Abstract
Cervical cancer remains a significant public health challenge, disproportionately affecting women in low- and middle-income countries (LMICs). Persistent infection with high-risk types of human papillomavirus (HPV), particularly HPV16 and HPV18, is the central cause of cervical carcinogenesis, driven by the viral oncoproteins E6 and E7, which disrupt the host tumor suppressors p53 and retinoblastoma protein (pRb). Advances in molecular understanding have catalyzed effective primary and secondary prevention strategies. Prophylactic HPV vaccination, especially the nonavalent formulation, has demonstrated high efficacy in reducing HPV infections and cervical precancer. Concurrently, HPV deoxyribonucleic acid (DNA) testing, self-sampling, and screen-and-treat protocols are transforming screening paradigms, particularly in resource-limited settings. However, global disparities in vaccine access, screening coverage, and health infrastructure persist, impeding progress toward the World Health Organization’s (WHO) 90–70–90 elimination targets. By synthesizing recent advances in virology, prevention strategies, and implementation innovations, such as therapeutic vaccines, artificial-intelligence (AI)-driven diagnostics, and mobile health solutions, this review sheds light on their potential to narrow these equity gaps.
Keywords: human papillomavirus, cervical cancer, HPV vaccination, screening and prevention, global health disparities
1. Introduction
Cervical cancer remains a major global public health challenge. In 2022, approximately 660,000 new cases and 350,000 deaths were reported worldwide, making it the fourth most common cancer and cancer-related cause of death among women [1,2,3]. Alarmingly, nearly 90% of new cervical cancer cases occur in low- and middle-income countries (LMICs), largely due to inequities in access to vaccination, screening, and treatment [3]. In regions with an elevated prevalence of human immunodeficiency virus (HIV), particularly sub-Saharan Africa, women living with HIV are up to six times more likely to develop cervical cancer, underscoring the interaction of biological and social determinants [3].
At the heart of cervical cancer etiology is human papillomavirus (HPV). Over 99% of cases are linked to persistent infection with high-risk HPV (hrHPV) strains, notably HPV16 and HPV18, which together contribute to approximately 70% of invasive cancers [4,5,6,7]. While HPV infection is exceedingly common, showing global prevalence rates ranging from 11–12% overall, it is typically transient, meaning that viral DNA becomes undetectable, and cytologic abnormalities resolve without clinical intervention. Clearance is achieved through a combination of innate immune activation (including interferon release, natural killer cell activity, and local cytokine production) and adaptive immunity, particularly the development of type-specific HPV antibodies and cytotoxic T lymphocytes targeting early viral proteins (E1, E2, E6, E7). Only a fraction of persistent infections progress into precancerous lesions and, over a decade or two, invasive carcinoma [2,8,9,10,11,12]. Oncoproteins E6 and E7 play pivotal roles in this transformation by inactivating tumor suppressor p53 and retinoblastoma protein; disrupting cell cycle control; and promoting malignant progression [13,14,15,16,17,18]. Figure 1 illustrates a conceptual mind map of HPV’s process in the human organism.
Figure 1.
HPV: pathogenesis, prevention, and global health challenges. Conceptual mind map (figure created in BioRender). This mind map encapsulates the biological, clinical, and societal aspects of HPV. It not only covers viral pathogenesis and its link to cervical cancer but also integrates modern prevention strategies, such as vaccination and screening, while acknowledging global challenges like disparities in healthcare access and education. It is a valuable tool for health professionals, educators, and policymakers aiming to understand and communicate the comprehensive landscape of HPV control and cervical cancer elimination.
Understanding this pathogenesis underpins crucial prevention strategies. Primary prevention through prophylactic HPV vaccination, particularly against types 16 and 18, has been transformative [19,20,21]. Vaccination programs are now expanding to include boys, following evidence that gender-neutral immunization enhances herd immunity and addresses HPV-associated cancers in men [20,21,22,23,24].
Secondary prevention hinges on early detection via cervical screening. Traditional cytology (pap smears) and HPV deoxyribonucleic acid (DNA) testing has reduced its incidence and mortality by up to 80% in high-income settings. However, access remains limited in resource-poor contexts. Innovative low-cost technologies, such as HPV self-sampling, point-of-care HPV testing, and portable visualization devices, have shown promise in bridging these gaps. The World Health Organization (WHO) now advocates a streamlined screen-and-treat approach tailored to low-resource settings to maximize early intervention and reduce attrition [25,26,27,28,29,30].
Despite these advances, awareness remains a critical barrier. Surveys indicate that 40% of women globally are unaware that HPV causes most cervical cancers—women lacking this awareness are less likely to undergo screening. Moreover, HPV-vaccinated individuals are, paradoxically, more likely to adhere to screening recommendations, perhaps reflecting broader engagement in preventive health behaviors [31,32,33,34].
Lastly, in 2020, the WHO launched the Global Strategy for Cervical Cancer Elimination, setting the “90–70–90” targets to be achieved by 2030: 90% of girls fully vaccinated with an HPV vaccine by age 15; 70% of women screened with a high-performance test at ages 35 and 45; and 90% of women identified with cervical disease receiving the appropriate treatment [35]. Mathematical modeling in countries like China suggests that reaching these targets, especially with adult catch-up vaccination, could drive elimination by mid-century. Yet sustained effort is needed to overcome vaccine supply shortages, screening infrastructure deficits, and socio-cultural resistance [27,35,36].
2. Epidemiology
2.1. Global Incidence and Mortality
Cervical cancer remains the fourth most commonly diagnosed cancer and the fourth leading cause of cancer death in women globally. Importantly, approximately 90% of these deaths occur in LMICs, highlighting profound global disparities [3,4,37,38,39]. This surge reflects rapid population growth in high-burden LMICs, persistently low HPV vaccination and screening coverage, high HIV co-infection rates in some regions, and entrenched socioeconomic and cultural barriers that limit access to preventive care [1,3,40,41].
2.2. Regional and National Trends
Incidence and mortality rates show stark regional variation. Sub-Saharan Africa bears the highest burden, followed by Central America and Southeast Asia. In contrast, North America and Western Europe maintain a relatively low incidence due to established screening and vaccination programs. In the United States (US), the age-adjusted incidence is around 7.7 per 100,000 women/year, with a death rate of 2.2 per 100,000 women/year based on Surveillance, Epidemiology, and End Results Program (SEER) data. The predictions for 2025 include 13,360 new cases and 4320 deaths. Notably, the highest incidence occurs among women aged 35–44, and more than 20% of cases involve women over 65 [3,42,43,44].
Longitudinal US data (1975–2018) show annual declines in its incidence by about 1.9% per year, slowing to −0.5% annually post-2006, while mortality has decreased by ~1% yearly. However, the incidence of distant-stage squamous cell carcinoma is rising (~1.1% per year), indicating potential delays in diagnosis [10,37,45,46,47].
2.3. HPV Prevalence and High-Risk Types
The prevalence of hrHPV varies geographically, reflecting population immunity, sexual behavior, and co-factors. Regionally, its prevalence is highest in sub-Saharan Africa (24%), followed by Latin America and the Caribbean (16%) and Eastern Europe and Southeast Asia (14% each) [8,42,48].
The most common hrHPV genotypes detected in the general female population are HPV16 and HPV18, which together account for about 70% of invasive cervical cancer cases worldwide [46,49,50].
In a large cohort of over 111,000 women undergoing primary HPV screening, hrHPV was detected in approximately 14% of participants—consistent with the broader range of 14–18% depending on assay sensitivity and genotyping depth [51,52].
2.4. Age-Specific and Histology Patterns
HPV prevalence exhibits a U-shaped age curve: peaking in younger women (<25 years), declining in mid-adulthood, and occasionally rising again post-menopause—likely due to persistent infection or cohort effects. Conversely, the incidence of cervical cancer peaks between ages 35 and 44, with a median age at diagnosis near 50 [3,37,42,43,47,53].
Histologically, squamous cell carcinoma (SCC) remains the dominant subtype, comprising ~70–80% of all invasive cervical cancers. Adenocarcinomas are increasing in many high-income regions, potentially due to the screening methods being less effective at detecting glandular precancers. In the U.S., although the overall incidence of SCC has decreased, distant-stage SCC has risen [43,45,54,55].
2.5. Risk Factors Beyond HPV
While hrHPV is necessary for cervical cancer development, additional co-factors influence its progression [3,41,42,56,57,58,59,60]:
HIV co-infection: Women living with HIV (WLWH) have an up to six-fold higher risk of cervical cancer, partly due to compromised immunity and hrHPV persistence; globally, an estimated 5% of all cervical cancer cases are attributable to HIV co-infection;
Sexual behavior: Early sexual debut, a higher number of partners, and the infection status of partners increase hrHPV acquisition;
Smoking: Tobacco use contributes independently to cervical neoplasia progression;
Immunosuppression: Aside from HIV, medical immunosuppression (e.g., transplant, steroids) raises its risk;
Socioeconomic factors: Poverty, limited access to screening/vaccines, and education deficits amplify its risk, especially in marginalized communities.
Moreover, further co-factors that modulate the progression to high-grade lesions and invasive cancer have been mentioned, such as genetic susceptibility, hormonal influences, nutritional deficiencies, co-infections, and microbiota alterations [61,62,63,64,65,66,67,68,69,70,71,72,73,74]:
Human leukocyte antigen (HLA) polymorphisms: Certain human leukocyte antigen variants may yield proteins with a lower affinity for HPV antigens, impeding immune clearance and increasing persistence risk;
The TP53 codon 72 polymorphism: A variant in TP53 (P72R) has been linked to heightened vulnerability; studies report that the Arginine/Arginine (Arg/Arg) genotype is associated with increased risk of HPV-associated cervical cancer;
Long-term oral contraceptive (OC) use: Among women with hrHPV infections, OC use over 5 years might double to quadruple their cervical cancer risk;
High parity: A meta-analysis found that women with high parity (many full-term pregnancies) have significantly elevated odds of developing cervical cancer compared to that in low-parity women;
Lower levels of vitamins A, C, E, and folate have been associated with a higher risk of cervical dysplasia and cancer, highlighting the role of antioxidant defenses in modifying HPV-related progression;
Chlamydia trachomatis co-infection: Meta-analyses reveal that women with concurrent HPV and Chlamydia trachomatis infection have a substantially higher risk of cervical cancer;
The depletion of protective Lactobacillus species and overrepresentation of anaerobes (e.g., Gardnerella, Prevotella, Sneathia) have been associated with higher rates of hrHPV infection and progression to precancer/cancer. Metagenomic studies observed a correlation between such dysbiosis and a higher severity of cervical lesions;
An elevated vaginal pH (>5), often accompanying microbial shifts, has also been linked to a 10–20% increase in HPV positivity risk in premenopausal women.
3. Virology and Pathogenesis
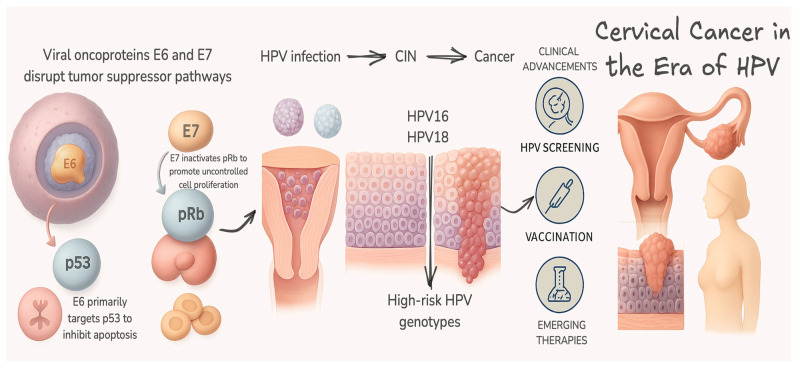
Human papillomaviruses (HPVs) are small, non-enveloped, double-stranded DNA viruses (~8000 bp) belonging to the Papillomaviridae family. Their icosahedral capsid comprises 72 L1 pentamers and accessory L2 proteins, facilitating epithelial tropism and immune evasion, particularly via capsid stability in cutaneous and mucosal environments [75,76,77,78,79,80]. To visually consolidate the interplay between viral oncogenesis and clinical intervention, Figure 2 provides an integrated schematic of HPV-driven cervical carcinogenesis and its translational implications for screening, prevention, and future therapies.
Figure 2.
Molecular and clinical overview of human papillomavirus (HPV)-induced cervical carcinogenesis (figure created in BioRender). This schematic integrates molecular and clinical perspectives on HPV-mediated cervical cancer. Upon infection with high-risk types of HPV (16 and 18), the viral oncoproteins E6 and E7 are expressed and disrupt key tumor suppressor pathways. E6 promotes the degradation of p53, inhibiting apoptosis, while E7 inactivates retinoblastoma protein (pRb), driving uncontrolled cell proliferation. These alterations lead to the accumulation of genetic damage and progression from cervical intraepithelial neoplasia (CIN) to invasive cervical cancer. Clinically, understanding these mechanisms informs prevention and management strategies: HPV vaccination, high-performance screening, and novel therapeutic approaches targeting E6/E7 are reshaping cervical cancer control. Linking molecular pathways to public health interventions underscores the importance of translating biological insights into global elimination efforts.
To consolidate the significant themes of this review, Table 1 provides an integrated overview of the biological, clinical, and preventive dimensions of HPV-driven cervical cancer.
Table 1.
