Abstract
The nuclear receptors LXRα and LXRβ have been implicated in the control of cholesterol and fatty acid metabolism in multiple cell types. Activation of these receptors stimulates cholesterol efflux in macrophages, promotes bile acid synthesis in liver, and inhibits intestinal cholesterol absorption, actions that would collectively be expected to reduce atherosclerotic risk. However, synthetic LXR ligands have also been shown to induce lipogenesis and hypertriglyceridemia in mice, raising questions as to the net effects of these compounds on the development of cardiovascular disease. We demonstrate here that the nonsteroidal LXR agonist GW3965 has potent antiatherogenic activity in two different murine models. In LDLR−/− mice, GW3965 reduced lesion area by 53% in males and 34% in females. A similar reduction of 47% was observed in male apoE−/− mice. Long-term (12-week) treatment with LXR agonist had differential effects on plasma lipid profiles in LDLR−/− and apoE−/− mice. GW3965 induced expression of ATP-binding cassettes A1 and G1 in modified low-density lipoprotein-loaded macrophages in vitro as well as in the aortas of hyperlipidemic mice, suggesting that direct actions of LXR ligands on vascular gene expression are likely to contribute to their antiatherogenic effects. These observations provide direct evidence for an atheroprotective effect of LXR agonists and support their further evaluation as potential modulators of human cardiovascular disease.
Recent work has identified the nuclear receptors LXRα and LXRβ as central regulators of lipid homeostasis. The physiologic ligands for these receptors are likely to be specific intermediates in the cholesterol biosynthetic pathway such as 24(S),25-epoxycholesterol (1–3). LXRα is expressed primarily in liver, intestine, adipose tissue, and macrophages, whereas LXRβ is expressed in many cell types (4). In peripheral cells such as macrophages, LXRs seem to coordinate a physiologic response to cellular cholesterol loading. LXRs directly control transcription of several genes involved in the cholesterol efflux pathway, including ATP-binding cassette (ABC) A1 (5–8), ABCG1 (9), and apolipoprotein E (apoE) (10). In the intestine, ligand activation of LXR/RXR heterodimers dramatically reduces dietary cholesterol absorption, an effect postulated to be mediated by ABCA1 (6).
In the liver, LXRs seem to regulate both cholesterol and fatty acid metabolism. Mice carrying a targeted disruption of the Lxrα gene fail to induce transcription of the gene encoding cholesterol 7α-hydroxylase (CYP7A1) in response to dietary cholesterol, implicating LXRs in the control of bile acid synthesis (11). Mice lacking LXRα were also observed to be deficient in expression of fatty acid synthase, steroyl-coA desaturase 1, acyl-coA carboxylase, and sterol regulatory element binding protein-1, suggesting an additional role in lipogenesis. This hypothesis was supported by the subsequent demonstration that the synthetic LXR ligand T1317 induces expression of lipogenic genes and raises plasma triglyceride levels in mice (12). The recent observation that both the sterol regulatory element binding protein-1c and fatty acid synthase promoters are direct targets for LXR/RXR heterodimers provides a potential mechanism for these effects (13–15). Hepatic cholesterol ester transfer protein and lipoprotein lipase expression are also regulated by LXRs (16, 17).
The ability of LXR-signaling pathways to have an impact on multiple aspects of systemic lipid metabolism makes the long-term effects of synthetic LXR agonists difficult to predict. On the one hand, the promotion of reverse cholesterol transport and inhibition of intestinal cholesterol absorption would be expected to reduce atherosclerotic risk. On the other hand, elevation of plasma triglyceride levels would be expected to have unfavorable effects on the development of metabolic disease. Indeed, significant controversy exists as to whether LXR agonists or antagonists would be the more useful agents for the modulation of human lipid metabolism (6, 8, 12). In the present study, we have analyzed the effect of a synthetic LXR ligand in two different murine models of atherosclerosis. Chronic administration of GW3965 significantly reduced atherosclerosis in both LDLR−/− mice and apoE−/− mice, providing direct evidence for an atheroprotective effect of LXR agonists. These observations suggest that LXR ligands may represent promising agents for intervention in human cardiovascular disease.
Materials and Methods
Cell Culture and RNA Analysis.
