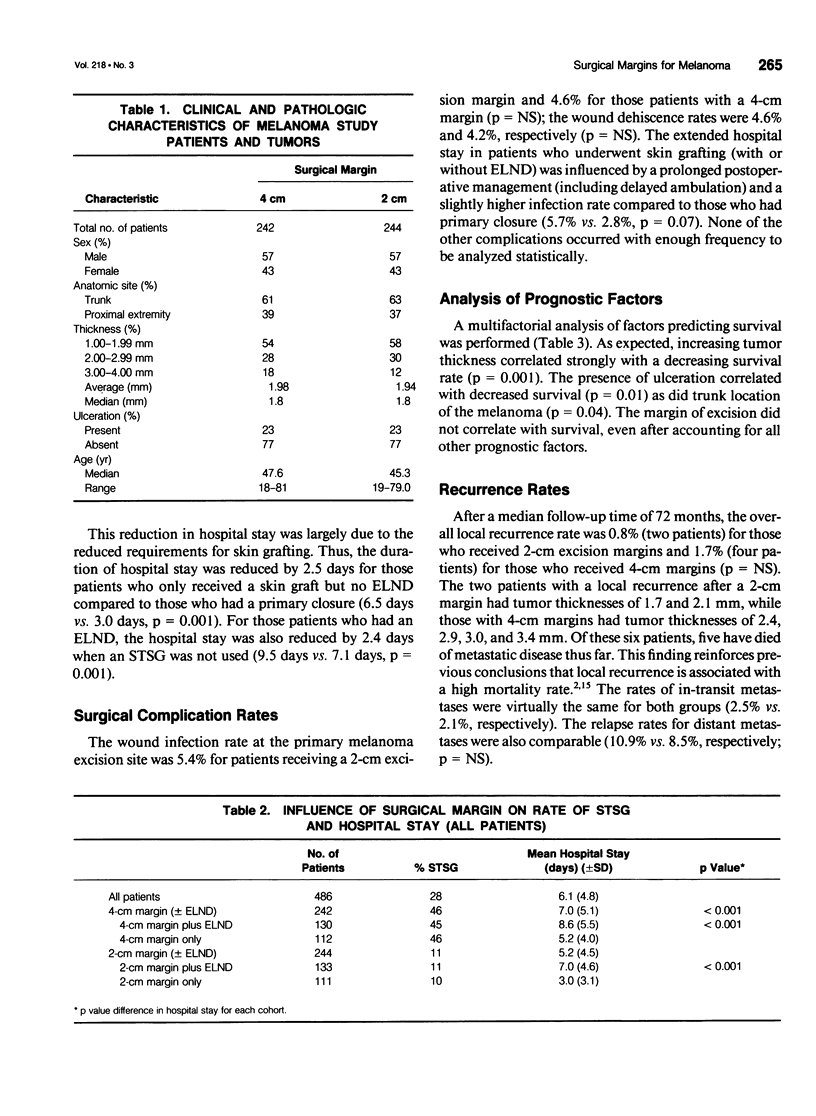
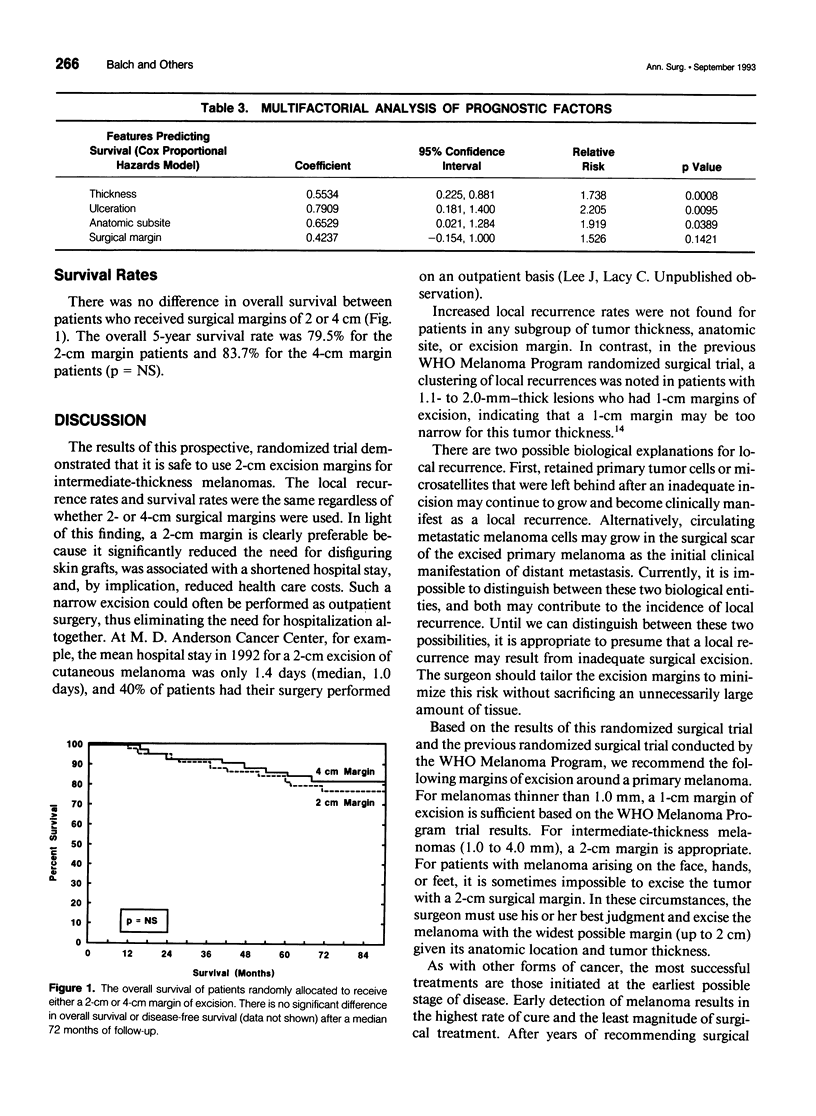
Abstract
BACKGROUND: A prospective, multi-institutional, randomized surgical trial involving 486 localized melanoma patients was conducted to determine whether excision margins for intermediate-thickness melanomas (1.0 to 4.0 mm) could be safely reduced from the standard 4-cm radius. METHODS: Patients with 1- to 4-mm-thick melanomas on the trunk or proximal extremities were randomly assigned to receive either a 2- or 4-cm surgical margin. RESULTS: The median follow-up time was 6 years. The local recurrence rate was 0.8% for 2-cm margins and 1.7% for 4-cm margins (p value not significant [NS]). The rates of in-transit metastases were 2.1% and 2.5%, respectively (p = NS). Of the six patients with local recurrences, five have died. Recurrence rates did not correlate with surgical margins, even among stratified thickness groups. The overall 5-year survival rate was 79.5% for the 2-cm margin patients and 83.7% for the 4-cm margin patients (p = NS). The need for skin grafting was reduced from 46% with 4-cm surgical margins to 11% with 2-cm surgical margins (p < 0.001). The hospital stay was shortened from 7.0 days for patients receiving 4-cm surgical margins to 5.2 days for those receiving 2-cm margins (p = 0.0001). This reduction was largely due to reduced need for skin grafting, since the hospital stay for those who had a skin graft was 2.5 days longer than that for those who had a primary wound closure (p < 0.01). CONCLUSION: Margins of excision can be safely reduced to 2 cm for patients with intermediate-thickness melanomas. The narrower margins significantly reduced the need for skin grafting and shortened the hospital stay.