Abstract
Studies over the last two decades have shown that mammalian nonmacrophagic liver endothelial cells clear the blood from numerous physiological and foreign waste macromolecules, such as polysaccharides and proteins released during extracellular matrix turnover, intracellular macromolecules, modified serum proteins, and bacterial and fungal proteins [Smedsrød, B., Pertoft, H., Gustafson, S. & Laurent, T. C. (1990) Biochem. J. 266, 313–327]. These macromolecules are released daily in gram-amounts in a normal human body and are effectively taken up and degraded by the liver endothelial cells. Recent studies show that bony fishes harbor a similar system of specialized nonmacrophagic scavenger endothelial cells in either kidney [Smedsrød, B., Gjøen, T., Sveinbjørnsson, B. & Berg, T. (1993) J. Fish Biol. 42, 279–291] or heart [Sørensen, K. K., Melkko, J. & Smedsrød, B. (1998) J. Exp. Biol. 201, 1707–1718], but not in liver. Using specific and extremely effective endocytosis, these fish scavenger endothelial cells function as their mammalian counterpart to eliminate soluble waste macromolecules from the circulation. We show here that species from all seven vertebrate classes carry a population of nonmacrophagic scavenger endothelial cells that efficiently eliminate an array of circulating waste macromolecules. Thus representing an important part of the vertebrate innate immune system, these scavenger endothelial cells display the following distribution in the different vertebrate classes: Gills in Agnatha and Chondrichtyes; heart or kidney in Osteichtyes; and liver in Amphibia, Reptilia, Aves, and Mammalia.
In his pioneering work on the ultrastructure of the hepatic sinusoid 30 years ago, Wisse (1) observed numerous “smooth-walled macropinocytic vesicles” in the sinusoidal liver endothelial cells (LEC), suggestive of active pinocytic scavenging of blood plasma proteins. The subsequent finding that the specific activities of lysosomal enzymes in LEC were as high as in the Kupffer cells (2), and for some enzymes even higher, substantiated Wisse's notion. In a series of papers over the next 20 years, it was shown that LEC eliminate an array of soluble macromolecular physiologic and foreign waste products from the circulation by receptor-mediated endocytosis (3–7). It is now well established that the population of LEC in mammals serves as the most important site of elimination of an array of circulating soluble macromolecular waste products. To carry out this scavenger function, LEC express at least five types of specific receptors for endocytosis of major physiologic waste products: (i) the hyaluronan receptor for major matrix polysaccharides and proteoglycans such as hyaluronan and chondroitin sulfate (8); (ii) the collagen alpha-chain receptor for collagen alpha-chains of several types of collagen (9); (iii) the scavenger receptor for amino-terminal propeptides of types I and III procollagen (4), modified macromolecules such as atherogenic advanced glycation end (AGE) products (5) and oxidized LDL (6), oligodeoxynucleotides (10), and blood proteins modified by the process of blood clotting and platelet activation (11); (iv) the mannose receptor for carboxy-terminal propeptide of type I procollagen (7) and tissue plasminogen activator (12); and (v) the Fc-γ receptor for IgG-antigen immune complexes (13). It is interesting to note that the LEC are able to clear all major categories of biological macromolecules (proteins, polysaccharides, lipids, and nucleic acids) by means of these five types of endocytosis receptors. The blood concentration of most of these substances is normally very low (ng/ml) because of highly efficient elimination by endocytosis receptors on LEC.
On the basis of several studies showing that LEC represent an important nonphagocytic scavenger cell in mammals, it has been suggested that the hepatic scavenger function is shared between Kupffer cells, which eliminate insoluble waste by phagocytosis, and LEC, which remove soluble colloidal or macromolecular waste (3). Hypothesizing that the scavenger function of LEC in mammals be represented by similar nonmacrophagic, nonphagocytic scavenger endothelia in animals of all vertebrate classes, we set out to screen animal species from the seven major vertebrate classes for the presence of scavenger cells equivalent of mammalian LEC.