A summary of key molecular, clinical, and public health elements in HPV-related cervical cancer and its prevention. [1,42,81,82,83,84,85,86,87].
| Domain | Component | Details/Significance |
|---|---|---|
| Etiology | High-Risk HPV Types | HPV16 and HPV18 account for ~70% of cervical cancer cases worldwide |
| Transmission | Primarily through sexual contact; peak incidence shortly after sexual debut | |
| Molecular Mechanism | E6 Oncoprotein | Binds E6AP to degrade p53, impairing apoptosis and DNA repair |
| E7 Oncoprotein | Inactivates pRb, driving cell cycle progression and genomic instability | |
| Natural History | Infection Progression | Mostly clears spontaneously; persistent infection may lead to CIN and invasive cancer |
| Co-Factors | Immunosuppression (e.g., HIV), smoking, early sexual activity, parity, OC use | |
| Prevention | Prophylactic Vaccines | Bivalent, quadrivalent, and nonavalent |
| WHO Vaccine Target | 90% of girls fully vaccinated by age 15 by 2030 | |
| Screening | Primary Testing Methods | Pap smear, HPV DNA test, co-testing, VIA |
| Emerging Tools | Self-sampling, methylation biomarkers, AI-enhanced cytology | |
| Global Disparities | LMIC Burden | 90% of cervical cancer deaths occur in LMICs |
| Access Gaps | Unequal access to vaccines, trained personnel, and diagnostic infrastructure | |
| Future Directions | Therapeutic Vaccines | Target E6/E7 in persistent HPV or CIN2/3; promising in early trials |
| Elimination Goals | WHO: <4 cases per 100,000 women/year (global elimination threshold) |
3.1. HPV Classification: Low-Risk vs. High-Risk
HPVs infect stratified squamous epithelia and are categorized into low-risk (lrHPV) and hrHPV based on their oncogenic potential. Low-risk types (e.g., HPV6 and HPV11) typically cause benign lesions like genital warts and low-grade dysplasia. However, rare cases of squamous cell carcinoma associated with HPV6/11 have been documented, though without evidence of viral genome integration. In contrast, high-risk types (notably HPV 16, 18, 31, 33, 45, 52, and 58) account for more than 70% of invasive cervical cancers, with HPV16 and HPV18 being most prevalent [16,88,89,90,91].
hrHPV types, most notably HPV16 and HPV18, are strongly linked to cervical cancer and other anogenital and oropharyngeal malignancies. A subset of types classified as “possible high-risk” is less frequently associated with cancer but remains under investigation due to emerging evidence. In contrast, low-risk types such as HPV6 and HPV11 are primarily associated with benign conditions, including genital warts and low-grade cervical lesions. This classification underpins both vaccine development and risk-based screening strategies [92,93,94,95]. To provide a clearer understanding of the oncogenic potential of different HPV genotypes, Table 2 categorizes HPV types by their associated clinical risk and disease outcomes.
Table 2.
| Category | HPV Types |
|---|---|
| Group 1 carcinogens | 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59 |
| (carcinogenic to humans) | |
| Considered high-risk | |
| Group 2A carcinogens | 68 |
| (probably carcinogenic to humans) | |
| Group 2B carcinogens | 26, 30, 34, 53, 66, 67, 69, 70, 73, 82, 85, 97 |
| (possibly carcinogenic to humans) | |
| Group 3 | 6, 11 |
| (low-risk) |
3.2. Infection Mechanisms and the Viral Life Cycle
HPV infections initiate in micro-abrasions, exposing the basal keratinocytes. L1 and L2 mediate virus attachment and internalization via sulfated glycans and α6-integrins, followed by endosomal trafficking and nuclear entry. The viral genome is maintained at low copy numbers in the basal cells and replicates episomally during early infection. As infected keratinocytes differentiate, late gene expression (L1/L2) leads to virion assembly and shedding. Occasionally, HPV DNA integrates into the host genome, disrupting regulatory elements like the E2 gene and promoting oncogene overexpression [91,98,99,100].
3.3. Oncoproteins E6 and E7: Central Drivers of Transformation
The oncogenic potential of hrHPV is primarily mediated by the early proteins E6 and E7 [99].
E6 is a ~151-amino-acid zinc-finger protein that forms a ternary complex with host E3 ubiquitin ligase E6AP, targeting p53 for proteasomal degradation. This impairs the DNA damage response and apoptosis, allowing for the survival and proliferation of mutated cells. E6 also activates human telomerase (hTERT); interrupts PDZ-domain-containing cell polarity proteins; and dysregulates the cell cycle and differentiation [10,16,91].
E7 binds retinoblastoma protein (pRb), releasing Early Region 2 binding factor (E2F) transcription factors and promoting S-phase progression. Loss of pRb leads to unchecked cell division and genomic instability. E7 also modulates DNA damage pathways to facilitate viral replication and contributes to epigenetic reprogramming [91,101,102].
E6/E7 synergism leads to the evasion of cell cycle checkpoints, apoptosis resistance, and genomic instability—hallmarks of carcinogenesis. E5, a minor oncoprotein, further supports these processes by activating growth factor receptors [101,102,103].
The integration of hrHPV DNA into the host genome is a critical turning point toward malignancy. Disruption of the E2 regulatory region increases the expression of E6/E7, reinforcing oncogenesis. Structural studies confirm that the long control region (LCR) is altered in integrated genomes, boosting viral transcription [16,88,91,98,104].
3.4. Beyond E6/E7: Additional Viral and Host Factors
Recent studies highlight the emerging roles of other viral genes (like E5) and host genomic and epigenetic changes in tumor progression. Variants of HPV16 also differ in their carcinogenicity, with non-European strains showing higher risk profiles. E6/E7 expression levels correlate with gene clusters associated with aggressive phenotypes and poorer prognoses [16,102,105].
HPV16 and HPV18 are etiologically linked to the development of several epithelial malignancies, most notably cervical cancer. Their oncogenic potential is primarily driven by the coordinated actions of three early viral proteins (E5, E6, and E7), which subvert key host cellular pathways to promote viral persistence, immune evasion, and uncontrolled cell proliferation. E6 targets the tumor suppressor p53 for degradation, E7 inactivates pRb to deregulate the cell cycle, and E5 enhances growth factor signaling while impairing antigen presentation. Table 3 summarizes the primary molecular targets, biological effects, and phenotypic outcomes associated with these viral proteins [99,106,107].
Table 3.
| Viral Protein | Cellular Target(s) | Biological Effect | Resulting Phenotype |
|---|---|---|---|
| E5 | EGFR, MHC-I | Enhances growth factor signaling, downregulates antigen presentation | Immune evasion, cell proliferation |
| E6 | p53 | Promotes ubiquitin-mediated degradation of p53 | Inhibits apoptosis, impairs DNA repair |
| E7 | pRb | Disrupts the pRb-E2F complex | Uncontrolled cell cycle progression |
MHC-I: Major Histocompatibility Complex Class I; EGRF: epidermal growth factor receptor.
4. Natural History and Screening
4.1. The Course of HPV Infection: Transient Versus Persistent
Most hrHPV infections are transient and are cleared by the immune system within 12–24 months in approximately 70–90% of women. A 2024 global meta-analysis on the genotype-specific persistence of HPV revealed that around half of persistent infections resolve within two years, validating the rationale for a 24-month surveillance approach before interventions are initiated. The natural history model of HPV infection is now understood to encompass latent states with potential reactivation, challenging the idea that a negative test equals the assumption of an all-clear [2,111,112,113,114].
In contrast, a smaller subset of infections becomes persistent, especially when involving hrHPV types, immunosuppression (e.g., HIV), smoking, or co-existing sexually transmitted infections (STIs). This persistent infection is the key precursor to cervical intraepithelial neoplasia (CIN) lesions and eventual malignant transformation [115,116,117].
4.2. Cervical Intraepithelial Neoplasia
CIN represents histopathological steps in cervical carcinogenesis [111,118,119,120,121,122,123,124]:
CIN1 (low-grade dysplasia): Often transient and usually regresses spontaneously.
CIN2/3 (high-grade dysplasia): A risk of progression to invasive carcinoma—natural history data emphasize close monitoring of CIN2 in young women due to frequent regression, while CIN3 is generally treated promptly due to a higher progression risk. Management aligns with the guidelines, balancing the risks of overtreatment versus progression to cancer.
4.3. Post-Treatment Surveillance
Effective post-treatment surveillance is essential for women treated for CIN2+ lesions, as these individuals remain at an elevated risk of residual or recurrent disease. hrHPV DNA testing, either alone or in combination with cytology (co-testing), is recommended due to its superior sensitivity compared to that of cytology alone, supporting its integration into follow-up protocols extending over a minimum of two years. To abridge, the natural history of hrHPV typically involves transient infection, but persistent hrHPV is the critical pivot toward CIN and cancer. Accurate detection of CIN2/3 via HPV DNA, liquid-based cytology (LBC), or visual inspection with acetic acid (VIA) remains essential. The international guidelines are shifting toward primary HPV testing with co-testing or LBC triage, and self-sampling models are increasing reach. Post-treatment, hrHPV testing ensures the early detection of recurrence, optimizing the long-term outcomes [125,126,127,128,129].
5. Prevention Strategies
5.1. HPV Vaccination
Prophylactic HPV vaccination remains the most effective primary prevention method against cervical cancer. As of 2025, 148 countries (76% of WHO member states) have included HPV vaccines in their national immunization schedules [21,130,131].
Three vaccines are widely used [132,133,134]:
Bivalent (HPV-16/18);
Quadrivalent (HPV-6/11/16/18);
Nonavalent (HPV-6/11/16/18/31/33/45/52/58).
The nonavalent vaccine offers expanded protection and has demonstrated 97.5% efficacy against hrHPV types in a single-dose schedule. Ten-year follow-up data confirm durable immunogenicity and safety for the nonavalent vaccine in adolescents and young adults [135,136,137,138].
Innovations include single-dose schedules, which maintain ≥85% efficacy compared to that of traditional three-dose regimens. While this simplifies implementation and accessibility, global vaccination coverage remains inequitable, with catch-up programs and male inclusion lagging [136].
Table 4 summarizes the principal HPV vaccines available globally, including their valency and dosing recommendations.
Table 4.
| Product (Maker) | HPV Types (Valency) | Label Dosing (Regulatory) |
|---|---|---|
| Gardasil 9 (MSD/Merck) | 6, 11, 16, 18, 31, 33, 45, 52, 58 (9-valent) | Two doses (9–14 y) or three doses (≥15 y/immunocompromised) per label |
| Gardasil/Silgard (MSD)—quadrivalent | 6, 11, 16, 18 (4-valent) | Two doses (9–13 y) or three doses (≥14 y/immunocompromised) per label |
| Cervarix (GSK)—bivalent | 16, 18 (2-valent) | Two doses (9–14 y) or three doses (≥15 y/immunocompromised) per label |
| Cecolin (Innovax, China)—bivalent | 16, 18 (2-valent) | Two doses (9–14 y) or three doses (≥15 y/immunocompromised) per label |
| Walrinva (Zerun/Walvax, China)—bivalent | 16, 18 (2-valent) | Standard 2/3 doses by age |
| CERVAVAC (Serum Institute of India)—quadrivalent | 6, 11, 16, 18 (4-valent) | Indian authorization of 2/3 doses by age |
Table 5 summarizes national surveillance data from countries that have achieved and sustained high HPV vaccine coverage, showing changes in the prevalence of HPV16/18, high-grade cervical lesions, and the incidence of cervical cancer in the decade following program implementation. These examples illustrate the substantial population-level benefits achieved when vaccination is introduced early, delivered before sexual debut, and maintained at high uptake.
Table 5.
Real-world impact of high-coverage HPV vaccination programs on HPV infection, cervical intraepithelial neoplasia, and cervical cancer incidence.
| Country/Setting | Vaccine/Program (Cohorts) | Outcome Measured | Key Real-World Impact | Study Period |
|---|---|---|---|---|
|
England (national) [148] |
bHPV vaccine then qHPV vaccine/9vHPV vaccine; routine at 12–13 y | Cervical cancer; CIN3 | Marked reductions across all deprivation groups; strongest when vaccinated at routine age | From 2006 up to 2020 follow-up |
|
England (surveillance) [149] |
Initially bHPV vaccine (Cervarix) at 12–13 y; changed to qHPV vaccine Gardasil in 2012 (HPV 16/18/6/11) | HPV16/18 infection prevalence | Around a 90% reduction in HPV16/18 in young women offered vaccination | 2010–2020 |
|
Scotland (national) [150] |
bHPV vaccine; high school program | CIN2 and CIN3 | Reduction in pre-invasive cervical disease in vaccinated cohorts | Women aged between 20 and 60 years by 2016 |
|
Sweden (national cohort) [151] |
qHPV vaccine | Invasive cervical cancer | Vaccination associated with substantially lower cervical cancer risk; strongest when vaccinated <17 y | 2006–2017 |
|
Australia (national, school-based) [152] |
qHPV vaccine | HPV-related disease, including high-grade abnormalities | Large population-level reductions in HPV-related outcomes; early decline in cervical abnormalities within 5 years | 2007–2016 |
|
United States (HPV-IMPACT, CDC) [153] |
Mixed vaccines: bHPV vaccine, qHPV vaccine and 9vHPV vaccine | CIN2 and CIN3 (screen-detected) | Among women 20–24 y, CIN2+ ↓79% and CIN3+ ↓80% from 2008 to 2022; has also declined in 25–29 y | 2008–2022 |
|
Denmark (population data) [154] |
National program | HPV-16/18 infection prevalence | HPV16/18 prevalence in vaccinated women fell from 15–17% pre-vaccine to <1% by 2021 | 2017–2024 |
|
Norway (regional, real-world) [155] |
National program | CIN2+/CIN3+ | CIN2+ incidence decreased markedly after initial rise; no cervical cancers recorded in vaccinated cohorts in this series | 2008–2022 |
|
Multi-country meta-analysis [156] |
Programs with high coverage | HPV infections; genital warts; CIN2+ | Countries with multi-cohort, high-coverage programs show larger direct impact and herd effects; significant reductions in HPV infection and CIN2+ | 2014–2018 |
HPV—human papillomavirus; CIN2+—cervical intraepithelial neoplasia grade 2 or higher; qHPV vaccine—quadrivalent HPV vaccine (targets types 6, 11, 16, and 18); bHPV vaccine—bivalent HPV vaccine (targets types 16 and 18); 9vHPV vaccine—nonavalent HPV vaccine (targets types 6, 11, 16, 18, 31, 33, 45, 52, and 58), ↓—decrease.
