent cell-mediated cytotoxicity
References
- 1.Sirisinha S, Eisen H N. Proc Natl Acad Sci USA. 1971;68:3130–3135. doi: 10.1073/pnas.68.12.3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sakato N, Eisen H N. J Exp Med. 1975;141:1411–1426. doi: 10.1084/jem.141.6.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Janeway C A, Jr, Sakato N, Eisen H N. Proc Natl Acad Sci USA. 1975;72:2357–2360. doi: 10.1073/pnas.72.6.2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jørgensen T, Hannestad K. Eur J Immunol. 1977;7:426–431. doi: 10.1002/eji.1830070705. [DOI] [PubMed] [Google Scholar]
- 5.Jørgensen T, Hannestad K. Nature (London) 1980;288:396–397. doi: 10.1038/288396a0. [DOI] [PubMed] [Google Scholar]
- 6.Jørgensen T, Hannestad K. J Exp Med. 1982;155:1587–1596. doi: 10.1084/jem.155.6.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kristoffersen G, Hannestad K. Eur J Immunol. 1988;18:1785–1790. doi: 10.1002/eji.1830181120. [DOI] [PubMed] [Google Scholar]
- 8.Reitan S K, Hannestad K. Eur J Immunol. 2001;31:2143–2153. doi: 10.1002/1521-4141(200107)31:7<2143::aid-immu2143>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 9.Iverson G M, Dresser D W. Nature (London) 1970;227:274–276. doi: 10.1038/227274a0. [DOI] [PubMed] [Google Scholar]
- 10.Reitan S K, Hannestad K. Eur J Immunol. 1995;25:1601–1608. doi: 10.1002/eji.1830250620. [DOI] [PubMed] [Google Scholar]
- 11.Hannestad K, Andreassen K, Kristoffersen G. Eur J Immunol. 1992;22:321–327. doi: 10.1002/eji.1830220206. [DOI] [PubMed] [Google Scholar]
- 12.Spira G, Bargellesi A, Teillaud J L, Scharff M D. J Immunol Methods. 1984;74:307–315. doi: 10.1016/0022-1759(84)90298-9. [DOI] [PubMed] [Google Scholar]
- 13.Mizuta T R, Suzuki N, Shimizu A, Honjo T. J Biol Chem. 1991;266:12514–12521. [PubMed] [Google Scholar]
- 14.Ravetch J V, Clynes R A. Annu Rev Immunol. 1998;16:421–432. doi: 10.1146/annurev.immunol.16.1.421. [DOI] [PubMed] [Google Scholar]
- 15.Gilliland L K, Walsh L A, Frewin M R, Wise M P, Tone M, Hale G, Kioussis D, Waldmann H. J Immunol. 1999;162:3663–3671. [PubMed] [Google Scholar]
- 16.Delacroix D, Malburny G, Vaerman J P. Eur J Immunol. 1985;15:893–898. doi: 10.1002/eji.1830150906. [DOI] [PubMed] [Google Scholar]
- 17.Koertge T E, Butler J E. Scand J Immunol. 1986;24:567–574. doi: 10.1111/j.1365-3083.1986.tb02172.x. [DOI] [PubMed] [Google Scholar]
- 18.Dempsey P W, Allison M E D, Akkaraju S, Goodnow C C, Fearon D T. Science. 1996;271:348–350. doi: 10.1126/science.271.5247.348. [DOI] [PubMed] [Google Scholar]
- 19.de Fougerolles A R, Batista F, Johnsson E, Fearon D T. Eur J Immunol. 2001;31:2189–2199. doi: 10.1002/1521-4141(200107)31:7<2189::aid-immu2189>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 20.Frazer J K, Capra J D. In: Fundamental Immunology. Paul W E, editor. Philadelphia: Lippincott; 1999. pp. 37–74. [Google Scholar]
- 21.Campbell K A, Lees A, Finkelman F D, Conrad D H. Eur J Immunol. 1992;22:2107–2112. doi: 10.1002/eji.1830220822. [DOI] [PubMed] [Google Scholar]
- 22.Phillips N E, Parker D C. J Immunol. 1984;132:627–632. [PubMed] [Google Scholar]
- 23.Ravetch J V, Lanier L L. Science. 2000;290:84–89. doi: 10.1126/science.290.5489.84. [DOI] [PubMed] [Google Scholar]
- 24.Minskoff S A, Matter K, Mellman I. J Immunol. 1998;161:2079–2083. [PubMed] [Google Scholar]
- 25.Wagle N M, Faassen A E, Kim J H, Pierce S K. J Immunol. 1999;162:2732–2740. [PubMed] [Google Scholar]
