Abstract
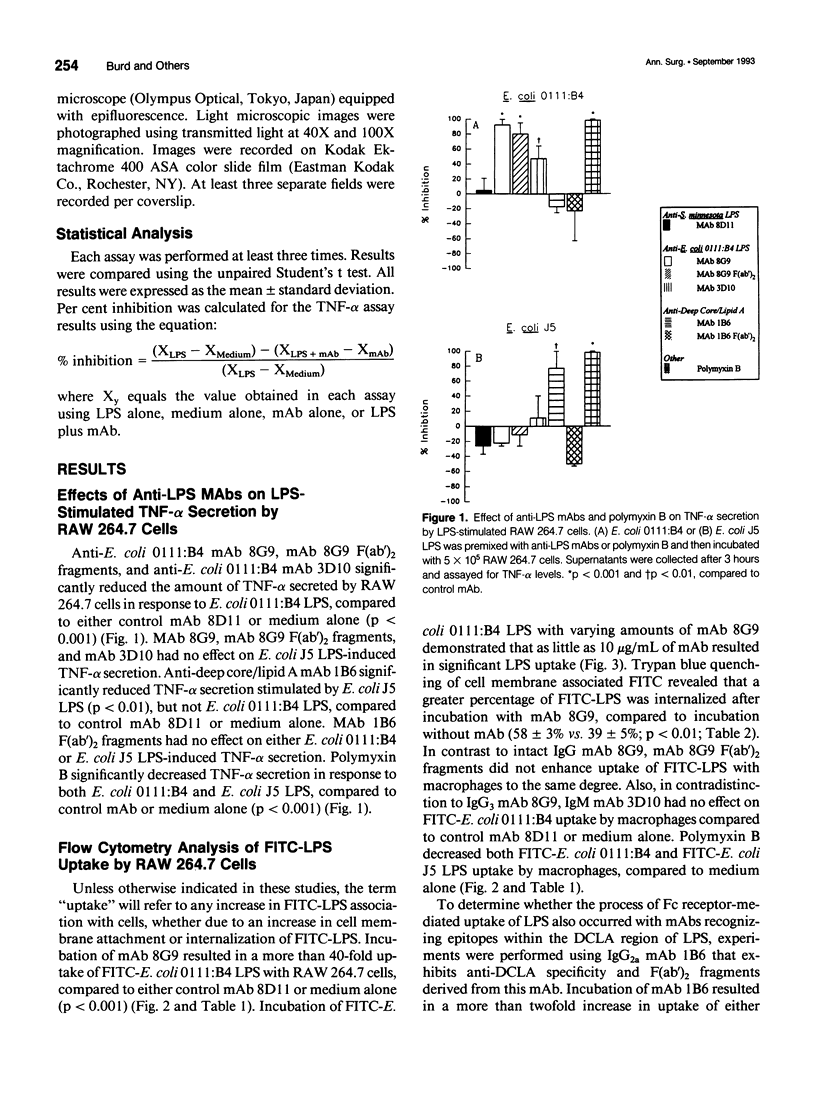
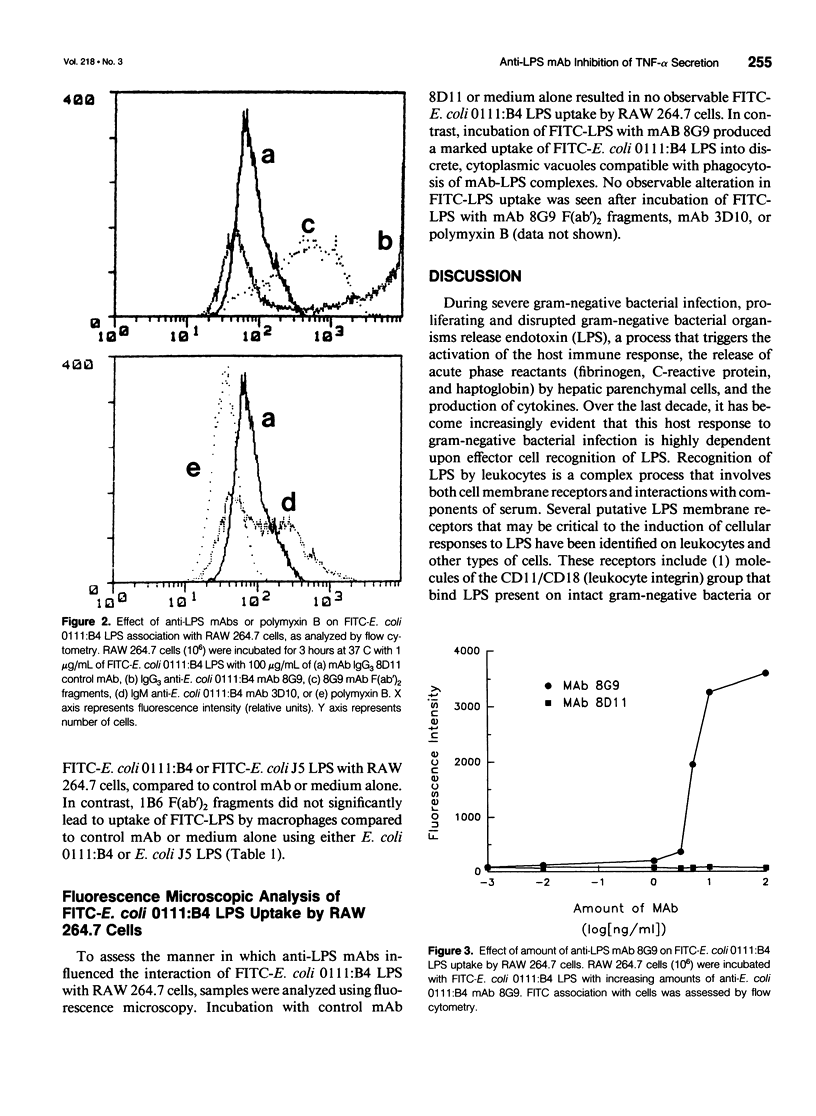
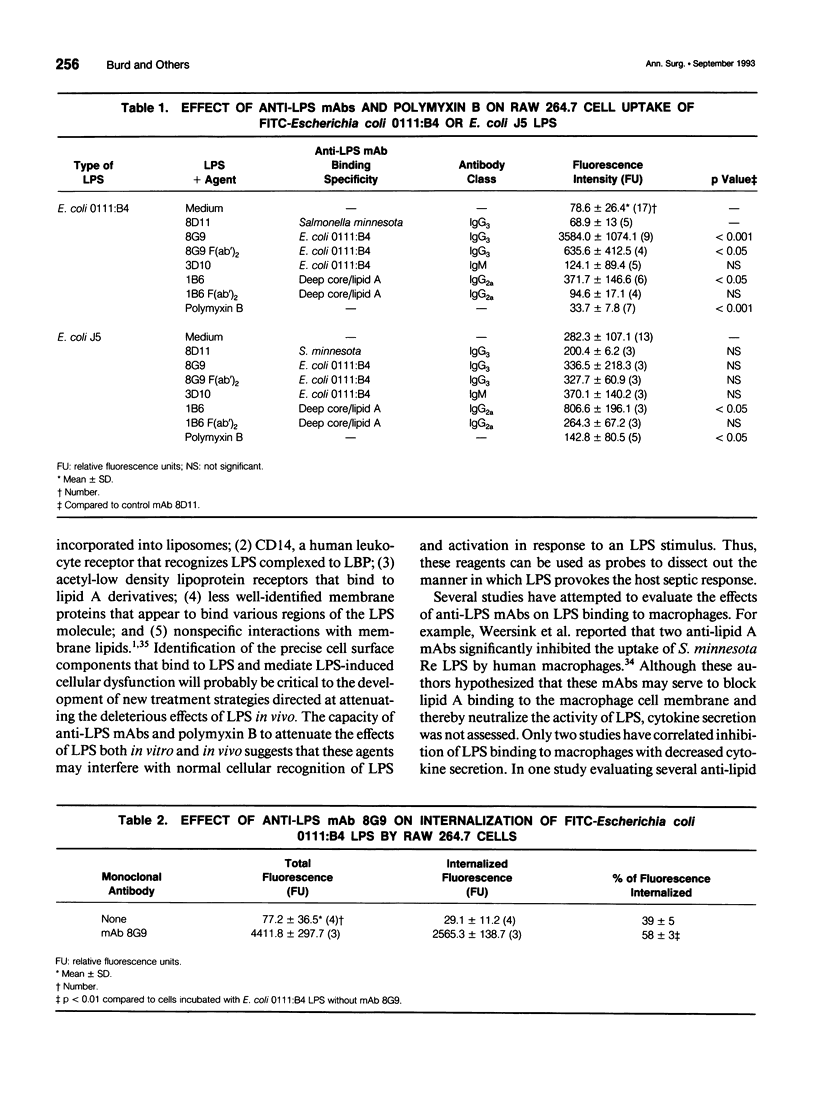
OBJECTIVE: To determine whether monoclonal antibodies (mAbs) directed against lipopolysaccharide (LPS, endotoxin) act by promoting LPS neutralization, LPS uptake by macrophages, or both processes, the authors assessed the effects of these agents on LPS-induced cytokine secretion and cellular uptake of LPS. SUMMARY BACKGROUND DATA: MAbs directed against LPS have been shown to attenuate LPS-induced macrophage tumor necrosis factor-alpha (TNF-alpha) secretion, a process that may contribute to protective capacity. The mechanisms by which this process occurs have not been established. METHODS: MAbs directed against LPS were evaluated in vitro for their capacity to (1) inhibit TNF-alpha secretion, and (2) alter fluorescein isothiocyanate-labeled LPS uptake (employing flow cytometry analysis and fluorescence microscopy) by the macrophage-like cell line RAW 264.7. RESULTS: MAb 8G9, an IgG3 directed against the O-antigen polysaccharide region of Escherichia coli 0111:B4 LPS, significantly reduced LPS-induced TNF-alpha secretion and promoted a more than 40-fold increase in LPS uptake by macrophages. The authors established that this was mediated by a Fc receptor-mediated process because 8G9 F(ab')2 fragments that lack the Fc portion of the IgG molecule were capable of inhibiting TNF-alpha secretion, but did not promote increased LPS uptake to the same degree. Cross-reactive, anti-deep core/lipid A mAb 1B6, an IgG2a, also promoted uptake of E. coli 0111:B4 LPS and O-antigen polysaccharide-deficient E. coli J5 LPS, but only inhibited TNF-alpha secretion induced by E. coli J5 LPS to which it binds most efficiently. MAb 3D10, an IgM also directed against the O-antigen polysaccharide region of E. coli 0111:B4 LPS, inhibited TNF-alpha secretion but did not increase cellular uptake of LPS, presumably acting solely due to LPS neutralization. Polymyxin B, an antibiotic that binds stoichiometrically to