The findings from these studies strongly suggest the presence of a nonmacrophagic system of scavenger endothelial cells that clear the blood from an array of circulating waste macromolecules and colloids. These scavenger endothelial cells make up the backbone of the reticuloendothelial system (RES), and thus represent an important part of the innate immune system.
Materials and Methods
Animals.
The following animal species were used: mouse (Mus musculus, Mammalia); rat (Rattus norvegicus, Mammalia); chicken (Gallus gallus, Aves); lizard (Anolus carolinensis, Reptilia); frog (Rana temporaria, Amphibia); Atlantic cod (Gadus morhua, Osteichtyes); Atlantic salmon (Salmo salar, Osteichtyes); Crucian carp (Carassius carassius, Osteichtyes); ray (Raja radiata, Chondrichtyes); lamprey (Lampetra fluviatilis, Agnatha); hagfish (Myxine glutinosa, Agnatha). Experimental protocols were approved by the Norwegian Ethics Committee for Research on Animals.
Probes to Study Nonphagocytic Endocytosis in Scavenger Endothelial Cells and Phagocytosis in Macrophages.
Collagen alpha chains, a ligand for the collagen alpha chain receptor, were prepared from bovine collagen (Collagen Biomaterials, Palo Alto, CA) or from hagfish (14). BSA (Sigma) was treated with formaldehyde as described (15) to make it a ligand for the scavenger receptor. Aminated latex beads (2 μm diameter), to study phagocytosis in macrophages, were a generous gift of J. Ugelstad and A. Berge (University of Trondheim, Norway).
Labeling of Soluble and Particular Probes with Fluorescence or Radioactivity.
Collagen alpha chains, formaldehyde-treated BSA, and aminated latex beads were labeled with fluorescein isothiocyanate as described (16–18). Collagen alpha chains and formaldehyde-treated BSA were conjugated to the trap-label 125I-tyraminyl cellobiose as described (19) to prevent the radiolabel from escaping from the cellular site of uptake.
Isolation and Cultivation of Cells.
Scavenger endothelial cells from rat and mouse liver were isolated and cultivated using in situ collagenase perfusion, density sedimentation, and selective adherence (ref. 20; B. Hansen, B. Arteta, and B.S., unpublished work). Isolation and culture of cod heart scavenger endothelial cells were accomplished by combined collagenase and trypsin treatment followed by differential sedimentation and selective adherence (22).
Results and Discussion
First, ligands labeled with the trap label 125I-tyramine cellobiose were administered, and the anatomical distribution of radioactivity determined after 24 h (Table 1). Collagen alpha chains and formaldehyde-treated BSA, endocytosed by mammalian LEC collagen alpha chain and scavenger receptors, respectively (3), were taken up mainly or exclusively in the liver of chicken, lizard, and frog. In cod, uptake was in heart (both atrium and ventricle; refs. 16 and 18); in salmon (23) and carp the ligands accumulated in head and trunk kidney. In vertebrates of highest phylogenetic age (ray, lamprey, and hagfish), ligands accumulated in the gills. Cod and salmon were also injected with ligands for the hyaluronan and mannose receptors. Hyaluronan accumulated exclusively in salmon kidney sinusoidal endothelium (unpublished) and cod endocardium (17). Ligands for the mannose receptor accumulated in heart of cod and kidney of the salmonid and rainbow trout, and also in liver of the two fishes (24–26).
Table 1.