5.2. Breakthrough Disease After HPV Vaccination
Although prophylactic HPV vaccination markedly reduces the incidence of high-grade cervical lesions and invasive cancer, rare cases of precancer and cancer have been documented in vaccinated individuals. These events typically occur due to one of three mechanisms [157,158]:
Infection with HPV types not included in the administered vaccine;
Vaccination initiated after HPV exposure;
Incomplete vaccination series or an older age at first dose.
There were several documented cases and implications:
A 19-year-old woman developed biopsy-confirmed CIN3 two years after completing the quadrivalent HPV vaccine series; genotyping revealed a non-vaccine HPV type [159]. Similarly, a 33-year-old woman who had received a quadrivalent vaccination in 2006 was later diagnosed with CIN3 [160]. Another report described a 30-year-old woman, vaccinated after sexual debut, who was diagnosed with adenocarcinoma in situ in the cervix—highlighting that vaccination does not treat pre-existing infections [161].
Larger cohort and trial data corroborate that lesions can still emerge in vaccinated populations, albeit at much lower rates. In the Costa Rica HPV Vaccine Trial (years 7–11), vaccinated women experienced a reduction in overall high-grade disease but showed a relative increase in CIN2+/CIN3+ caused by non-vaccine HPV types, consistent with type replacement or unmasking [162]. A Swedish nationwide cohort reported an incidence of invasive cervical cancer of 47 per 100,000 in vaccinated women compared with 94 per 100,000 in unvaccinated women—confirming strong but incomplete protection [151].
Importantly, the timing and completeness of vaccination influence breakthrough risk. Scottish data indicate that one or two doses at ages 12–13 confer measurable protection, whereas those at an older age generally require three doses for the optimal effect; infections and lesions can still occur during the vaccination course, especially if the exposure precedes completion [148,150].
5.3. Screening Programs, Screening Methods, and the Impact of Vaccination
HPV vaccination has significantly altered epidemiological patterns. In vaccinated cohorts aged 20–24 years, reports indicate declines of 79% in CIN2+ and 80% in CIN3+ from 2008 to 2022. Additionally, a nationwide U.S. study showed reduced cervical cancer incidence and mortality in girls aged 15–24 after introducing the vaccine [137,153,163,164].
Screening programs (pap and HPV testing) have similarly reduced the incidence in high-income countries by up to 80%. However, in LMICs, inadequate screening infrastructure contributes substantially to a persistently high disease burden [3,27,40,165].
The conventional pap smear has been the cornerstone of cervical screening since the mid-20th century. One review highlighted that while cytology detected high-grade lesions with fair accuracy, it underperformed in cases with concurrent inflammation or a low viral load, underscoring the limitations in isolation [115,117,166,167,168]. LBC, now the standard (e.g., ThinPrep, SurePath), improves sample adequacy, reduces artifacts, and enables reflex HPV testing [167].
HPV DNA testing directly detects high-risk viral strains and offers higher sensitivity but lower specificity than cytology. In a review, it was reported that randomized trials with HPV testing reduced the detection of CIN2+ both at 6 months and 36 months compared to that with VIA and offered better long-term reassurance. In the US, the United States Preventive Services Task Force (USPSTF) now recommends primary hrHPV testing every 5 years for women aged 30–65, with cytology alone every 3 years for ages 21–29. A 2024 update in England likewise extends HPV-based screening intervals to five years for ages 25–49, shifting to clinician-collected and self-sampling methods [169,170,171,172,173,174,175].
In some settings, co-testing (HPV+cytology) every 5 years continues, offering maximal lesion detection but increased costs and follow-up burden. The detection of hrHPV is often followed by reflex cytology to identify high-risk cases requiring colposcopy [176].
VIA remains a low-cost, single-visit option for LMICs. VIA demonstrates varying sensitivity and efficacy, which may be influenced by the administering healthcare professional. The WHO guidelines recommend integrating VIA with HPV testing or deploying screen-and-treat models for low-resource settings [27,113,166,167,171,176].
Self-collected vaginal swabs for HPV DNA testing are gaining traction: randomized trials show comparable sensitivity to that of clinician-collected samples, increasing screening uptake among underscreened women. Food and Drug Administration (FDA)-approved at-home kits offer promising access improvements, though positive results require an in-clinic follow-up [177,178,179,180]. Self-sampling has emerged as a game-changer in enhancing screening reach. A global review noted that vaginal self-swabs yielded 94.6% sensitivity and 91.6–96.8% specificity for detecting HPV, with costs reduced by 32–48%, particularly benefiting underserved populations. A U.S. intervention using the “3R” behavioral model saw 75% uptake of self-testing among medically underserved women [87,135]. Community-led initiatives, such as education culturally tailored to non-Hispanic Black women, significantly boosted uptake. A scoping review emphasized involving trusted community leaders to effectively engage indigenous, newcomer, and rural populations. In India, Accredited Social Health Activist (ASHA) workers facilitated door-to-door kit delivery under a Reach, Effectiveness, Adoption, Implementation, and Maintenance (RE-AIM) framework, enhancing its feasibility and community acceptance [181,182,183].
5.4. Education and Behavioral Interventions
Education and behavioral strategies are crucial to improving both vaccination and screening uptake [87,181,184,185]:
Social mobilization models, such as the Information–Motivation–Behavioral Skills model, have improved community awareness and empowerment for self-sampling initiatives;
Studies in the U.S. have found that women educated through culturally competent interventions were more likely to engage in self-sampling and referral follow-up;
Awareness barriers persist, as only around 6.5% of some communities had heard of self-sampling, though 75% felt confident in performing it after education;
Digital platforms and artificial intelligence (AI)-enhanced systems are being introduced to prompt eligible individuals, support training, and facilitate follow-up referrals;
Moving forward, scaling universal vaccine coverage, enabling accessible screening, and investing in culturally informed education are essential to advancing toward the WHO target of cervical cancer elimination.
6. Global Disparities and Challenges
6.1. Inequities in Vaccine and Screening Access
Cervical cancer is a stark marker of global health inequity. Nearly 94% of cervical cancer deaths in 2022 occurred in LMICs, where screening and treatment infrastructure is lacking. While HPV vaccination has been introduced in 97% of high-income nations, only 42% of low-income countries have introduced HPV vaccination into their national immunization programs. Although vaccination programs now cover 75 countries with single-dose schedules, many remain off track to meet the WHO’s target of 90% of girls being vaccinated by age 15, a key goal of the “90-70-90” strategy [186,187,188,189].
Similarly, the screening coverage is uneven. Only 70% of women globally receive a high-performance screening test by ages 35 and 45—the second pillar of WHO’s strategy. Most LMICs fall short due to limited infrastructure, funding challenges, and a lack of trained personnel [186,187].
6.2. Socioeconomic, Cultural, and Health System Barriers
Barriers span several dimensions [190,191,192,193,194,195,196,197]:
Low awareness of HPV and its link to cervical cancer is widespread, as seen in Ghana and Venezuela, where knowledge gaps persist even among healthcare personnel;
Cultural barriers and stigma related to sexual health deter vaccine uptake and participation in pelvic exams, especially in conservative communities;
Health system limitations, such as shortages of skilled pathologists and a lack of screening logistics, exacerbate inequities. For instance, Ghana relies heavily on a small number of urban-based pathologists, depriving rural areas of services.
These multifaceted barriers hinder progress toward global elimination targets.
6.3. Innovations Addressing Disparities
Promising strategies aim to close these gaps [30,87,186,187,188,191,198,199,200] include:
Self-sampling for HPV has emerged as a scalable alternative in both high- and low-resource settings;
Self-sampling is cost-effective in LMICs—studies in India and China show that combined annual self-testing and nonavalent vaccination offers superior health and economic outcomes;
Mailed kits in the U.S. safety net significantly boost screening in underserved communities;
The WHO-endorsed screen-and-treat model replaces multiple visits with point-of-care testing and treatment in a single visit—crucial for LMICs.
6.4. The WHO Cervical Cancer Elimination Initiative
Established in 2018 and formalized by the 2020 World Health Assembly, the WHO Cervical Cancer Elimination Initiative established targets of 90% HPV vaccination, 70% screening, and 90% treatment by 2030, aiming to reduce its incidence to below 4 per 100,000 women. As of mid-2025, 194 countries have endorsed the WHO strategy, and 75 have adopted single-dose schedules. However, significant gaps persist: modeling predicts that without accelerated progress, LMICs, especially in sub-Saharan Africa, will miss the 2030 benchmarks, perpetuating high mortality rates [186,187,188,190,198,199,201].
6.5. Steps Forward
The following steps could help close inequity gaps [186,187,188,198,200,202,203,204]:
Scaling single-dose vaccination, simplifying logistics and reducing cost barriers;
Expanding self-sampling, integrating it into HIV and primary care services, supported by community education;
Investing in the capacity of the health system, including data systems, workforce training, and supply chain logistics;
Utilizing innovative delivery models, such as mobile clinics and AI-based image analysis, to reach underserved populations;
Engaging communities and women leaders, empowering them to increase the acceptance and supervision of prevention programs;
Securing funding, supported by global pledges;
In essence, cervical cancer disproportionally impacts women in LMICs, reflecting systemic inequities in vaccine access, screening capacity, and healthcare infrastructure. Barriers that range from cultural stigma to resource shortages compound the crisis. Nonetheless, digital health tools, self-sampling, simplified vaccination strategies, and WHO’s ambitious elimination framework offer a roadmap to equity and control. The imperative now is swift, integrated action and sustained investment.
7. Future Directions
As we advance toward the elimination of cervical cancer, emerging technologies and strategic innovations will enhance prevention, detection, and treatment, bridging the current gaps and charting the course for a future with a minimal disease burden.
7.1. Therapeutic HPV Vaccines and Immunotherapies
While prophylactic HPV vaccines prevent new infections, therapeutic vaccines aim to eliminate established HPV infections and treat high-grade lesions and cancers. Recent clinical trials are highly promising [95,198,205,206,207,208]:
A novel therapeutic vaccine (Vvax001) targeting HPV16 E6/E7 showed lesion regression in 17 of 18 CIN3 patients, with half experiencing complete regression and durable HPV clearance up to 19 weeks following vaccination;
In-depth reviews describe multiple platforms—peptide, DNA, ribonucleic acid (RNA), and viral/inactivated vectors—currently in phase II and III trials, designed for CIN and invasive cancer treatment;
New candidates like mHTV-03E2, an messenger RNA (mRNA)-based vaccine targeting HPV-16/18, have shown potent immunogenicity and strong preclinical efficacy;
Industry efforts include Transgene’s TG4001 (HPV16 E6/E7), in phase II for metastatic cervical cancers.
Alongside therapeutic vaccines, cellular therapies like TCR-engineered T cells are under exploration for refractory HPV-associated malignancies. Additionally, immune checkpoint inhibitors combined with vaccine strategies could enhance antitumor responses, potentially transforming recurrent and metastatic cervical cancer therapy [209].
7.2. Precision Diagnostics: Biomarkers and AI
Advances in molecular diagnostics promise more precise screening and triage [198,209,210]:
DNA methylation markers and HPV integration assays offer improved specificity in distinguishing remote/transient infections from lesions with malignant potential;
Liquid biopsies detecting circulating HPV DNA may enable the early detection of cancer and real-time monitoring of treatment responses;
AI-based image analyses (e.g., self-supervised learning on cytology slides) enhance automated triage and extend the diagnostic capacity to low-resource settings;
These advances are especially beneficial in regions with a limited presence of specialists, allowing telepathology and remote diagnostics to close care gaps.
7.3. Expanded Vaccination Strategies
Efforts are ongoing to optimize vaccine delivery and coverage [95,136,209,211,212]:
Single-dose regimens for the nonavalent vaccine have demonstrated efficacy, offering cost-effective strategies for LMICs;
Gender-neutral vaccination campaigns are gaining momentum, strengthening herd immunity and reducing non-cervical HPV cancers;
Boosting vaccine equity requires immune profiling, affordable generics, cold-chain adaptation, and political commitment.
7.4. Self-Sampling and Decentralized Screening: The Integration of Prevention and Cancer Care, Policy, and Implementation Science
A holistic approach binds the following [85,87,95,191,212,213,214,215,216,217]:
The FDA-approved Teal Health at-home HPV test delivers clinical-equivalent sensitivity and the potential to close screening gaps, particularly among underserved populations;
Combining therapeutic vaccinations post-surgery may reduce recurrence rates, while observational data support its efficacy;
Point-of-care integrations: Merging HIV services, self-sampling, and AI tools, focusing on high-risk groups, and maximizing the screening yield;
Modeling studies emphasize that scaling the 90-70-90 strategies with adult catch-ups could lead to elimination by 2061;
Essential enablers include political will, funding, cold chain logistics for vaccines, community education, and accurate data systems;
By reducing the reliance on clinic-based visits, these tools democratize early detection.
The future of cervical cancer control is multidisciplinary: therapeutic vaccines, precision diagnostics, equitable vaccine deployment, self-sampling, digital automation, system integration, and an underpinning of robust policy. Together, these innovations can help realize the WHO’s vision of global elimination.
8. Conclusions
Cervical cancer remains a preventable malignancy, yet it continues to cause significant morbidity and mortality, especially in underserved regions. The foundation of its control rests on a three-pronged strategy: primary prevention via vaccination, secondary prevention through screening, and the equitable global implementation of both.
Despite scientific advances, global inequities persist. The WHO Cervical Cancer Elimination Initiative aims to reduce the incidence of cervical cancer to below 4 per 100,000 women annually through a 90–70–90 framework by 2030. However, its success depends critically on policy adoption, investments in the cold chain and workforce, community engagement, and strong data systems.
Looking ahead, continued innovation is essential. Emerging technologies, including therapeutic vaccines, AI-supported diagnostics, liquid biopsies, and advanced biomarkers, promise to enhance its detection, treatment, and monitoring, particularly in under-resourced settings. Likewise, integrated approaches combining vaccinations with screening offer faster progress, as demonstrated by population-level trials.