the lipid A portion of LPS, inhibited TNF-alpha secretion and prevented cellular LPS uptake. CONCLUSIONS: These results suggest that IgG and IgM anti-LPS mAbs exert protective capacity by extracellular neutralization of LPS, while IgG Fc receptor-mediated cellular uptake also may serve to bypass macrophage activation and TNF-alpha secretion by promoting internalization and intracellular neutralization.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appelmelk B. J., Verweij-van Vught A. M., Maaskant J. J., Schouten W. F., De Jonge A. J., Thijs L. G., Maclaren D. M. Production and characterisation of mouse monoclonal antibodies reacting with the lipopolysaccharide core region of gram-negative bacilli. J Med Microbiol. 1988 Jun;26(2):107–114. doi: 10.1099/00222615-26-2-107. [DOI] [PubMed] [Google Scholar]
- Bannatyne R. M., Harnett N. M., Lee K. Y., Biggar W. D. Inhibition of the biologic effects of endotoxin on neutrophils by polymyxin B sulfate. J Infect Dis. 1977 Oct;136(4):469–474. doi: 10.1093/infdis/136.4.469. [DOI] [PubMed] [Google Scholar]
- Baumgartner J. D., Heumann D., Gerain J., Weinbreck P., Grau G. E., Glauser M. P. Association between protective efficacy of anti-lipopolysaccharide (LPS) antibodies and suppression of LPS-induced tumor necrosis factor alpha and interleukin 6. Comparison of O side chain-specific antibodies with core LPS antibodies. J Exp Med. 1990 Mar 1;171(3):889–896. doi: 10.1084/jem.171.3.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burd R. S., Cody C. S., Raymond C. S., Dunn D. L. Anti-endotoxin monoclonal antibodies protect by enhancing bacterial and endotoxin clearance. Arch Surg. 1993 Feb;128(2):145–151. doi: 10.1001/archsurg.1993.01420140022004. [DOI] [PubMed] [Google Scholar]
- Burd R. S., Raymond C. S., Dunn D. L. Endotoxin promotes synergistic lethality during concurrent Escherichia coli and Candida albicans infection. J Surg Res. 1992 Jun;52(6):537–542. doi: 10.1016/0022-4804(92)90125-j. [DOI] [PubMed] [Google Scholar]