Anatomical distribution of soluble radiolabeled macromolecules in different classes of vertebrates
| Vertebrate | Heart | Gills | Liver | Spleen | Kidney | Blood |
|---|---|---|---|---|---|---|
| Agnatha | ||||||
| Hagfish* (Myxine glutinosa) | − | + | + | − | − | |
| Lamprey* (Lampetra fluviatilis) | − | + | − | − | − | |
| Chondrichthyes | ||||||
| Ray* (Raja radiata) | − | + | − | − | − | − |
| Osteichthyes | ||||||
| Crucian carp* (Carassius carassius) | − | − | − | − | + | − |
| Atlantic salmon† (Salmo salar) | − | − | − | − | + | − |
| Atlantic cod‡ (Gadus morhua) | + | − | − | − | − | − |
| Amphibia | ||||||
| Frog* (Rana temporaria) | − | + | ||||
| Reptilia | ||||||
| Lizard* (Anolis carolinensis) | − | + | − | − | − | |
| Aves | ||||||
| Chicken* (Gallus gallus) | − | + | − | − | − | |
| Mammalia | ||||||
| Rat§ (Rattus norvegicus) | − | + | − | − | − | |
| Mouse¶ (Mus musculus) | − | + | − | − | − |
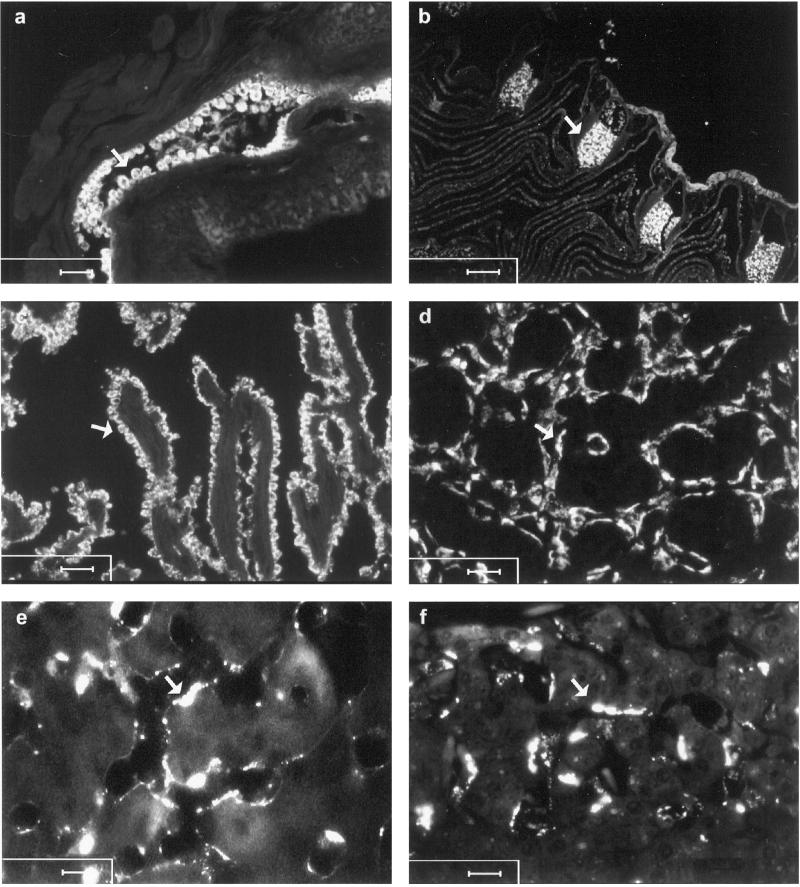
In the next set of experiments, fluorescently labeled ligands were administered to trace the cellular localization of these waste molecules. In hagfish, the probe was taken up exclusively in the high endothelium of the radial artery of the gills (Fig. 1a). In lamprey (Fig. 1b) and ray (not shown), the probes accumulated in the cavernous body, which is a branch of the afferent artery of the gill. In cod, the cells that stained positively in heart were identified as endocardium (Fig. 1c). In salmon (Fig. 1d) and crucian carp (not shown), the cells that accumulated fluorescence were identified as endothelial cells lining blood sinusoids in trunk (Fig. 1) and head kidney. All terrestrial vertebrates studied accumulated the probes in LEC (Fig. 1 e and f). Administration of fluorescently labeled particles (2 μm diameter) in selected species showed insignificant colocalization of particles and soluble test ligands (not shown). In hagfish and cod, beads and soluble probes distributed to different organs. Thus, soluble probes were taken up in endocardium of cod, whereas particles in kidney were recovered almost entirely (16). In hagfish, particles were taken up by liver macrophages, whereas soluble probes accumulated in high endothelium of gill arteries.