In summary, the trajectory against cervical cancer is promising and grounded in robust scientific advances and validated by decreasing disease trends. To capitalize on this momentum, global efforts must prioritize:
Equitable vaccine delivery, including simplified dosing strategies and broader gender coverage;
Accessible screening, leveraging self-sampling and HPV-based methods embedded into health systems;
Innovative implementation, supporting diagnostics, treatment, and data systems tailored to low-resource contexts;
Global solidarity, ensuring political commitment and sustained financing to close the equity gap.
Acknowledgments
Mohamed-Zakaria Assani and Lidia Boldeanu share equal contributions and status as main/first authors.
Abbreviations
The following abbreviations are used in this manuscript:
| 2-valent/bvalent/bHPV vaccine | Bivalent (HPV-16/18) |
| 4-valent/qvalent/qHPV vaccine | Quadrivalent (HPV-6/11/16/18) |
| 9-valent/9vHPV vaccine | Nonavalent (HPV-6/11/16/18/31/33/45/52/58) |
| AI | Artificial Intelligence |
| ASHA | Accredited Social Health Activist |
| Arg | Arginine |
| CIN | Cervical intraepithelial neoplasia |
| CIN1 | Cervical intraepithelial neoplasia grade 1 |
| CIN2 | Cervical intraepithelial neoplasia grade 2 |
| CIN3 | Cervical intraepithelial neoplasia grade 3 |
| DNA | Deoxyribonucleic acid |
| E2F | Early Region 2 binding factor |
| E6AP | ubiquitin-protein ligase E3A |
| EGFR | Epidermal growth factor receptor |
| FDA | Food and Drug Administration |
| HIV | Human immunodeficiency virus |
| HLA | Human leukocyte antigen |
| HPV | Human papillomavirus |
| HPVs | Human papillomaviruses |
| hrHPV | High-risk HPV |
| hTERT | Human telomerase |
| LBC | Liquid-based cytology |
| LCR | Long control region |
| LMICs | Low- and middle-income countries |
| lrHPV | Low-risk HPV |
| MHC-I | Major Histocompatibility Complex Class I |
| mRNA | Messenger RNA |
| OC | Oral contraceptive |
| OR | Odds ratio |
| pRb | Retinoblastoma protein |
| RE-AIM | Reach, Effectiveness, Adoption, Implementation, and Maintenance |
| RNA | Ribonucleic acid |
| SCC | Squamous cell carcinoma |
| SEER | Surveillance, Epidemiology, and End Results Program |
| STIs | Sexually transmitted infections |
| UK | United Kingdom |
| US | United States |
| USPSTF | United States Preventive Services Task Force |
| VIA | Visual inspection with acetic acid |
| WLWH | Women living with HIV |
| WHO | World Health Organization |
Author Contributions
Conceptualization: M.-Z.A. and L.B.; methodology: A.-D.A. and M.-M.M.; software: M.-M.M.; validation: C.-C.V., L.B. and I.S.; resources: C.-C.V., M.-Z.A. and M.V.B.; data curation: M.-M.M. and M.V.B.; writing—original draft preparation: M.-Z.A., A.L.D. and L.B.; writing—review and editing: L.B., I.S. and M.-M.M.; visualization: A.-D.A., M.-M.M. and M.V.B.; supervision: M.-Z.A. and A.L.D.; project administration: A.L.D. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
The Article Processing Charges were funded by the University of Medicine and Pharmacy of Craiova, Romania.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Zhou L., Li Y., Wang H., Qin R., Han Z., Li R. Global cervical cancer elimination: Quantifying the status, progress, and gaps. BMC Med. 2025;23:67. doi: 10.1186/s12916-025-03897-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jafari Sales A., Hosseini-Karkaj K., Soleymanpour K., Kadkhodaei-Ilkhechi G., Farahnaki-Sadabadi M., Pashazadeh M. Human Papillomavirus and Cervical Cancer: A Narrative Review. Mod. Care J. 2025;22:e159690. doi: 10.5812/mcj-159690. [DOI] [Google Scholar]
- 3.Cervical Cancer. [(accessed on 4 July 2025)]. Available online: https://www.who.int/news-room/fact-sheets/detail/cervical-cancer.
- 4.Wu J., Jin Q., Zhang Y., Ji Y., Li J., Liu X., Duan H., Feng Z., Liu Y., Zhang Y., et al. Global burden of cervical cancer: Current estimates, temporal trend and future projections based on the GLOBOCAN 2022. J. Natl. Cancer Cent. 2025;5:322–329. doi: 10.1016/j.jncc.2024.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ramakrishnan S., Partricia S., Mathan G. Overview of high-risk HPV’s 16 and 18 infected cervical cancer: Pathogenesis to prevention. Biomed. Pharmacother. 2015;70:103–110. doi: 10.1016/j.biopha.2014.12.041. [DOI] [PubMed] [Google Scholar]
- 6.Okunade K.S. Human papillomavirus and cervical cancer. J. Obs. Gynaecol. 2020;40:602–608. doi: 10.1080/01443615.2019.1634030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cervical Cancer Causes, Risk Factors, and Prevention. [(accessed on 4 July 2025)]; Available online: https://www.cancer.gov/types/cervical/causes-risk-prevention.
- 8.Osmani V., Hörner L., Nkurunziza T., Rank S., Tanaka L.F., Klug S.J. Global prevalence of cervical human papillomavirus in women aged 50 years and older with normal cytology: A systematic review and meta-analysis. Lancet Microbe. 2025;6:100955. doi: 10.1016/j.lanmic.2024.100955. [DOI] [PubMed] [Google Scholar]
- 9.Skinner S.R., Wheeler C.M., Romanowski B., Castellsagué X., Lazcano-Ponce E., Del Rosario-Raymundo M.R., Vallejos C., Minkina G., Pereira Da Silva D., McNeil S., et al. Progression of HPV infection to detectable cervical lesions or clearance in adult women: Analysis of the control arm of the VIVIANE study. Int. J. Cancer. 2016;138:2428–2438. doi: 10.1002/ijc.29971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Win T.Z.K., Simms K., Thinn M.M., Tin K.N., Aung S., Feletto E., Bateson D., Canfell K. A Review of Human Papillomavirus Prevalence and Cervical Cancer in Myanmar. J. Epidemiol. Glob. Health. 2025;15:89. doi: 10.1007/s44197-025-00436-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Borda H., Bloem P., Akaba H., Guillaume D., Willens V., Jurgensmeyer M., Muralidharan K., Limaye R. Status of HPV disease and vaccination programmes in LMICs: Introduction to special issue. Vaccine. 2024;42:S1–S8. doi: 10.1016/j.vaccine.2023.10.062. [DOI] [PubMed] [Google Scholar]
- 12.Forman D., de Martel C., Lacey C.J., Soerjomataram I., Lortet-Tieulent J., Bruni L., Vignat J., Ferlay J., Bray F., Plummer M., et al. Global burden of human papillomavirus and related diseases. Vaccine. 2012;30((Suppl. S5)):F12–F23. doi: 10.1016/j.vaccine.2012.07.055. [DOI] [PubMed] [Google Scholar]
- 13.Kennedy E.M., Kornepati A.V.R., Goldstein M., Bogerd H.P., Poling B.C., Whisnant A.W., Kastan M.B., Cullen B.R. Inactivation of the Human Papillomavirus E6 or E7 Gene in Cervical Carcinoma Cells by Using a Bacterial CRISPR/Cas RNA-Guided Endonuclease. J. Virol. 2014;88:11965–11972. doi: 10.1128/JVI.01879-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bhattacharjee R., Das S.S., Biswal S.S., Nath A., Das D., Basu A., Malik S., Kumar L., Kar S., Singh S.K., et al. Mechanistic role of HPV-associated early proteins in cervical cancer: Molecular pathways and targeted therapeutic strategies. Crit. Rev. Oncol. Hematol. 2022;174:103675. doi: 10.1016/j.critrevonc.2022.103675. [DOI] [PubMed] [Google Scholar]
- 15.Fischer M., Uxa S., Stanko C., Magin T.M., Engeland K. Human papilloma virus E7 oncoprotein abrogates the p53-p21-DREAM pathway. Sci. Rep. 2017;7:2603. doi: 10.1038/s41598-017-02831-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Y., Qiu K., Ren J., Zhao Y., Cheng P. Roles of human papillomavirus in cancers: Oncogenic mechanisms and clinical use. Signal Transduct. Target. Ther. 2025;10:44. doi: 10.1038/s41392-024-02083-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huber J., Mueller A., Sailer M., Regidor P.A. Human papillomavirus persistence or clearance after infection in reproductive age. What is the status? Review of the literature and new data of a vaginal gel containing silicate dioxide, citric acid, and selenite. Womens Health. 2021;17:17455065211020702. doi: 10.1177/17455065211020702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rodríguez A.C., Schiffman M., Herrero R., Wacholder S., Hildesheim A., Castle P.E., Solomon D., Burk R., Proyecto Epidemiológico Guanacaste Group Rapid Clearance of Human Papillomavirus and Implications for Clinical Focus on Persistent Infections. JNCI J. Natl. Cancer Inst. 2008;100:513–517. doi: 10.1093/jnci/djn044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yusupov A., Popovsky D., Mahmood L., Kim A.S., Akman A.E., Yuan H. The nonavalent vaccine: A review of high-risk HPVs and a plea to the CDC. Am. J. Stem. Cells. 2019;8:52–64. [PMC free article] [PubMed] [Google Scholar]
- 20.Bruni L., Saura-Lázaro A., Montoliu A., Brotons M., Alemany L., Diallo M.S., Afsar O.Z., LaMontagne D.S., Mosina L., Contreras M., et al. HPV vaccination introduction worldwide and WHO and UNICEF estimates of national HPV immunization coverage 2010–2019. Prev. Med. 2021;144:106399. doi: 10.1016/j.ypmed.2020.106399. [DOI] [PubMed] [Google Scholar]
- 21.Han J., Zhang L., Chen Y., Zhang Y., Wang L., Cai R., Li M., Dai Y., Dang L., Chen H., et al. Global HPV vaccination programs and coverage rates: A systematic review. EClinicalMedicine. 2025;84:103290. doi: 10.1016/j.eclinm.2025.103290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aggarwal S., Agarwal P., Gupta N. A comprehensive narrative review of challenges and facilitators in the implementation of various HPV vaccination program worldwide. Cancer Med. 2024;13:e6862. doi: 10.1002/cam4.6862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bhalerao V., Gotarkar S., Muneshwar K. The Impact of HPV Vaccination on Cervical Cancer in adolescent females: A narrative review. J. Fam. Med. Prim. Care. 2024;13:4775–4782. doi: 10.4103/jfmpc.jfmpc_235_24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Martel C., Plummer M., Vignat J., Franceschi S. Worldwide burden of cancer attributable to HPV by site, country and HPV type. Int. J. Cancer. 2017;141:664–670. doi: 10.1002/ijc.30716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cervical Cancer Screening. [(accessed on 4 July 2025)]. Available online: https://www.who.int/data/gho/indicator-metadata-registry/imr-details/3240.
- 26.Cervical Cancer Screening. [(accessed on 4 July 2025)]; Available online: https://www.ncbi.nlm.nih.gov/books/NBK65734/
- 27.Gopalkrishnan K., Karim R. Addressing Global Disparities in Cervical Cancer Burden: A Narrative Review of Emerging Strategies. Curr. HIV/AIDS Rep. 2025;22:18. doi: 10.1007/s11904-025-00727-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eun T.J., Perkins R.B. Screening for Cervical Cancer. Med. Clin. N. Am. 2020;104:1063–1078. doi: 10.1016/j.mcna.2020.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kamath Mulki A., Withers M. Human Papilloma Virus self-sampling performance in low- and middle-income countries. BMC Women’s Health. 2021;21:12. doi: 10.1186/s12905-020-01158-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Duan R., Yang H., Zhai X., Yu J., Lu C., Yang W., Long T., Dao Y., Li G., Zhou Y., et al. Accuracy and acceptability of self-sampling HPV testing in cervical cancer screening: A population-based study in rural Yunnan, China. Sci. Rep. 2025;15:26390. doi: 10.1038/s41598-025-09292-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.2023 Global State of Cervical Cancer Awareness Report. [(accessed on 4 July 2025)]. Available online: https://www.bgi.com/global/news/bgi-genomics-global-2023-state-of-cervical-cancer-awareness-report.
- 32.Ssentongo P., McCall-Hosenfeld J.S., Calo W.A., Moss J., Lengerich E.J., Chinchilli V.M., Ba D.M. Association of human papillomavirus vaccination with cervical cancer screening: A systematic review and meta-analysis. Medicine. 2022;101:e29329. doi: 10.1097/MD.0000000000029329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Di Giuseppe G., Folcarelli L., Lanzano R., Napolitano F., Pavia M. HPV Vaccination and Cervical Cancer Screening: Assessing Awareness, Attitudes, and Adherence in Detained Women. Vaccines. 2022;10:1280. doi: 10.3390/vaccines10081280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin W., Wang Y., Liu Z., Chen B., Yuan S., Wu B., Gong L. Awareness and attitude towards human papillomavirus and its vaccine among females with and without daughter(s) who participated in cervical cancer screening in Shenzhen, China. Trop. Med. Int. Health. 2019;24:1054–1063. doi: 10.1111/tmi.13283. [DOI] [PubMed] [Google Scholar]
- 35.Global Strategy to Accelerate the Elimination of Cervical Cancer as a Public Health Problem. [(accessed on 4 July 2025)]. Available online: https://www.who.int/publications/i/item/9789240014107.
- 36.Ji L., Chen M., Yao L. Strategies to eliminate cervical cancer in China. Front. Oncol. 2023;13:1105468. doi: 10.3389/fonc.2023.1105468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang X., Qi S., Dai L., Ye Q., Li X. Trends in HPV-positive cervical cancer prevalence: A retrospective study from 2013 to 2020. Virol. J. 2025;22:199. doi: 10.1186/s12985-025-02830-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Canfell K., Kim J.J., Brisson M., Keane A., Simms K.T., Caruana M., Burger E.A., Martin D., Nguyen D.T.N., Bénard É., et al. Mortality impact of achieving WHO cervical cancer elimination targets: A comparative modelling analysis in 78 low-income and lower-middle-income countries. Lancet. 2020;395:591–603. doi: 10.1016/S0140-6736(20)30157-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.5 fast facts about HPV and cervical cancer. [(accessed on 4 July 2025)]. Available online: https://www.unicef.org/stories/fast-facts-hpv-cervical-cancer.