- Burd R. S., Raymond C. S., Ratz C. A., Dunn D. L. A rapid procedure for purifying IgM monoclonal antibodies from murine ascites using a DEAE-disk. Hybridoma. 1993 Feb;12(1):135–142. doi: 10.1089/hyb.1993.12.135. [DOI] [PubMed] [Google Scholar]
- Cody C. S., Burd R. S., Mayoral J. L., Dunn D. L. Protective anti-lipopolysaccharide monoclonal antibodies inhibit tumor necrosis factor production. J Surg Res. 1992 Apr;52(4):314–319. doi: 10.1016/0022-4804(92)90109-d. [DOI] [PubMed] [Google Scholar]
- Dunn D. L., Bogard W. C., Jr, Cerra F. B. Enhanced survival during murine gram-negative bacterial sepsis by use of a murine monoclonal antibody. Arch Surg. 1985 Jan;120(1):50–53. doi: 10.1001/archsurg.1985.01390250044007. [DOI] [PubMed] [Google Scholar]
- Dunn D. L. Immunotherapeutic advances in the treatment of gram-negative bacterial sepsis. World J Surg. 1987 Apr;11(2):233–240. doi: 10.1007/BF01656407. [DOI] [PubMed] [Google Scholar]
- Fong Y., Lowry S. F. Tumor necrosis factor in the pathophysiology of infection and sepsis. Clin Immunol Immunopathol. 1990 May;55(2):157–170. doi: 10.1016/0090-1229(90)90094-7. [DOI] [PubMed] [Google Scholar]
- Glauser M. P., Zanetti G., Baumgartner J. D., Cohen J. Septic shock: pathogenesis. Lancet. 1991 Sep 21;338(8769):732–736. doi: 10.1016/0140-6736(91)91452-z. [DOI] [PubMed] [Google Scholar]
- Greenman R. L., Schein R. M., Martin M. A., Wenzel R. P., MacIntyre N. R., Emmanuel G., Chmel H., Kohler R. B., McCarthy M., Plouffe J. A controlled clinical trial of E5 murine monoclonal IgM antibody to endotoxin in the treatment of gram-negative sepsis. The XOMA Sepsis Study Group. JAMA. 1991 Aug 28;266(8):1097–1102. [PubMed] [Google Scholar]
- Harris R. L., Musher D. M., Bloom K., Gathe J., Rice L., Sugarman B., Williams T. W., Jr, Young E. J. Manifestations of sepsis. Arch Intern Med. 1987 Nov;147(11):1895–1906. [PubMed] [Google Scholar]
- Hed J., Hallden G., Johansson S. G., Larsson P. The use of fluorescence quenching in flow cytofluorometry to measure the attachment and ingestion phases in phagocytosis in peripheral blood without prior cell separation. J Immunol Methods. 1987 Jul 16;101(1):119–125. doi: 10.1016/0022-1759(87)90224-9. [DOI] [PubMed] [Google Scholar]