Figure 1.
Cellular distribution of fluorescein isothiocyanate (FITC)-labeled formaldehyde-treated serum albumin 24 h after injection. Arrows point to accumulation of fluorescence in hagfish (a, high endothelium of the radial artery of the gills); lamprey (b, cavernous body, which is a branch of the afferent artery of the gill); cod (c, endocardium); salmon (d, endothelial cells lining blood sinusoids in trunk kidney); and lizard and rat (e and f, sinusoidal liver endothelial cells). [Scale bars, 50 μm (a and b), 25 μm (c and d), and 10 μm (e and f).]
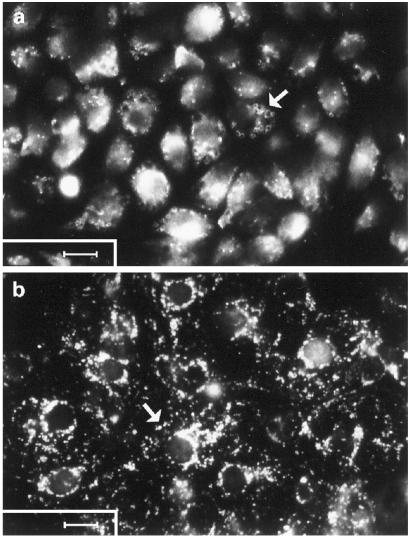
A recently developed method (22) to isolate and culture cod atrial endocardial endothelial cells was used to show that these cells avidly take up fluorescently labeled bovine collagen alpha chains in vitro (Fig. 2a). Similarly, a well established technique (20) to prepare primary cultures of rat LEC was used to show that these cells actively take up fluorescently labeled hagfish collagen alpha chains in vitro (Fig. 2b).
Figure 2.
Uptake of FITC-labeled ligands in primary cultures of cod endocardium and rat sinusoidal liver endothelial cells. (a) Cultured cod endocardial endothelial cells after a 1-h incubation with FITC-labeled formaldehyde-treated BSA (100 μg/ml) at 12°C. (b) cultured rat liver sinusoidal endothelial cells after a 1-h incubation with FITC-labeled hagfish collagen alpha chains (100 μg/ml) at 37°C. (Arrows, accumulation of probe in vesicles; Scale bars, 10 μm.)
When Aschoff (27) introduced the concept of the RES, that is a system of cells with scavenger function, about 80 years ago, both macrophages and other types of cells that accumulated intravenously administered vital stains were included. During the decades that followed, a comparatively large body of research was carried out in the field of macrophage biology, and the macrophage came to be regarded as the only cell type making up the RES. However, a recent re-evaluation of the vital stain method revealed that the cells in rat that accumulate intravenously administered stain are almost exclusively LEC (28). Thus, if RES is defined strictly on the basis of accumulation of vital stain, macrophages should not be considered part of the RES of liver. This is, of course, a rather controversial conclusion that may confuse rather than clarify. To avoid this difficulty, one should look more closely into the kind of test substances that are used to distinguish the cells of RES. Particles are cleared from blood almost exclusively by macrophages lining the blood vessels of organs that are traditionally associated with RES function (i.e., liver and spleen). If, on the other hand, soluble macromolecules such as colloidal vital stains are injected, the material will be scavenged almost exclusively by LEC. Thus, by including clearance of both particulate and soluble test material as criteria, RES should be defined as the system of scavenger cells that remove particles and soluble macromolecular waste material from the circulation. By applying these criteria for the definition of RES, both Kupffer cells and LEC should be viewed as equally important members of mammalian hepatic RES.