Peritoneal macrophages were obtained from thioglycolate-injected mice as described (9) and cultured in DMEM containing 10% FBS. For ligand treatments, cells were cultured in DMEM supplemented with 5% lipoprotein-deficient serum (Intracel, Frederick, MD) and ligand for 48 h. In some experiments, cells were preloaded with 50 μg/ml protein AcLDL (Intracel) for 24 h. Total RNA was isolated by using Trizol (Life Technologies, Rockville, MD). Northern analysis was performed as described (18) by using radiolabeled cDNA probes. Real-time quantitative PCR assays were performed by using an Applied Biosystems 7700 sequence detector as in ref. 19. In brief, 1 μg of total RNA was reverse transcribed with random hexamers by using Taqman Reverse Transcription Reagents Kit (Applied Biosystems). Each amplification mixture (50 μl) contained 50 ng cDNA, 900 nM forward primer, 900 nM reverse primer, 100 nM fluorogenic probe (Applied Biosystems), and 25 μl of Universal PCR Master Mix. Samples were analyzed for 36B4 or 18S rRNA expression in parallel in the same run. Quantitative expression values were extrapolated from separate standard curves for controls and unknowns generated with 10-fold dilutions of cDNA. Assays were performed in duplicate. Primer and probe sequences are available on request.
Animals and Diets.
LDLR knockout mice on a C57BL/6 background were obtained from The Jackson Laboratory and fed ad libitum a western diet (Research Diets, D12079B; 21% fat, 1.5% cholesterol) with or without GW3965, as indicated. ApoE knockout male mice on a C57BL/6 background were maintained on normal chow (RM1 maintenance diet; SDS, London, U.K.). Body weight and food intake monitored at regular intervals. Experiments were performed on age-matched mice of 2.5–4 months of age. Where indicated, diets were supplemented with GW3965 [EC50 190 nM on LXRα (20)], at a level sufficient to provide the appropriate milligrams per kilogram body weight (mpk) dose on consumption of a 5-g diet by a 30-g mouse per day. Animal experiments were approved by the Institutional Animal Care and Research Advisory Committee of the University of California, Los Angeles.
Plasma Lipids.
Mice were fasted overnight and euthanized; blood was collected from the abdominal vena cava. Aliquots of plasma were subjected to lipid analysis by using commercially available enzymatic kits, ILab600 Clinical Chemistry Analyzer (Instrumentation Laboratory, Lexington, MA) or by HPLC (Kroma System 2000, Bio-Tek Kontron Instruments, Winooski, VT). Values are reported as mean ± SEM. Comparisons between control and treated mice were made by using the Student t test for independent samples (two-tailed) and ANOVA.
Histology and Lesion Analysis.
For en face analysis, mice were euthanized and the aorta dissected out, opened longitudinally from heart to the iliac arteries, and stained with Sudan IV to determine lesion area (21). Images were captured by use of a Sony 3-CCD video camera and analyzed by a single technician who was blinded to the study protocol and used imagepro image analysis software. The extent of lesion formation is expressed as the percentage of the total aortic surface area covered by lesions. Atherosclerotic lesions at the aortic valve were analyzed as described (22). The upper portion of the heart and proximal aorta were obtained, embedded in OCT compound (Fisher, Tustin, CA), and stored at −70°C. For LDLR−/− mice, serial 10-μm-thick cryosections of aorta, beginning at the aortic root, were collected for a distance of 400 μm. For apoE−/− mice, 4-μm sections were analyzed for a distance of 600 μm. Sections were stained with Oil Red O and hematoxylin. The lipid-staining areas on 25 sections were determined in a blinded fashion by light microscopy. The mean value of lesion area of aortic wall per section was then calculated. Comparisons between control and treated mice were made by using the Student t test for independent samples (two-tailed) and ANOVA. Immunohistochemical analyses of atherosclerotic lesions in the aortic root were performed as described (23). In brief, 10-μm-thick cryosections were fixed in acetone and incubated with rabbit polyclonal antibody to mouse ABCA1 (a gift of O. Francone, Pfizer) (24), rat monoclonal antibody to mouse macrophages (MOMA-2, Accurate Chemicals), or control rabbit IgG, followed by incubation with biotinylated anti-rabbit or antirat secondary antibodies. Signals were detected with peroxidase chromogen and alkaline phosphatase, respectively (Vector Laboratories).