- Heumann D., Gallay P., Barras C., Zaech P., Ulevitch R. J., Tobias P. S., Glauser M. P., Baumgartner J. D. Control of lipopolysaccharide (LPS) binding and LPS-induced tumor necrosis factor secretion in human peripheral blood monocytes. J Immunol. 1992 Jun 1;148(11):3505–3512. [PubMed] [Google Scholar]
- Karkhanis Y. D., Zeltner J. Y., Jackson J. J., Carlo D. J. A new and improved microassay to determine 2-keto-3-deoxyoctonate in lipopolysaccharide of Gram-negative bacteria. Anal Biochem. 1978 Apr;85(2):595–601. doi: 10.1016/0003-2697(78)90260-9. [DOI] [PubMed] [Google Scholar]
- Lam C., Schütze E., Walzl H., Basalka E. Protection of mice against lethal endotoxemia by lipid X is mediated through inhibition of neutrophil function. Circ Shock. 1987;22(4):311–321. [PubMed] [Google Scholar]
- Lamoyi E. Preparation of F(ab')2 fragments from mouse IgG of various subclasses. Methods Enzymol. 1986;121:652–663. doi: 10.1016/0076-6879(86)21064-2. [DOI] [PubMed] [Google Scholar]
- Lasfargues A., Tahri-Jouti M. A., Pedron T., Girard R., Chaby R. Effects of lipopolysaccharide on macrophages analyzed with anti-lipid A monoclonal antibodies and polymyxin B. Eur J Immunol. 1989 Dec;19(12):2219–2225. doi: 10.1002/eji.1830191207. [DOI] [PubMed] [Google Scholar]
- Leroy O., Beuscart C., Mouton Y. Gram-negative nosocomial infection: incidence, pathogens, compromised host. Br J Clin Pract Suppl. 1988 Feb;57:27–35. [PubMed] [Google Scholar]
- Macdonald R. A., Hosking C. S., Jones C. L. The measurement of relative antibody affinity by ELISA using thiocyanate elution. J Immunol Methods. 1988 Feb 10;106(2):191–194. doi: 10.1016/0022-1759(88)90196-2. [DOI] [PubMed] [Google Scholar]
- Marra M. N., Wilde C. G., Griffith J. E., Snable J. L., Scott R. W. Bactericidal/permeability-increasing protein has endotoxin-neutralizing activity. J Immunol. 1990 Jan 15;144(2):662–666. [PubMed] [Google Scholar]