It was noted already 120 years ago (29) that insects carry a scavenger cell type, the so-called pericardial cell, that apparently serves the same clearance function as scavenger endothelial cells of vertebrates. In a study published by Crossley in 1972 (30), it was concluded that these insect pericardial cells operate in much the same way as vertebrate scavenger endothelial cells in that they take up only soluble macromolecules and colloids, not particles. In contrast, the insect haemocytes operate as vertebrate macrophages in that they phagocytose particles. Thus, the RES of insects and vertebrates share the feature of consisting of two functionally different cell types: one cell type is geared to endocytosis of soluble macromolecules and colloids (scavenger endothelial cells in vertebrates and pericardial cells in insects), and a different cell type is specialized on phagocytosis of larger insoluble matter (macrophages of vertebrates and haemocytes of insects).
The findings in this present paper can be summarized as follows: (i) Species from the major vertebrate classes are furnished with a population of specialized scavenger endothelium that plays a central role in the catabolism of physiologic and unphysiologic soluble waste macromolecules. (ii) Scavenger endothelial cells of all seven vertebrate classes avidly take up ligands for the collagen alpha chain and scavenger receptors. In addition, the hyaluronan and mannose receptors have been demonstrated in scavenger endothelial cells of mammals (several species) and bony fishes [salmonids and cod (21)]. (iii) Scavenger endothelia in all vertebrate classes are geared to endocytosis of colloids and soluble macromolecules; phagocytic uptake of particles is performed mainly by macrophages. Therefore, the scavenger system of vertebrates is based on two groups of cells, namely scavenger endothelial cells (scavenging by pinocytosis of soluble macromolecules or colloids) and macrophages (scavenging by phagocytosis of particulate material). Vertebrate scavenger endothelial cells and macrophages appear to carry the same function as insect pericardial cells and haemocytes, respectively. (iv) The most primitive vertebrates, Agnatha (hagfish and lamprey) and Elasmobranchia (ray), carry their scavenger endothelial cells in specialized gill arteries; scavenger endothelial cells of the phylogenetically younger Osteichtyes (salmonids, carp, cod) are located in either kidney sinusoids or endocardium; and in the four classes of terrestrial vertebrates, scavenger endothelial cells are located in the liver sinusoids. On this basis we propose the name Scavenger Endothelial Cells to distinguish these reticuloendothelial cells from other types of endothelia and highlight their role as a major scavenger apparatus, representing an important part of the vertebrate innate immune system.
Acknowledgments
This work was supported by The Norwegian Research Council.
Abbreviations
- LEC
liver endothelial cells
- RES
reticuloendothelial system
References
- 1.Wisse E. J Ultrastruct Res. 1972;38:528–562. doi: 10.1016/0022-5320(72)90089-5. [DOI] [PubMed] [Google Scholar]
- 2.Knook D L, Sleyster E C. Biochem Biophys Res Commun. 1980;96:250–257. doi: 10.1016/0006-291x(80)91207-3. [DOI] [PubMed] [Google Scholar]