- 40.Petersen Z., Jaca A., Ginindza T.G., Maseko G., Takatshana S., Ndlovu P., Zondi N., Zungu N., Varghese C., Hunting G., et al. Barriers to uptake of cervical cancer screening services in low-and-middle-income countries: A systematic review. BMC Women’s Health. 2022;22:486. doi: 10.1186/s12905-022-02043-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stelzle D., Tanaka L.F., Lee K.K., Ibrahim Khalil A., Baussano I., Shah A.S.V., McAllister D.A., Gottlieb S.L., Klug S.J., Winkler A.S., et al. Estimates of the global burden of cervical cancer associated with HIV. Lancet Glob. Health. 2020;9:e119. doi: 10.1016/S2214-109X(20)30459-9. Correction in Lancet Glob. Health 2021, 9, e161–e169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Human papillomavirus and cancer. [(accessed on 4 July 2025)]. Available online: https://www.who.int/news-room/fact-sheets/detail/human-papilloma-virus-and-cancer.
- 43.Cancer Stat Facts: Cervical Cancer. [(accessed on 4 July 2025)]; Available online: https://seer.cancer.gov/statfacts/html/cervix.html.
- 44.Key Statistics for Cervical Cancer. [(accessed on 4 July 2025)]. Available online: https://www.cancer.org/cancer/types/cervical-cancer/about/key-statistics.html.
- 45.Cheng X., Wang P., Cheng L., Zhao F., Liu J. Trends in cervical cancer incidence and mortality in the United States, 1975–2018: A population-based study. Front. Med. 2025;12:1579446. doi: 10.3389/fmed.2025.1579446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chan C.K., Aimagambetova G., Ukybassova T., Kongrtay K., Azizan A. Human Papillomavirus Infection and Cervical Cancer: Epidemiology, Screening, and Vaccination—Review of Current Perspectives. J. Oncol. 2019;2019:3257939. doi: 10.1155/2019/3257939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang S., Xu H., Zhang L., Qiao Y. Cervical cancer: Epidemiology, risk factors and screening. Chin. J. Cancer Res. 2020;32:720–728. doi: 10.21147/j.issn.1000-9604.2020.06.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.HPV PREVENTION AT A GLANCE. [(accessed on 4 July 2025)]. Available online: https://hpvcentre.net/hpvatglance.php.
- 49.Human Papillomavirus and Related Diseases Report. [(accessed on 4 July 2025)]. Available online: https://hpvcentre.net/statistics/reports/XWX.pdf.
- 50.Berza N., Zodzika J., Kivite-Urtane A., Baltzer N., Curkste A., Pole I., Nygård M., Pärna K., Stankunas M., Tisler A., et al. Understanding the high-risk human papillomavirus prevalence and associated factors in the European country with a high incidence of cervical cancer. Eur. J. Public Health. 2024;34:826–832. doi: 10.1093/eurpub/ckae075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mazurec K., Trzeszcz M., Mazurec M., Kobierzycki C., Jach R., Halon A. Distribution of 14 High-Risk HPV Types and p16/Ki67 Dual-Stain Status in Post-Colposcopy Histology Results: Negative, Low- and High-Grade Cervical Squamous Intraepithelial Lesions. Cancers. 2024;16:3401. doi: 10.3390/cancers16193401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shi R., Qi W., Wang Z., Cai J., Zhao M., Wang Z. High-risk human papillomavirus genotype distribution and attribution to cervical lesions in a Shanxi Province screening population. Sci. Rep. 2025;15:28217. doi: 10.1038/s41598-025-14228-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Aden D., Zaheer S., Khan S., Jairajpuri Z.S., Jetley S. Navigating the landscape of HPV-associated cancers: From epidemiology to prevention. Pathol.-Res. Pract. 2024;263:155574. doi: 10.1016/j.prp.2024.155574. [DOI] [PubMed] [Google Scholar]
- 54.Li S., Liu C., Weng L. Exploring Cervical Adenocarcinoma: Epidemiological Insights, Diagnostic and Therapeutic Challenges, and Pathogenetic Mechanisms. Cancer Med. 2025;14:e70620. doi: 10.1002/cam4.70620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Islami F., Fedewa S.A., Jemal A. Trends in cervical cancer incidence rates by age, race/ethnicity, histological subtype, and stage at diagnosis in the United States. Prev. Med. 2019;123:316–323. doi: 10.1016/j.ypmed.2019.04.010. [DOI] [PubMed] [Google Scholar]
- 56.Broshkevitch C.J., Barnabas R.V., Liu G., Palanee-Phillips T., Rao D.W. Enhanced cervical cancer and HIV interventions reduce the disproportionate burden of cervical cancer cases among women living with HIV: A modeling analysis. PLoS ONE. 2024;19:e0301997. doi: 10.1371/journal.pone.0301997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Luvián-Morales J., Gutiérrez-Enríquez S.O., Granados-García V., Torres-Poveda K. Risk factors for the development of cervical cancer: Analysis of the evidence. Front. Oncol. 2024;14:1378549. doi: 10.3389/fonc.2024.1378549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fonseca-Moutinho J.A. Smoking and cervical cancer. ISRN Obs. Gynecol. 2011;2011:847684. doi: 10.5402/2011/847684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bowden S.J., Doulgeraki T., Bouras E., Markozannes G., Athanasiou A., Grout-Smith H., Kechagias K.S., Ellis L.B., Zuber V., Chadeau-Hyam M., et al. Risk factors for human papillomavirus infection, cervical intraepithelial neoplasia and cervical cancer: An umbrella review and follow-up Mendelian randomisation studies. BMC Med. 2023;21:274. doi: 10.1186/s12916-023-02965-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Khalil A., Mpunga T., Wei F., Baussano I., de Martel C., Bray F., Stelzle D., Dryden-Peterson S., Jaquet A., Horner M.J., et al. Age-specific burden of cervical cancer associated with HIV: A global analysis with a focus on sub-Saharan Africa. Int. J. Cancer. 2021;150:761–772. doi: 10.1002/ijc.33841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li X., Wu J., Wu Y., Duan Z., Luo M., Li L., Li S., Jia Y. Imbalance of Vaginal Microbiota and Immunity: Two Main Accomplices of Cervical Cancer in Chinese Women. Int. J. Womens Health. 2023;15:987–1002. doi: 10.2147/IJWH.S406596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mazinani S., Aghazadeh M., Poortahmasebi V., Arafi V., Hasani A. Cervical cancer pathology and vaginal and gut microbiota: Conception of the association. Lett. Appl. Microbiol. 2025;78:ovaf088. doi: 10.1093/lambio/ovaf088. [DOI] [PubMed] [Google Scholar]
- 63.Peng Y., Tang Q., Wu S., Zhao C. Associations of Atopobium, Garderella, Megasphaera, Prevotella, Sneathia, and Streptococcus with human papillomavirus infection, cervical intraepithelial neoplasia, and cancer: A systematic review and meta-analysis. BMC Infect. Dis. 2025;25:708. doi: 10.1186/s12879-025-10851-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mitra A., MacIntyre D.A., Marchesi J.R., Lee Y.S., Bennett P.R., Kyrgiou M. The vaginal microbiota, human papillomavirus infection and cervical intraepithelial neoplasia: What do we know and where are we going next? Microbiome. 2016;4:58. doi: 10.1186/s40168-016-0203-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chen H., Luo L., Wen Y., He B., Ling H., Shui J., He P., Hou X., Tang S., Li Z. Chlamydia trachomatis and Human Papillomavirus Infection in Women From Southern Hunan Province in China: A Large Observational Study. Front. Microbiol. 2020;11:827. doi: 10.3389/fmicb.2020.00827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhu H., Shen Z., Luo H., Zhang W., Zhu X. Chlamydia Trachomatis Infection-Associated Risk of Cervical Cancer: A Meta-Analysis. Medicine. 2016;95:e3077. doi: 10.1097/MD.0000000000003077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Javadi K., Ferdosi-Shahandashti E., Rajabnia M., Khaledi M. Vaginal microbiota and gynecological cancers: A complex and evolving relationship. Infect. Agent. Cancer. 2024;19:27. doi: 10.1186/s13027-024-00590-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wei Z.-T., Chen H.-L., Wang C.-F., Yang G.-L., Han S.-M., Zhang S.-L. Depiction of Vaginal Microbiota in Women with High-Risk Human Papillomavirus Infection. Front. Public Health. 2021;8:587298. doi: 10.3389/fpubh.2020.587298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Koster S., Gurumurthy R.K., Kumar N., Prakash P.G., Dhanraj J., Bayer S., Berger H., Kurian S.M., Drabkina M., Mollenkopf H.-J., et al. Modelling Chlamydia and HPV co-infection in patient-derived ectocervix organoids reveals distinct cellular reprogramming. Nat. Commun. 2022;13:1030. doi: 10.1038/s41467-022-28569-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tekalegn Y., Sahiledengle B., Woldeyohannes D., Atlaw D., Degno S., Desta F., Bekele K., Aseffa T., Gezahegn H., Kene C. High parity is associated with increased risk of cervical cancer: Systematic review and meta-analysis of case-control studies. Womens Health. 2022;18:17455065221075904. doi: 10.1177/17455065221075904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bovo A.C., Pedrão P.G., Guimarães Y.M., Godoy L.R., Resende J.C.P., Longatto-Filho A., Reis R.D. Combined Oral Contraceptive Use and the Risk of Cervical Cancer: Literature Review. Rev. Bras. Ginecol. Obstet. 2023;45:e818–e824. doi: 10.1055/s-0043-1776403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang S.S., Bratti M.C., Rodríguez A.C., Herrero R., Burk R.D., Porras C., González P., Sherman M.E., Wacholder S., Lan Z.E., et al. Common Variants in Immune and DNA Repair Genes and Risk for Human Papillomavirus Persistence and Progression to Cervical Cancer. J. Infect. Dis. 2009;199:20–30. doi: 10.1086/595563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hu X., Zhang Z., Ma D., Huettner P.C., Massad L.S., Nguyen L., Borecki I., Rader J.S. TP53, MDM2, NQO1, and susceptibility to cervical cancer. Cancer Epidemiol. Biomark. Prev. 2010;19:755–761. doi: 10.1158/1055-9965.EPI-09-0886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Adebamowo S.N., Adeyemo A., Adebayo A., Achara P., Alabi B., Bakare R.A., Famooto A.O., Obende K., Offiong R., Olaniyan O., et al. Genome, HLA and polygenic risk score analyses for prevalent and persistent cervical human papillomavirus (HPV) infections. Eur. J. Hum. Genet. 2024;32:708–716. doi: 10.1038/s41431-023-01521-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Human Papillomavirus (HPV) Infection. [(accessed on 4 July 2025)]; Available online: https://www.ncbi.nlm.nih.gov/books/NBK321770/
- 76.Haręża D.A., Wilczyński J.R., Paradowska E. Human Papillomaviruses as Infectious Agents in Gynecological Cancers. Oncogenic Properties of Viral Proteins. Int. J. Mol. Sci. 2022;23:1818. doi: 10.3390/ijms23031818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cerqueira C., Schiller J.T. Papillomavirus assembly: An overview and perspectives. Virus Res. 2017;231:103–107. doi: 10.1016/j.virusres.2016.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Egawa N., Egawa K., Griffin H., Doorbar J. Human Papillomaviruses; Epithelial Tropisms, and the Development of Neoplasia. Viruses. 2015;7:3863–3890. doi: 10.3390/v7072802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kirk A., Graham S.V. The human papillomavirus late life cycle and links to keratinocyte differentiation. J. Med. Virol. 2024;96:e29461. doi: 10.1002/jmv.29461. [DOI] [PubMed] [Google Scholar]
- 80.Finnen R.L., Erickson K.D., Chen X.S., Garcea R.L. Interactions between papillomavirus L1 and L2 capsid proteins. J Virol. 2003;77:4818–4826. doi: 10.1128/JVI.77.8.4818-4826.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wang M., Ma Y., Zhang N., Li L., Fu Y., Li L., Zhai Q. Methylation testing versus cervical cytology for triage of HPV-positive women: A comparative study. Diagn. Microbiol. Infect. Dis. 2025;113:116917. doi: 10.1016/j.diagmicrobio.2025.116917. [DOI] [PubMed] [Google Scholar]
- 82.Wang J., Elfström K.M., Dillner J. Human papillomavirus-based cervical screening and long-term cervical cancer risk: A randomised health-care policy trial in Sweden. Lancet Public Health. 2024;9:e886–e895. doi: 10.1016/S2468-2667(24)00218-4. [DOI] [PubMed] [Google Scholar]
- 83.Jenkins D. A review of cross-protection against oncogenic HPV by an HPV-16/18 AS04-adjuvanted cervical cancer vaccine: Importance of virological and clinical endpoints and implications for mass vaccination in cervical cancer prevention. Gynecol. Oncol. 2008;110:S18–S25. doi: 10.1016/j.ygyno.2008.06.027. [DOI] [PubMed] [Google Scholar]
- 84.Cho E.H., Park M.S., Woo H.Y., Park H., Kwon M.J. Evaluation of clinical usefulness of HPV-16 and HPV-18 genotyping for cervical cancer screening. J. Gynecol. Oncol. 2024;35:e72. doi: 10.3802/jgo.2024.35.e72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zou Z., Fairley C.K., Ong J.J., Shen M., Chow E.P.F., Liu H., Xia R., Li R., Hocking J., Zhuang G., et al. Impact of achieving WHO’s 90-70-90 targets on cervical cancer elimination and potential benefits in preventing other HPV-related cancers in China: A modelling study. EClinicalMedicine. 2024;77:102878. doi: 10.1016/j.eclinm.2024.102878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sumiec E.G., Yim Z.Y., Mohy-Eldin H., Nedjai B. The current state of DNA methylation biomarkers in self-collected liquid biopsies for the early detection of cervical cancer: A literature review. Infect. Agent. Cancer. 2024;19:62. doi: 10.1186/s13027-024-00623-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gomes M., Provaggi E., Pembe A.B., Olaitan A., Gentry-Maharaj A. Advancing Cervical Cancer Prevention Equity: Innovations in Self-Sampling and Digital Health Technologies Across Healthcare Settings. Diagnostics. 2025;15:1176. doi: 10.3390/diagnostics15091176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zolfi E., Khaleghi Mehr F., Emtiazi N., Moradi Y. A review of the carcinogenic potential of human papillomavirus (HPV) in urological cancers. Virol. J. 2025;22:53. doi: 10.1186/s12985-025-02682-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Williams G.A., 2nd, Wu A.A., Eugene H.C., Tsai Y.C., Wong M., Nonogaki H., Roden R.B.S., Hung C.F., Wu T.C., Vang R., et al. Clinicopathologic Features and Viral Status of Low-risk HPV6 and HPV11-Associated Squamous Cell Carcinoma of the Uterine Cervix and Vulva. Am. J. Surg. Pathol. 2025;49:458–470. doi: 10.1097/PAS.0000000000002367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mlynarczyk-Bonikowska B., Rudnicka L. HPV Infections—Classification, Pathogenesis, and Potential New Therapies. Int. J. Mol. Sci. 2024;25:7616. doi: 10.3390/ijms25147616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wu M., Huang H., Tang Y., Ren X., Jiang X., Tian M., Li W. Unveiling the multifaceted realm of human papillomavirus: A comprehensive exploration of biology, interactions, and advances in cancer management. Front. Immunol. 2024;15:1430544. doi: 10.3389/fimmu.2024.1430544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Human papillomavirus vaccines: WHO position paper. [(accessed on 4 July 2025)]. Available online: https://www.who.int/publications/i/item/who-wer9750-645-672.