- Mayoral J. L., Dunn D. L. Cross-reactive murine monoclonal antibodies directed against the core/lipid A region of endotoxin inhibit production of tumor necrosis factor. J Surg Res. 1990 Oct;49(4):287–292. doi: 10.1016/0022-4804(90)90022-t. [DOI] [PubMed] [Google Scholar]
- Mayoral J. L., Schweich C. J., Dunn D. L. Decreased tumor necrosis factor production during the initial stages of infection correlates with survival during murine gram-negative sepsis. Arch Surg. 1990 Jan;125(1):24–28. doi: 10.1001/archsurg.1990.01410130026003. [DOI] [PubMed] [Google Scholar]
- Sagawa T., Hitsumoto Y., Kanoh M., Utsumi S., Kimura S. Mechanisms of neutralization of endotoxin by monoclonal antibodies to O and R determinants of lipopolysaccharide. Adv Exp Med Biol. 1990;256:341–344. doi: 10.1007/978-1-4757-5140-6_29. [DOI] [PubMed] [Google Scholar]
- Silva A. T., Appelmelk B. J., Buurman W. A., Bayston K. F., Cohen J. Monoclonal antibody to endotoxin core protects mice from Escherichia coli sepsis by a mechanism independent of tumor necrosis factor and interleukin-6. J Infect Dis. 1990 Aug;162(2):454–459. doi: 10.1093/infdis/162.2.454. [DOI] [PubMed] [Google Scholar]
- Skelly R. R., Munkenbeck P., Morrison D. C. Stimulation of T-independent antibody responses by hapten-lipopolysaccharides without repeating polymeric structure. Infect Immun. 1979 Feb;23(2):287–293. doi: 10.1128/iai.23.2.287-293.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahri-Jouti M. A., Chaby R. Specific binding of lipopolysaccharides to mouse macrophages--I. Characteristics of the interaction and inefficiency of the polysaccharide region. Mol Immunol. 1990 Aug;27(8):751–761. doi: 10.1016/0161-5890(90)90084-d. [DOI] [PubMed] [Google Scholar]
- Tahri-Jouti M. A., Mondange M., Le Dur A., Auzanneau F. I., Charon D., Girard R., Chaby R. Specific binding of lipopolysaccharides to mouse macrophages--II. Involvement of distinct lipid a substructures. Mol Immunol. 1990 Aug;27(8):763–770. doi: 10.1016/0161-5890(90)90085-e. [DOI] [PubMed] [Google Scholar]