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balch C. M., Murad T. M., Soong S. J., Ingalls A. L., Richards P. C., Maddox W. A. Tumor thickness as a guide to surgical management of clinical stage I melanoma patients. Cancer. 1979 Mar;43(3):883–888. doi: 10.1002/1097-0142(197903)43:3<883::aid-cncr2820430316>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Breslow A., Macht S. D. Optimal size of resection margin for thin cutaneous melanoma. Surg Gynecol Obstet. 1977 Nov;145(5):691–692. [PubMed] [Google Scholar]
- Cosimi A. B., Sober A. J., Mihm M. C., Fitzpatrick T. B. Conservative surgical management of superficially invasive cutaneous melanoma. Cancer. 1984 Mar 15;53(6):1256–1259. doi: 10.1002/1097-0142(19840315)53:6<1256::aid-cncr2820530607>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Day C. L., Jr, Mihm M. C., Jr, Sober A. J., Fitzpatrick T. B., Malt R. A. Narrower margins for clinical stage I malignant melanoma. N Engl J Med. 1982 Feb 25;306(8):479–482. doi: 10.1056/NEJM198202253060810. [DOI] [PubMed] [Google Scholar]
- Elder D. E., Guerry D., 4th, Heiberger R. M., LaRossa D., Goldman L. I., Clark W. H., Jr, Thompson C. J., Matozzo I., Van Horn M. Optimal resection margin for cutaneous malignant melanoma. Plast Reconstr Surg. 1983 Jan;71(1):66–72. doi: 10.1097/00006534-198301000-00015. [DOI] [PubMed] [Google Scholar]
- Goldman L. I., Byrd R. Narrowing resection margins for patients with low-risk melanoma. Am J Surg. 1988 Feb;155(2):242–244. doi: 10.1016/s0002-9610(88)80704-9. [DOI] [PubMed] [Google Scholar]