- 93.Burd E.M. Human papillomavirus and cervical cancer. Clin. Microbiol. Rev. 2003;16:1–17. doi: 10.1128/CMR.16.1.1-17.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Muñoz N., Bosch F.X., Sanjosé S.d., Herrero R., Castellsagué X., Shah K.V., Snijders P.J.F., Meijer C.J.L.M. Epidemiologic Classification of Human Papillomavirus Types Associated with Cervical Cancer. N. Engl. J. Med. 2003;348:518–527. doi: 10.1056/NEJMoa021641. [DOI] [PubMed] [Google Scholar]
- 95.Zheng Q., He M., Mao Z., Huang Y., Li X., Long L., Guo M., Zou D. Advancing the Fight Against Cervical Cancer: The Promise of Therapeutic HPV Vaccines. Vaccines. 2025;13:92. doi: 10.3390/vaccines13010092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Human Papillomavirus Type 51. [(accessed on 4 July 2025)]. Available online: https://www.sciencedirect.com/topics/biochemistry-genetics-and-molecular-biology/human-papillomavirus-type-51.
- 97.Nikolic N., Basica B., Strbac M., Terzic L., Patic A., Kovacevic G., Velicki R., Petrovic D., Mandic A., Petrovic V. Prevalence of Carcinogenic Genotypes of HPV-Infected Women in a Ten-Year Period (2014–2023) in Vojvodina, Serbia. Medicina. 2024;60:922. doi: 10.3390/medicina60060922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Liu Y., Ai H. Comprehensive insights into human papillomavirus and cervical cancer: Pathophysiology, screening, and vaccination strategies. Biochim. Biophys. Acta (BBA)-Rev. Cancer. 2024;1879:189192. doi: 10.1016/j.bbcan.2024.189192. [DOI] [PubMed] [Google Scholar]
- 99.Pavelescu L.A., Mititelu-Zafiu N.L., Mindru D.E., Vladareanu R., Curici A. Molecular Insights into HPV-Driven Cervical Cancer: Oncoproteins, Immune Evasion, and Epigenetic Modifications. Microorganisms. 2025;13:1000. doi: 10.3390/microorganisms13051000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Baba S.K., Alblooshi S.S.E., Yaqoob R., Behl S., Al Saleem M., Rakha E.A., Malik F., Singh M., Macha M.A., Akhtar M.K., et al. Human papilloma virus (HPV) mediated cancers: An insightful update. J. Transl. Med. 2025;23:483. doi: 10.1186/s12967-025-06470-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Downham L., Jaafar I., Rol M.L., Nyawira Nyaga V., Valls J., Baena A., Zhang L., Gunter M.J., Arbyn M., Almonte M. Accuracy of HPV E6/E7 oncoprotein tests to detect high-grade cervical lesions: A systematic literature review and meta-analysis. Br. J. Cancer. 2024;130:517–525. doi: 10.1038/s41416-023-02490-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Janiszewska J., Kostrzewska-Poczekaj M., Wierzbicka M., Brenner J.C., Giefing M. HPV-driven oncogenesis-much more than the E6 and E7 oncoproteins. J. Appl. Genet. 2025;66:63–71. doi: 10.1007/s13353-024-00883-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Obanya D.I., Wootton L.M., Morgan E.L. Advances in understanding the mechanisms of the human papillomavirus oncoproteins. Biochem. Soc. Trans. 2025;53:565–577. doi: 10.1042/BST20253041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Han F., Guo X.-y., Jiang M.-x., Xia N.-s., Gu Y., Li S.-w. Structural biology of the human papillomavirus. Structure. 2024;32:1877–1892. doi: 10.1016/j.str.2024.09.011. [DOI] [PubMed] [Google Scholar]
- 105.Mahendra I.N.B., Prayudi P.K.A., Dwija I., Suwiyoga K. HPV16-E6/E7 Oncogene Mutation and p53 Expression among Indonesian Women with Cervical Cancer. Asian Pac J Cancer Prev. 2022;23:2705–2711. doi: 10.31557/APJCP.2022.23.8.2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mesplède T., Gagnon D., Bergeron-Labrecque F., Azar I., Sénéchal H., Coutlée F., Archambault J. p53 degradation activity, expression, and subcellular localization of E6 proteins from 29 human papillomavirus genotypes. J. Virol. 2012;86:94–107. doi: 10.1128/JVI.00751-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mir B.A., Ahmad A., Farooq N., Priya M.V., Siddiqui A.H., Asif M., Manzoor R., Ishqi H.M., Alomar S.Y., Rahaman P.F. Increased expression of HPV-E7 oncoprotein correlates with a reduced level of pRb proteins via high viral load in cervical cancer. Sci. Rep. 2023;13:15075. doi: 10.1038/s41598-023-42022-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wang H., Mo P., Ren S., Yan C. Activating transcription factor 3 activates p53 by preventing E6-associated protein from binding to E6. J. Biol. Chem. 2010;285:13201–13210. doi: 10.1074/jbc.M109.058669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.de Freitas A.C., de Oliveira T.H.A., Barros M.R., Jr., Venuti A. hrHPV E5 oncoprotein: Immune evasion and related immunotherapies. J Exp Clin Cancer Res. 2017;36:71. doi: 10.1186/s13046-017-0541-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Liu X., Clements A., Zhao K., Marmorstein R. Structure of the Human Papillomavirus E7 Oncoprotein and Its Mechanism for Inactivation of the Retinoblastoma Tumor Suppressor*. J. Biol. Chem. 2006;281:578–586. doi: 10.1074/jbc.M508455200. [DOI] [PubMed] [Google Scholar]
- 111.Diakite I., Martins B., Owusu-Edusei K., Palmer C., Patterson-Lomba O., Gomez-Lievano A., Zion A., Simpson R., Daniels V., Elbasha E. Structured Literature Review to Identify Human Papillomavirus’s Natural History Parameters for Dynamic Population Models of Vaccine Impacts. Infect. Dis. Ther. 2024;13:965–990. doi: 10.1007/s40121-024-00952-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gravitt P.E., Winer R.L. Natural History of HPV Infection across the Lifespan: Role of Viral Latency. Viruses. 2017;9:267. doi: 10.3390/v9100267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zhao M., Kang P., Zhu L., Zhou D., Cui M., Zhang M., Jia J., Luo L. Global pattern of persistent human papillomavirus infection in female genital tract: An update system review and meta-analysis. iScience. 2024;27:110991. doi: 10.1016/j.isci.2024.110991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Graham S.V. The human papillomavirus replication cycle, and its links to cancer progression: A comprehensive review. Clin. Sci. 2017;131:2201–2221. doi: 10.1042/CS20160786. [DOI] [PubMed] [Google Scholar]
- 115.Ali M., Sinha R., Kumar A., Karim S., Irfan M., Kumar S., Sinha S., Kumar A., Ghosh A., Singh M. HPV DNA status and clinical history of patients are supplements for accurate reporting of the cytological Pap smear. Sci. Rep. 2024;14:17486. doi: 10.1038/s41598-024-68344-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Della Fera A.N., Warburton A., Coursey T.L., Khurana S., McBride A.A. Persistent Human Papillomavirus Infection. Viruses. 2021;13:321. doi: 10.3390/v13020321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kombe Kombe A.J., Li B., Zahid A., Mengist H.M., Bounda G.-A., Zhou Y., Jin T. Epidemiology and Burden of Human Papillomavirus and Related Diseases, Molecular Pathogenesis, and Vaccine Evaluation. Front. Public Health. 2021;8:552028. doi: 10.3389/fpubh.2020.552028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Perkins R.B., Guido R.S., Castle P.E., Chelmow D., Einstein M.H., Garcia F., Huh W.K., Kim J.J., Moscicki A.B., Nayar R., et al. 2019 ASCCP Risk-Based Management Consensus Guidelines for Abnormal Cervical Cancer Screening Tests and Cancer Precursors. J. Low. Genit. Tract. Dis. 2020;24:102–131. doi: 10.1097/LGT.0000000000000525. Erratum in J. Low. Genit. Tract. Dis. 2021, 25, 330–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sykes P.H., Simcock B.J., Innes C.R., Harker D., Williman J.A., Whitehead M., van der Griend R.A., Lawton B.A., Hibma M., Fitzgerald P., et al. Predicting regression of cervical intraepithelial neoplasia grade 2 in women under 25 years. Am. J. Obstet. Gynecol. 2022;226:222.e1–222.e13. doi: 10.1016/j.ajog.2021.09.009. [DOI] [PubMed] [Google Scholar]
- 120.Moscicki A.B., Ma Y., Wibbelsman C., Darragh T.M., Powers A., Farhat S., Shiboski S. Rate of and risks for regression of cervical intraepithelial neoplasia 2 in adolescents and young women. Obs. Gynecol. 2010;116:1373–1380. doi: 10.1097/AOG.0b013e3181fe777f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ehret A., Bark V.N., Mondal A., Fehm T.N., Hampl M. Regression rate of high-grade cervical intraepithelial lesions in women younger than 25 years. Arch. Gynecol. Obstet. 2023;307:981–990. doi: 10.1007/s00404-022-06680-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lee M.H., Finlayson S.J., Gukova K., Hanley G., Miller D., Sadownik L.A. Outcomes of Conservative Management of High Grade Squamous Intraepithelial Lesions in Young Women. J. Low. Genit. Tract Dis. 2018;22:212–218. doi: 10.1097/LGT.0000000000000399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.de Sanjosé S., Brotons M., Pavón M.A. The natural history of human papillomavirus infection. Best Pract. Res. Clin. Obstet. Gynaecol. 2018;47:2–13. doi: 10.1016/j.bpobgyn.2017.08.015. [DOI] [PubMed] [Google Scholar]
- 124.Bedell S.L., Goldstein L.S., Goldstein A.R., Goldstein A.T. Cervical Cancer Screening: Past, Present, and Future. Sex. Med. Rev. 2020;8:28–37. doi: 10.1016/j.sxmr.2019.09.005. [DOI] [PubMed] [Google Scholar]
- 125.Cuschieri K., Bhatia R., Cruickshank M., Hillemanns P., Arbyn M. HPV testing in the context of post-treatment follow up (test of cure) J. Clin. Virol. 2016;76:S56–S61. doi: 10.1016/j.jcv.2015.10.008. [DOI] [PubMed] [Google Scholar]
- 126.Ramírez A.T., Valls J., Baena A., Rojas F.D., Ramírez K., Álvarez R., Cristaldo C., Henríquez O., Moreno A., Reynaga D.C., et al. Performance of cervical cytology and HPV testing for primary cervical cancer screening in Latin America: An analysis within the ESTAMPA study. Lancet Reg. Health–Am. 2023;26:100593. doi: 10.1016/j.lana.2023.100593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Alonso I., Torné A., Puig-Tintoré L.M., Esteve R., Quinto L., Campo E., Pahisa J., Ordi J. Pre- and post-conization high-risk HPV testing predicts residual/recurrent disease in patients treated for CIN 2–3. Gynecol. Oncol. 2006;103:631–636. doi: 10.1016/j.ygyno.2006.04.016. [DOI] [PubMed] [Google Scholar]
- 128.Clarke M.A., Deshmukh A.A., Suk R., Roberts J., Gilson R., Jay N., Stier E.A., Wentzensen N. A systematic review and meta-analysis of cytology and HPV-related biomarkers for anal cancer screening among different risk groups. Int. J. Cancer. 2022;151:1889–1901. doi: 10.1002/ijc.34199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Mariani L., Sandri M.T., Preti M., Origoni M., Costa S., Cristoforoni P., Bottari F., Sideri M. HPV-Testing in Follow-up of Patients Treated for CIN2+ Lesions. J. Cancer. 2016;7:107–114. doi: 10.7150/jca.13503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Immunization Coverage. [(accessed on 4 July 2025)]. Available online: https://www.who.int/news-room/fact-sheets/detail/immunization-coverage.