- Teng N. N., Kaplan H. S., Hebert J. M., Moore C., Douglas H., Wunderlich A., Braude A. I. Protection against gram-negative bacteremia and endotoxemia with human monoclonal IgM antibodies. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1790–1794. doi: 10.1073/pnas.82.6.1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vacheron F., Mandine E., Lenaour R., Smets P., Zalisz R., Guenounou M. Inhibition of production of tumor necrosis factor by monoclonal antibodies to lipopolysaccharides. J Infect Dis. 1992 May;165(5):873–878. doi: 10.1093/infdis/165.5.873. [DOI] [PubMed] [Google Scholar]
- Weersink A. J., Van Kessel K. P., Torensma R., Van Strijp J. A., Verhoef J. Binding of rough lipopolysaccharides (LPS) to human leukocytes. Inhibition by anti-LPS monoclonal antibody. J Immunol. 1990 Jul 1;145(1):318–324. [PubMed] [Google Scholar]
- Wright S. D. Multiple receptors for endotoxin. Curr Opin Immunol. 1991 Feb;3(1):83–90. doi: 10.1016/0952-7915(91)90082-c. [DOI] [PubMed] [Google Scholar]
- Wright S. D., Ramos R. A., Tobias P. S., Ulevitch R. J., Mathison J. C. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science. 1990 Sep 21;249(4975):1431–1433. doi: 10.1126/science.1698311. [DOI] [PubMed] [Google Scholar]
- Zanetti G., Heumann D., Gérain J., Kohler J., Abbet P., Barras C., Lucas R., Glauser M. P., Baumgartner J. D. Cytokine production after intravenous or peritoneal gram-negative bacterial challenge in mice. Comparative protective efficacy of antibodies to tumor necrosis factor-alpha and to lipopolysaccharide. J Immunol. 1992 Mar 15;148(6):1890–1897. [PubMed] [Google Scholar]
- Ziegler E. J., Fisher C. J., Jr, Sprung C. L., Straube R. C., Sadoff J. C., Foulke G. E., Wortel C. H., Fink M. P., Dellinger R. P., Teng N. N. Treatment of gram-negative bacteremia and septic shock with HA-1A human monoclonal antibody against endotoxin. A randomized, double-blind, placebo-controlled trial. The HA-1A Sepsis Study Group. N Engl J Med. 1991 Feb 14;324(7):429–436. doi: 10.1056/NEJM199102143240701. [DOI] [PubMed] [Google Scholar]