- Milton G. W., Shaw H. M., Farago G. A., McCarthy W. H. Tumour thickness and the site and time of first recurrence in cutaneous malignant melanoma (stage I). Br J Surg. 1980 Aug;67(8):543–546. doi: 10.1002/bjs.1800670804. [DOI] [PubMed] [Google Scholar]
- Milton G. W., Shaw H. M., McCarthy W. H. Resection margins for melanoma. Aust N Z J Surg. 1985 Jun;55(3):225–226. doi: 10.1111/j.1445-2197.1985.tb00076.x. [DOI] [PubMed] [Google Scholar]
- Reintgen D. S., Vollmer R., Tso C. Y., Seigler H. F. Prognosis for recurrent stage I malignant melanoma. Arch Surg. 1987 Nov;122(11):1338–1342. doi: 10.1001/archsurg.1987.01400230126022. [DOI] [PubMed] [Google Scholar]
- Stage I melanoma of the skin: the problem of resection margins. W.H.O. Collaborating Centres for Evaluation of Methods of Diagnosis and Treatment of Melanoma. Eur J Cancer. 1980 Aug;16(8):1079–1085. [PubMed] [Google Scholar]
- Urist M. M., Balch C. M., Soong S., Shaw H. M., Milton G. W., Maddox W. A. The influence of surgical margins and prognostic factors predicting the risk of local recurrence in 3445 patients with primary cutaneous melanoma. Cancer. 1985 Mar 15;55(6):1398–1402. doi: 10.1002/1097-0142(19850315)55:6<1398::aid-cncr2820550639>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Veronesi U., Cascinelli N., Adamus J., Balch C., Bandiera D., Barchuk A., Bufalino R., Craig P., De Marsillac J., Durand J. C. Thin stage I primary cutaneous malignant melanoma. Comparison of excision with margins of 1 or 3 cm. N Engl J Med. 1988 May 5;318(18):1159–1162. doi: 10.1056/NEJM198805053181804. [DOI] [PubMed] [Google Scholar]
- Veronesi U., Cascinelli N. Narrow excision (1-cm margin). A safe procedure for thin cutaneous melanoma. Arch Surg. 1991 Apr;126(4):438–441. doi: 10.1001/archsurg.1991.01410280036004. [DOI] [PubMed] [Google Scholar]