- 3.Smedsrød B, Pertoft H, Gustafson S, Laurent T C. Biochem J. 1990;266:313–327. doi: 10.1042/bj2660313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Melkko J, Hellevik T, Risteli L, Risteli J, Smedsrød B. J Exp Med. 1994;179:405–412. doi: 10.1084/jem.179.2.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smedsrød B, Melkko J, Araki N, Sano H, Horiuchi S. Biochem J. 1997;322:567–573. doi: 10.1042/bj3220567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Van Berkel T J C, de Rijke Y B, Krujit J K. J Biol Chem. 1991;266:2282–2289. [PubMed] [Google Scholar]
- 7.Smedsrød B, Melkko J, Risteli L, Risteli J. Biochem J. 1990;271:345–350. doi: 10.1042/bj2710345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smedsrød B, Pertoft H, Eriksson S, Fraser J R E, Laurent T C. Biochem J. 1984;223:617–626. doi: 10.1042/bj2230617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smedsrød B, Johansson S, Pertoft H. Biochem J. 1985;228:415–424. doi: 10.1042/bj2280415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bijsterbosch M K, Manoharan M, Rump E T, De Vrueh R L A, van Veghel R, Tivel K L, Biessen E A L, Bennett C F, Cook P D, van Berkel T J C. Nucleic Acids Res. 1997;25:3290–3296. doi: 10.1093/nar/25.16.3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hansen B, Melkko J, Smedsrød B. Mol Cell Biochem. 2002;229:463–472. doi: 10.1023/a:1017919800347. [DOI] [PubMed] [Google Scholar]
- 12.Smedsrød B, Einarsson M. Thromb Haemostasis. 1990;63:60–66. [PubMed] [Google Scholar]
- 13.Skogh T, Blomhoff R, Eskild W, Berg T. Immunology. 1985;55:585–594. [PMC free article] [PubMed] [Google Scholar]
- 14.Lewis M S, Piez K A. J Biol Chem. 1964;239:3336–3340. [PubMed] [Google Scholar]
- 15.Mego J L, Bertini F, McQueen D J. J Cell Biol. 1967;32:699–707. doi: 10.1083/jcb.32.3.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smedsrød B, Olsen R, Sveinbjørnsson B. Cell Tissue Res. 1995;280:39–48. [Google Scholar]
- 17.Sørensen K K, Dahl L B, Smedsrød B. Cell Tissue Res. 1997;290:101–109. doi: 10.1007/s004410050912. [DOI] [PubMed] [Google Scholar]
- 18.Sørensen K K, Melkko J, Smedsrød B. J Exp Biol. 1998;201:1707–1718. doi: 10.1242/jeb.201.11.1707. [DOI] [PubMed] [Google Scholar]
- 19.Pittman R C, Carew T E, Glass C K, Green S R, Taylor C A, Jr, Attie A D. Biochem J. 1984;212:791–800. doi: 10.1042/bj2120791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smedsrød B, Pertoft H. J Leukocyte Biol. 1985;38:213–230. doi: 10.1002/jlb.38.2.213. [DOI] [PubMed] [Google Scholar]
- 21.Seternes T, Øynebråten I, Sørensen K, Smedsrød B. J Exp Biol. 2001;204:1537–1546. doi: 10.1242/jeb.204.9.1537. [DOI] [PubMed] [Google Scholar]
- 22.Koren C W R, Sveinbjørnsson B, Smedsrød B. Cell Tissue Res. 1997;290:89–99. doi: 10.1007/s004410050911. [DOI] [PubMed] [Google Scholar]
- 23.Smedsrød B, Gjøen T, Sveinbjørnsson B, Berg T. J Fish Biol. 1993;42:279–291. [Google Scholar]
- 24.Dannevig B H, Lauve A, Press C M, Landsverk T. Fish Shellfish Immunol. 1994;4:3–18. [Google Scholar]
- 25.Dannevig B H, Struksnæs G, Skogh T, Kindberg G, Berg T. Fish Physiol Biochem. 1990;8:229–238. doi: 10.1007/BF00004462. [DOI] [PubMed] [Google Scholar]
- 26.Sørensen K K, Tollersrud O K, Evjen G, Smedsrød B. Comp Biochem Physiol. 2001;129:615–630. doi: 10.1016/s1095-6433(01)00300-2. [DOI] [PubMed] [Google Scholar]
- 27.Aschoff L. Ergeb Inn Med Kinderheilkd. 1924;26:1–118. [Google Scholar]
- 28.Kawai Y, Smedsrød B, Elvevold K, Wake K. Cell Tissue Res. 1998;292:395–410. doi: 10.1007/s004410051069. [DOI] [PubMed] [Google Scholar]
- 29.Balbiani C R. C R Acad Sci. 1886;103:952–954. [Google Scholar]
- 30.Crossley A C. Tissue Cell. 1972;4:529–560. doi: 10.1016/s0040-8166(72)80029-6. [DOI] [PubMed] [Google Scholar]