- 131.Naidoo D., Govender K., Mantell J.E. Breaking barriers: Why including boys and men is key to HPV prevention. BMC Med. 2024;22:525. doi: 10.1186/s12916-024-03701-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kim J., Choe Y.J., Park J., Cho J., Cheong C., Oh J.-K., Park M., Shim E., Yu S.-Y. Comparative Effects of Bivalent, Quadrivalent, and Nonavalent Human Papillomavirus Vaccines in The Prevention of Genotype-Specific Infection: A Systematic Review and Network Meta-Analysis. Infect. Chemother. 2024;56:37–46. doi: 10.3947/ic.2023.0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Williamson A.L. Recent Developments in Human Papillomavirus (HPV) Vaccinology. Viruses. 2023;15:1440. doi: 10.3390/v15071440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kamolratanakul S., Pitisuttithum P. Human Papillomavirus Vaccine Efficacy and Effectiveness against Cancer. Vaccines. 2021;9:1413. doi: 10.3390/vaccines9121413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Asare M., Elizondo A., Dwumfour-Poku M., Mena C., Gutierrez M., Mamudu H.M. Intervention to Increase Cervical Cancer Screening Behavior among Medically Underserved Women: Effectiveness of 3R Communication Model. Healthcare. 2023;11:1323. doi: 10.3390/healthcare11091323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Song Y., Choi W., Shim E. Cost-Effectiveness of Human Papillomavirus Vaccination in the UK: Two Versus Single-Dose of Nonavalent HPV Vaccination. Am. J. Prev. Med. 2024;67:231–240. doi: 10.1016/j.amepre.2024.03.008. [DOI] [PubMed] [Google Scholar]
- 137.Harper D.M., Navarro-Alonso J.A., Bosch F.X., Paavonen J., Stanley M., Sasieni P., Yébenes M., Martínez-Martínez N., Rodriguez Á., García A., et al. Impact of human papillomavirus vaccines in the reduction of infection, precursor lesions, and cervical cancer: A systematic literature review. Hum. Vaccin. Immunother. 2025;21:2497608. doi: 10.1080/21645515.2025.2497608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Rostami Varnousfaderani M., Khoshnazar Z., Zeratie H., Hosseini Koukamari P. Optimizing HPV vaccine effectiveness: Impact of vaccination age and dose schedule on immunogenicity and cervical cancer prevention. Front. Public Health. 2025;13:1544220. doi: 10.3389/fpubh.2025.1544220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Gardasil 9. [(accessed on 4 July 2025)]. Available online: https://www.merck.com/product/usa/pi_circulars/g/gardasil_9/gardasil_9_pi.pdf.
- 140.Gardasil. [(accessed on 4 July 2025)]; Available online: https://www.fda.gov/files/vaccines%2C%20blood%20%26%20biologics/published/Package-Insert---Gardasil.pdf.
- 141.Cervavarix. [(accessed on 4 July 2025)]; Available online: https://www.fda.gov/media/78013/download?attachment.
- 142.Zaman K., Schuind A.E., Adjei S., Antony K., Aponte J.J., Buabeng P.B., Qadri F., Kemp T.J., Hossain L., Pinto L.A., et al. Safety and immunogenicity of Innovax bivalent human papillomavirus vaccine in girls 9–14 years of age: Interim analysis from a phase 3 clinical trial. Vaccine. 2024;42:2290–2298. doi: 10.1016/j.vaccine.2024.02.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Walrinvax. [(accessed on 4 July 2025)]. Available online: https://extranet.who.int/prequal/vaccines/p/walrinvaxr.
- 144.Cervavac. [(accessed on 4 July 2025)]. Available online: https://www.seruminstitute.com/product_ind_cervavac.php.
- 145.Gardasil. [(accessed on 4 July 2025)]. Available online: https://www.ema.europa.eu/en/documents/product-information/gardasil-epar-product-information_en.pdf.
- 146.Cervarix. [(accessed on 4 July 2025)]. Available online: https://www.ema.europa.eu/en/documents/product-information/cervarix-epar-product-information_en.pdf.
- 147.WHO PUBLIC ASSESSMENT REPORT (WHOPAR) Cecolin. [(accessed on 4 July 2025)]. Available online: https://extranet.who.int/prequal/sites/default/files/vwa_vaccine/FVP-P-389_HVP_Innovax_WHOPAR_2025.pdf.
- 148.Falcaro M., Soldan K., Ndlela B., Sasieni P. Effect of the HPV vaccination programme on incidence of cervical cancer and grade 3 cervical intraepithelial neoplasia by socioeconomic deprivation in England: Population based observational study. BMJ. 2024;385:e077341. doi: 10.1136/bmj-2023-077341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Checchi M., Mesher D., Panwar K., Anderson A., Beddows S., Soldan K. The impact of over ten years of HPV vaccination in England: Surveillance of type-specific HPV in young sexually active females. Vaccine. 2023;41:6734–6744. doi: 10.1016/j.vaccine.2023.10.002. [DOI] [PubMed] [Google Scholar]
- 150.Palmer T., Wallace L., Pollock K.G., Cuschieri K., Robertson C., Kavanagh K., Cruickshank M. Prevalence of cervical disease at age 20 after immunisation with bivalent HPV vaccine at age 12–13 in Scotland: Retrospective population study. BMJ. 2019;365:l1161. doi: 10.1136/bmj.l1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Lei J., Ploner A., Elfström K.M., Wang J., Roth A., Fang F., Sundström K., Dillner J., Sparén P. HPV Vaccination and the Risk of Invasive Cervical Cancer. N. Engl. J. Med. 2020;383:1340–1348. doi: 10.1056/NEJMoa1917338. [DOI] [PubMed] [Google Scholar]
- 152.Patel C., Brotherton J.M., Pillsbury A., Jayasinghe S., Donovan B., Macartney K., Marshall H. The impact of 10 years of human papillomavirus (HPV) vaccination in Australia: What additional disease burden will a nonavalent vaccine prevent? Eurosurveillance. 2018;23:1700737. doi: 10.2807/1560-7917.ES.2018.23.41.1700737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Gargano J.W., Stefanos R., Dahl R.M., Castilho J.L., Bostick E.A., Niccolai L.M., Park I.U., Blankenship S., Brackney M.M., Chan K., et al. Trends in Cervical Precancers Identified Through Population-Based Surveillance-Human Papillomavirus Vaccine Impact Monitoring Project, Five Sites, United States, 2008–2022. MMWR Morb. Mortal. Wkly. Rep. 2025;74:96–101. doi: 10.15585/mmwr.mm7406a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Nonboe M.H., Napolitano G.M., Schroll J.B., Andersen B., Bennetsen M.H., Christiansen S., Frandsen A.P., Rygaard C., Salmani R., Høgdall E.V.S., et al. Human papillomavirus prevalence in first, second and third cervical cell samples from women HPV-vaccinated as girls, Denmark, 2017 to 2024: Data from the Trial23 cohort study. Eurosurveillance. 2025;30:2400820. doi: 10.2807/1560-7917.ES.2025.30.27.2400820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Mikalsen M.P., Simonsen G.S., Sørbye S.W. Impact of HPV Vaccination on the Incidence of High-Grade Cervical Intraepithelial Neoplasia (CIN2+) in Women Aged 20–25 in the Northern Part of Norway: A 15-Year Study. Vaccines. 2024;12:421. doi: 10.3390/vaccines12040421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Drolet M., Bénard É., Pérez N., Brisson M., Ali H., Boily M.-C., Baldo V., Brassard P., Brotherton J.M.L., Callander D., et al. Population-level impact and herd effects following the introduction of human papillomavirus vaccination programmes: Updated systematic review and meta-analysis. Lancet. 2019;394:497–509. doi: 10.1016/S0140-6736(19)30298-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Reuschenbach M., Doorbar J., del Pino M., Joura E.A., Walker C., Drury R., Rauscher A., Saah A.J. Prophylactic HPV vaccines in patients with HPV-associated diseases and cancer. Vaccine. 2023;41:6194–6205. doi: 10.1016/j.vaccine.2023.08.047. [DOI] [PubMed] [Google Scholar]
- 158.Arbyn M., Xu L., Simoens C., Martin-Hirsch P.P. Prophylactic vaccination against human papillomaviruses to prevent cervical cancer and its precursors. Cochrane Database Syst. Rev. 2018;5:Cd009069. doi: 10.1002/14651858.CD009069.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Sladič M., Taneska P., Cvjetičanin B., Velikonja M., Smrkolj V., Smrkolj Š. Cervical Intraepithelial Neoplasia Grade 3 in a HPV-Vaccinated Patient: A Case Report. Medicina. 2022;58:339. doi: 10.3390/medicina58030339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.McLucas B., Vail E., Chua K.J., Walt G. Cervical intraepithelial neoplasia grade 3 in a patient following Gardasil vaccination. BMJ Case Rep. 2019;12:e230366. doi: 10.1136/bcr-2019-230366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Makris G.M., Karakitsos P., Kotsifa E., Margari N., Poulakaki N., Sergentanis T.N., Battista M.J., Chrelias C., Papantoniou N. Endocervical Carcinogenesis and HPV Vaccination: An Occasional Circumstance or a Gap in the Chain? Case Rep. Obstet. Gynecol. 2017;2017:4976741. doi: 10.1155/2017/4976741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Shing J.Z., Hu S., Herrero R., Hildesheim A., Porras C., Sampson J.N., Schussler J., Schiller J.T., Lowy D.R., Sierra M.S., et al. Precancerous cervical lesions caused by non-vaccine-preventable HPV types after vaccination with the bivalent AS04-adjuvanted HPV vaccine: An analysis of the long-term follow-up study from the randomised Costa Rica HPV Vaccine Trial. Lancet Oncol. 2022;23:940–949. doi: 10.1016/S1470-2045(22)00291-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Tabibi T., Barnes J.M., Shah A., Osazuwa-Peters N., Johnson K.J., Brown D.S. Human Papillomavirus Vaccination and Trends in Cervical Cancer Incidence and Mortality in the US. JAMA Pediatr. 2022;176:313–316. doi: 10.1001/jamapediatrics.2021.4807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Gupta C., Dolma K.G., Sherpa M.L., Bag A., Byahut A. Human papillomavirus vaccine: Success and challenges. J. Biosci. 2025;50:51. doi: 10.1007/s12038-025-00493-8. [DOI] [PubMed] [Google Scholar]
- 165.Malone C., Barnabas R.V., Buist D.S.M., Tiro J.A., Winer R.L. Cost-effectiveness studies of HPV self-sampling: A systematic review. Prev. Med. 2020;132:105953. doi: 10.1016/j.ypmed.2019.105953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Sachan P.L., Singh M., Patel M.L., Sachan R. A Study on Cervical Cancer Screening Using Pap Smear Test and Clinical Correlation. Asia Pac. J. Oncol. Nurs. 2018;5:337–341. doi: 10.4103/apjon.apjon_15_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Lucksom P.G., Sherpa M.L., Pradhan A., Lal S., Gupta C. Advances in HPV Screening Tests for Cervical Cancer-A Review. J. Obstet. Gynaecol. India. 2022;72:13–18. doi: 10.1007/s13224-021-01569-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Tambvekar S.E., Balsarkar G. Modernizing Cervical Cytology Screening with Liquid-Based Methods at Community-Level Hospitals: A Much-Needed Breakthrough for India. J. Obstet. Gynecol. India. 2024;74:371–377. doi: 10.1007/s13224-024-02051-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.NHS Rolls Out More Personalised Cervical Screening for Millions. [(accessed on 4 July 2025)]. Available online: https://www.england.nhs.uk/2025/06/nhs-rolls-out-more-personalised-cervical-screening-for-millions/
- 170.U.S. Preventive Services Task Force Issues Draft Recommendation Statement on Screening for Cervical Cancer. [(accessed on 4 July 2025)]. Available online: https://www.uspreventiveservicestaskforce.org/uspstf/sites/default/files/file/supporting_documents/cervical-cancer-screening-draft-rec-bulletin_0.pdf.
- 171.Bouvard V., Wentzensen N., Mackie A., Berkhof J., Brotherton J., Giorgi-Rossi P., Kupets R., Smith R., Arrossi S., Bendahhou K., et al. The IARC Perspective on Cervical Cancer Screening. N. Engl. J. Med. 2021;385:1908–1918. doi: 10.1056/NEJMsr2030640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Zhang J., Zhao Y., Dai Y., Dang L., Ma L., Yang C., Li Y., Kong L., Wei L., Zhang S., et al. Effectiveness of High-risk Human Papillomavirus Testing for Cervical Cancer Screening in China: A Multicenter, Open-label, Randomized Clinical Trial. JAMA Oncol. 2021;7:263–270. doi: 10.1001/jamaoncol.2020.6575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Denny L., Kuhn L., Hu C.-C., Tsai W.-Y., Wright T.C. Human Papillomavirus–Based Cervical Cancer Prevention: Long-term Results of a Randomized Screening Trial. JNCI J. Natl. Cancer Inst. 2010;102:1557–1567. doi: 10.1093/jnci/djq342. [DOI] [PubMed] [Google Scholar]
- 174.Ogilvie G.S., van Niekerk D., Krajden M., Smith L.W., Cook D., Gondara L., Ceballos K., Quinlan D., Lee M., Martin R.E., et al. Effect of Screening with Primary Cervical HPV Testing vs Cytology Testing on High-grade Cervical Intraepithelial Neoplasia at 48 Months: The HPV FOCAL Randomized Clinical Trial. Jama. 2018;320:43–52. doi: 10.1001/jama.2018.7464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Moy L.M., Zhao F.-H., Li L.-Y., Ma J.-F., Zhang Q.-M., Chen F., Song Y., Hu S.-Y., Balasubramanian A., Pan Q.-J., et al. Human papillomavirus testing and cervical cytology in primary screening for cervical cancer among women in rural China: Comparison of sensitivity, specificity, and frequency of referral. Int. J. Cancer. 2010;127:646–656. doi: 10.1002/ijc.25071. [DOI] [PubMed] [Google Scholar]
- 176.Kaya Terzi N., Yulek O. Assessment of Cervicovaginal Smear and HPV DNA Co-Test for Cervical Cancer Screening: Implications for Diagnosis and Follow-Up Strategies. Diagnostics. 2024;14:611. doi: 10.3390/diagnostics14060611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Comparing Clinician-Collected and Self-Collected Tests for Detecting High-Risk HPV Infection among Female-to-Male Transgender Adults. [(accessed on 4 July 2025)]; Available online: https://www.ncbi.nlm.nih.gov/books/NBK595846/ [PubMed]
- 178.Ogilvie G.S., Patrick D.M., Schulzer M., Sellors J.W., Petric M., Chambers K., White R., FitzGerald J.M. Diagnostic accuracy of self collected vaginal specimens for human papillomavirus compared to clinician collected human papillomavirus specimens: A meta-analysis. Sex. Transm. Infect. 2005;81:207–212. doi: 10.1136/sti.2004.011858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Mathews C.S., Sargent A., Cuschieri K., Rebolj M., Brentnall A.R., Mackie A., Mills C., Martinelli C., Wright A.-M., Hunt K., et al. HPValidate—Human papillomavirus testing with DNA and mRNA assays on self-collected samples in cervical screening: Comparison of test characteristics on three self-sampling devices. Br. J. Cancer. 2025 doi: 10.1038/s41416-025-03102-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.FDA Roundup. [(accessed on 4 July 2025)]; Available online: https://www.fda.gov/news-events/press-announcements/fda-roundup-may-17-2024.