- 26.Kaminsky M S, Kitamura K, Maloney D G, Campbell M J, Levy R. J Immunol. 1986;136:1123–1130. [PubMed] [Google Scholar]
- 27.Clynes R A, Towers T L, Presta L G, Ravetch J V. Nat Med. 2000;6:443–446. doi: 10.1038/74704. [DOI] [PubMed] [Google Scholar]
- 28.Ravetch J V, Kinet J-P. Annu Rev Immunol. 1991;9:457–492. doi: 10.1146/annurev.iy.09.040191.002325. [DOI] [PubMed] [Google Scholar]
- 29.Ravetch J V, Bolland S. Annu Rev Immunol. 2001;19:275–290. doi: 10.1146/annurev.immunol.19.1.275. [DOI] [PubMed] [Google Scholar]
- 30.Yuan R, Clynes R, Oh J, Ravetch J V, Scharff M D. J Exp Med. 1998;187:641–648. doi: 10.1084/jem.187.4.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fossati-Jimack L, Ioan-Facsinay A, Reininger L, Chicheportiche Y, Watanabe N, Saito T, Hofhuis F, Gessner J, Schiller C, Schmidt R, et al. J Exp Med. 2000;191:1293–1302. doi: 10.1084/jem.191.8.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Davis R S, Wang Y-H, Kubagawa H, Cooper M D. Proc Natl Acad Sci USA. 2001;98:9772–9777. doi: 10.1073/pnas.171308498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moura I C, Centelles M N, Arcos-Fajardo M, Malheiros D M, Collawn J F, Cooper M D, Monteiro R C. J Exp Med. 2001;194:417–426. doi: 10.1084/jem.194.4.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shibuya A, Sakamoto N, Shimizu Y, Shibuya K, Osawa M, Hiroyama T, Eyre H J, Sutherland G R, Endo Y, Fujita T, et al. Nat Immunol. 2000;1:441–446. doi: 10.1038/80886. [DOI] [PubMed] [Google Scholar]
- 35.Sakamoto N, Shibuya K, Shimizu Y, Yotsumoto K, Miyabayashi T, Sakano S, Tsuji T, Nakayama E, Nakauchi H, Shibuya A. Eur J Immunol. 2001;31:1310–1316. doi: 10.1002/1521-4141(200105)31:5<1310::AID-IMMU1310>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 36.Seppälä I J T, Eichmann K. Eur J Immunol. 1979;9:243–250. doi: 10.1002/eji.1830090314. [DOI] [PubMed] [Google Scholar]
- 37.Pape K A, Khoruts A, Mondino A, Jenkins M K. J Immunol. 1997;159:591–598. [PubMed] [Google Scholar]
- 38.Eynon E E, Parker D C. J Exp Med. 1992;175:131–138. doi: 10.1084/jem.175.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fuchs E J, Matzinger P. Science. 1992;258:1156–1159. doi: 10.1126/science.1439825. [DOI] [PubMed] [Google Scholar]
- 40.Steinman R M, Nussenzweig M C. Proc Natl Acad Sci USA. 2002;99:351–358. doi: 10.1073/pnas.231606698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bretscher P, Cohn M. Science. 1970;169:1042–1049. doi: 10.1126/science.169.3950.1042. [DOI] [PubMed] [Google Scholar]
- 42.Schwartz R H. In: Fundamental Immunology. Paul W E, editor. Philadelphia: Lippincott; 1999. pp. 701–739. [Google Scholar]
- 43.Havas H F. Immunology. 1969;17:819–829. [PMC free article] [PubMed] [Google Scholar]
- 44.Golan D T, Borel Y. J Exp Med. 1971;134:1046–1061. doi: 10.1084/jem.134.4.1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schnare M, Barton G M, Holt A C, Takeda K, Akira S, Medzhitov R. Nat Immunol. 2001;2:947–950. doi: 10.1038/ni712. [DOI] [PubMed] [Google Scholar]
- 46.Jerne N K. Ann Inst Pasteur Immunol. 1974;125C:373–389. [PubMed] [Google Scholar]
- 47.Müller C, Rajewsky K. J Exp Med. 1984;159:758–772. doi: 10.1084/jem.159.3.758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hannestad K, Kristoffersen G, Briand J P. Eur J Immunol. 1986;16:889–893. doi: 10.1002/eji.1830160803. , and correction (1986) 16, 1470. [DOI] [PubMed] [Google Scholar]
- 49.Kearney J F. In: Fundamental Immunology. Paul W E, editor. New York: Raven; 1993. pp. 887–902. [Google Scholar]