- 181.Shastri S.S., McNeill L.H., Shete S. Culturally Competent Education and Human Papillomavirus Self-Sampling Achieves Healthy People 2030 Cervical Screening Target Among Low-Income Non-Hispanic Black and Hispanic Women. JCO Glob. Oncol. 2024:e2400005. doi: 10.1200/GO.24.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Fullerton M.M., Ford C., D’Silva C., Chiang B., Onobrakpor S.-I., Dievert H., Yang H., Cabaj J., Ivers N., Davidson S., et al. HPV self-sampling implementation strategies to engage under screened communities in cervical cancer screening: A scoping review to inform screening programs. Front. Public Health. 2024;12:1430968. doi: 10.3389/fpubh.2024.1430968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Hariprasad R., Srinivasan M., Ravi P., Tamang H., Sharma A., Pradhan S., Dhanasekaran K. A qualitative study on ASHA workers’ perspective on HPV self-sampling in Sikkim India. Sci. Rep. 2025;15:15985. doi: 10.1038/s41598-025-87041-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Choi H., Dion H., Huang M., Sathyan L., Herfel E., Makhulo B., Ambaka J., Huchko M.J. Applying the Information-Motivation-Behavioral Skills model to a video-assisted HPV intervention to promote self-screening uptake: A qualitative study in Western Kenya. BMJ Glob. Health. 2025;10:e017616. doi: 10.1136/bmjgh-2024-017616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Xiong S., Ghebre R., Kulasingam S., Mason S.M., Pratt R.J., Lazovich D. Exploring factors associated with preferences for human papillomavirus (HPV) self-sampling among racially- and ethnically-diverse women in Minnesota: A cross-sectional study. Prev. Med. Rep. 2023;34:102243. doi: 10.1016/j.pmedr.2023.102243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Global Cervical Cancer Elimination Forum. [(accessed on 4 July 2025)]. Available online: https://www.who.int/initiatives/cervical-cancer-elimination-initiative/cervical-cancer-forum/global-opportunity-and-challenge.
- 187.Cervical Cancer Elimination Initiative. [(accessed on 4 July 2025)]. Available online: https://www.who.int/initiatives/cervical-cancer-elimination-initiative.
- 188.Chidebe R.C.W., Osayi A., Torode J.S. The Global Fund, Cervical Cancer, and HPV infections: What can low- and middle-income countries do to accelerate progress by 2030? EClinicalmedicine. 2025;81:103127. doi: 10.1016/j.eclinm.2025.103127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Ginsburg O. Global disparities in HPV vaccination. Lancet Glob. Health. 2016;4:e428–e429. doi: 10.1016/S2214-109X(16)30107-3. [DOI] [PubMed] [Google Scholar]
- 190.Dorji T., Nopsopon T., Tamang S.T., Pongpirul K. Human papillomavirus vaccination uptake in low-and middle-income countries: A meta-analysis. EClinicalMedicine. 2021;34:100836. doi: 10.1016/j.eclinm.2021.100836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Serrano B., Ibáñez R., Robles C., Peremiquel-Trillas P., de Sanjosé S., Bruni L. Worldwide use of HPV self-sampling for cervical cancer screening. Prev. Med. 2022;154:106900. doi: 10.1016/j.ypmed.2021.106900. [DOI] [PubMed] [Google Scholar]
- 192.Ken-Amoah S., Blay Mensah L.B., Eliason S., Anane-Fenin B., Agbeno E.K., Essuman M.A., Essien-Baidoo S. Poor knowledge and awareness of human papillomavirus and cervical cancer among adult females in rural Ghana. Front. Trop. Dis. 2022;3:971266. doi: 10.3389/fitd.2022.971266. [DOI] [Google Scholar]
- 193.Ebu N.I., Abotsi-Foli G.E., Gakpo D.F. Nurses’ and midwives’ knowledge, attitudes, and acceptance regarding human papillomavirus vaccination in Ghana: A cross-sectional study. BMC Nurs. 2021;20:11. doi: 10.1186/s12912-020-00530-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Dedey F., Nsaful J., Nartey E., Labi J., Adu-Aryee N.A., Kuti C., Clegg-Lamptey J.-N. Assessing the impact of cervical cancer education in two high schools in Ghana. BMC Cancer. 2024;24:1359. doi: 10.1186/s12885-024-13134-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Nartey Y., Amo-Antwi K., Osei-Ntiamoah B., Hill P.C., Dassah E.T., Asmah R.H., Nyarko K.M., Agambire R., Konney T.O., Yarney J., et al. Knowledge of Human Papillomavirus, Risk Factors and Screening for Cervical Cancer Among Women in Ghana. Cancer Control. 2025;32:10732748251323765. doi: 10.1177/10732748251323765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Nyaaba J.A., Akurugu E. Knowledge, barriers and uptake towards Cervical Cancer screening among female health workers in Ghana: A perspective of the Health Belief Model. Int. J. Afr. Nurs. Sci. 2023;19:100587. doi: 10.1016/j.ijans.2023.100587. [DOI] [Google Scholar]
- 197.Appiah R.S., Boakye K., Appiah G., Aidoo A.A., Acquah-Hagan G., Singh B., Appiah F., Boateng D. Rural-urban variations in cervical cancer screening uptake among women in Ghana: Evidence from the 2022 Ghana Demographic and Health Survey. BMC Women’s Health. 2025;25:255. doi: 10.1186/s12905-025-03802-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Adebamowo C., Rossi P.G., Castle P.E. Challenges and Opportunities for Global Cervical Cancer Elimination: How Can We Build a Model for Other Cancers? Am. Soc. Clin. Oncol. Educ. Book. 2025;45:e473702. doi: 10.1200/EDBK-25-473702. [DOI] [PubMed] [Google Scholar]
- 199.Avila-Aguero M.L., Ospina-Henao S., Brenes-Chacon H., Espinal-Tejada C., Trejo-Varon R., Morice A. Human Papilloma Virus Vaccination as a Strategy to Eliminate Cervical Cancer: Challenges and Opportunities. Vaccines. 2025;13:297. doi: 10.3390/vaccines13030297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Wu Z.H., An Y.J., Chu Z.X., Li X.R., Hou A.Y., Jiang Y.J., Hu Q.H. Cost-effectiveness of self-sampling and enhanced strategies for HPV prevention among men who have sex with men in China: A modeling study. BMC Med. 2025;23:362. doi: 10.1186/s12916-025-04131-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Global Leaders Unite to Accelerate Cervical Cancer Elimination Efforts. [(accessed on 4 July 2025)]. Available online: https://www.who.int/westernpacific/news/item/19-06-2025-global-leaders-unite-to-accelerate-cervical-cancer-elimination-efforts.
- 202.Stanley M., Schuind A., Muralidharan K.K., Guillaume D., Willens V., Borda H., Jurgensmeyer M., Limaye R. Evidence for an HPV one-dose schedule. Vaccine. 2024;42:S16–S21. doi: 10.1016/j.vaccine.2024.01.046. [DOI] [PubMed] [Google Scholar]
- 203.Bula A.K., Mhango P., Tsidya M., Chimwaza W., Kaira P., Ghambi K., Heitner J., Lee F., McGue S., Chinula L., et al. Client perspectives and satisfaction with integrating facility and community-based HPV self-sampling for cervical cancer screening with family planning: A mixed method study. BMC Public Health. 2025;25:1718. doi: 10.1186/s12889-025-22761-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Arrossi S., Thouyaret L., Herrero R., Campanera A., Magdaleno A., Cuberli M., Barletta P., Laudi R., Orellana L. Effect of self-collection of HPV DNA offered by community health workers at home visits on uptake of screening for cervical cancer (the EMA study): A population-based cluster-randomised trial. Lancet Glob. Health. 2015;3:e85–e94. doi: 10.1016/S2214-109X(14)70354-7. [DOI] [PubMed] [Google Scholar]
- 205.Tourneau C.L., Rolland F., Capitain O., Cassier P.A., Fumet J.D., Salas S., Daste A., Manso L., Bermejo-Perez M.-J., Casado A., et al. Randomized phase II trial evaluating the combination of TG4001, an HPV16 therapeutic vaccine, and avelumab (ave) in patients (pts) with immunotherapy-naïve recurrent and/or metastatic (R/M) HPV16-positive cervical or anogenital cancer. J. Clin. Oncol. 2025;43:2638. doi: 10.1200/JCO.2025.43.16_suppl.2638. [DOI] [Google Scholar]
- 206.Zhao X., Zhang Y., Trejo-Cerro O., Kaplan E., Li Z., Albertsboer F., El Hammiri N., Mariz F.C., Banks L., Ottonello S., et al. A safe and potentiated multi-type HPV L2-E7 nanoparticle vaccine with combined prophylactic and therapeutic activity. npj Vaccines. 2024;9:119. doi: 10.1038/s41541-024-00914-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Wang J., Wang Q., Ma L., Lv K., Han L., Chen Y., Zhou R., Zhou H., Chen H., Wang Y., et al. Development of an mRNA-based therapeutic vaccine mHTV-03E2 for high-risk HPV-related malignancies. Mol. Ther. 2024;32:2340–2356. doi: 10.1016/j.ymthe.2024.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Eerkens A.L., Esajas M.D., Brummel K., Vledder A., van Rooij N., Plat A., Avalos Haro S.B., Paijens S.T., Slagter-Menkema L., Schuuring E., et al. Vvax001, a Therapeutic Vaccine, for Patients with HPV16-Positive High-grade Cervical Intraepithelial Neoplasia: A Phase II Trial. Clin Cancer Res. 2025;31:1016–1026. doi: 10.1158/1078-0432.CCR-24-1662. [DOI] [PubMed] [Google Scholar]
- 209.Xu M., Cao C., Wu P., Huang X., Ma D. Advances in cervical cancer: Current insights and future directions. Cancer Commun. 2025;45:77–109. doi: 10.1002/cac2.12629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Stegmüller T., Abbet C., Bozorgtabar B., Clarke H., Petignat P., Vassilakos P., Thiran J.P. Self-supervised learning-based cervical cytology for the triage of HPV-positive women in resource-limited settings and low-data regime. Comput. Biol. Med. 2024;169:107809. doi: 10.1016/j.compbiomed.2023.107809. [DOI] [PubMed] [Google Scholar]
- 211.Burmeister C.A., Khan S.F., Schäfer G., Mbatani N., Adams T., Moodley J., Prince S. Cervical cancer therapies: Current challenges and future perspectives. Tumour Virus Res. 2022;13:200238. doi: 10.1016/j.tvr.2022.200238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Wang R., Huang H., Yu C., Li X., Wang Y., Xie L. Current status and future directions for the development of human papillomavirus vaccines. Front. Immunol. 2024;15:1362770. doi: 10.3389/fimmu.2024.1362770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Fitzpatrick M.B., Behrens C.M., Hibler K., Parsons C., Kaplan C., Orso R., Parker L., Memmel L., Collins A., McNicholas C., et al. Clinical Validation of a Vaginal Cervical Cancer Screening Self-Collection Method for At-Home Use: A Nonrandomized Clinical Trial. JAMA Netw. Open. 2025;8:e2511081. doi: 10.1001/jamanetworkopen.2025.11081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Han L., Zhang B. Can prophylactic HPV vaccination reduce the recurrence of cervical lesions after surgery? Review and prospect. Infect. Agents Cancer. 2023;18:66. doi: 10.1186/s13027-023-00547-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Pruski D., Millert-Kalińska S., Jach R., Przybylski M. Effect of vaccination against HPV in the HPV-positive patients not covered by primary prevention on the disappearance of infection. Sci. Rep. 2025;15:12642. doi: 10.1038/s41598-025-92861-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Petráš M., Lomozová D., Dvořák V., Dvořák V., Jr., Malinová J., Trnková M., Fišer I., Dlouhý P., Rosina J., Lesná I.K. Early and long-term effects of prophylactic and post-excision human papillomavirus vaccination on recurrent high-grade cervical intraepithelial neoplasia relative to margin status: A retrospective cohort study in the Czech Republic. Lancet Reg. Health–Eur. 2025;55:101337. doi: 10.1016/j.lanepe.2025.101337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Alarid-Escudero F., Gracia V., Wolf M., Zhao R., Easterly C.W., Kim J.J., Canfell K., de Kok I.M.C.M., Barnabas R.V., Kulasingam S. State-level disparities in cervical cancer prevention and outcomes in the United States: A modeling study. JNCI J. Natl. Cancer Inst. 2024;117:737–746. doi: 10.1093/jnci/djae298. [DOI] [PMC free article] [PubMed] [Google Scholar]