Results
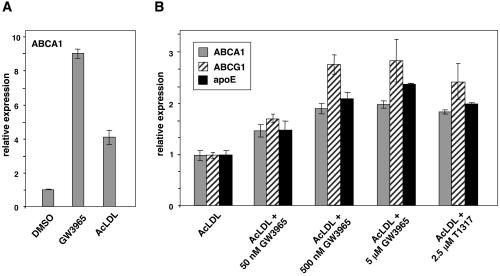
Previous work has identified LXRs as mediators of the effects of oxysterols on ABCA1, ABCG1, and apoE expression in macrophages. The synthetic LXR ligands T1317 and GW3965 have also been shown to induce expression of these genes (6, 9, 10, 19). However, the impact of LXR ligands on lipid-loaded macrophages that already contain high levels of LXR activators has not been explored. In theory, for synthetic agonists to exhibit utility for the promotion of cholesterol efflux from lipid-loaded cells, they must be more efficacious than the endogenous ligands present in such cells. As shown in Fig. 1A, GW3965 was significantly more effective than acetylated low-density lipoprotein (LDL) in inducing ABCA1 expression in thioglycolate-elicited murine peritoneal macrophages. Moreover, both GW3965 and the structurally unrelated agonist T1317 were able to induce expression of the LXR target genes ABCA1, ABCG1, and apoE further in cells that had been preloaded with acetylated LDL (Fig. 1B). This observation suggests that it may be possible to promote LXR target gene expression and cholesterol efflux from highly lipid-loaded macrophages in the context of an atherosclerotic lesion.
Figure 1.
Synthetic LXR ligands are more efficacious than endogenous LXR ligands in lipid-loaded macrophages. (A) Murine thioglycolate-elicited peritoneal macrophages from C57BL/6 mice were treated for 24 h with vehicle (DMSO), 5 μM GW3965, or 50 μg/ml protein AcLDL. (B) Peritoneal macrophages were cultured for 24 h in the presence of 50 μg/ml AcLDL and then treated for an additional 24 h with the indicated concentration of GW3965 or T1317. RNA was isolated and gene expression assayed by real time quantitative PCR. Data are presented as mRNA level relative to vehicle control.
The influence of GW3965 on the development of atherosclerosis was analyzed in both LDLR−/− and apoE−/− mice. In the first study, LDLR−/− mice of 12 weeks of age were fed an atherogenic high-fat diet for 12 weeks. One group received high-fat diet alone, one group received a high-fat diet containing 1 mpk GW3965, and one group received a high-fat diet containing 10 mpk GW3965. The 10-mpk dosing regimen was determined to result in plasma drug concentrations between 200 and 400 nM (EC50 on LXRα = 190 nm). No difference occurred in body weight or food consumption between groups (Table 1). After 12 weeks, mice treated with GW3965 showed a significant decrease in total cholesterol and unesterified cholesterol compared with controls (Table 1). Total cholesterol was also significantly reduced in the group receiving 1 mpk GW3965. In contrast to short-term treatment (data not shown), plasma triglycerides and high-density lipoprotein (HDL) cholesterol were not different from control after chronic treatment with LXR agonist. This observation is consistent with previous work in C57BL/6 mice showing that the marked hypertriglyceridemia induced by LXR ligands is largely transient (15).
Table 1.
Plasma lipid levels in LDLR−/− mice maintained for 12 weeks on a high-fat diet in the presence or absence of GW3965
| T = 0 Vehicle | 12 weeks
|
|||
|---|---|---|---|---|
| Vehicle | GW3965 (1 mpk) | GW3965 (10 mpk) | ||
| Males | ||||
| Total cholesterol, mg/dl | 319.8 ± 6.1 | 1233.9 ± 66.6 | 1014.3 ± 59.4* | 828.5 ± 109.6** |
| HDL cholesterol, mg/dl | 126.5 ± 1.3 | 73.4 ± 2.9 | 69.4 ± 4.1 | 63.7 ± 8.1 |
| Triglycerides, mg/dl | 96.9 ± 5.8 | 166.1 ± 22.6 | 172.0 ± 11.4 | 183.8 ± 28.8 |
| Unesterified cholesterol, mg/dl | 81.9 ± 2.6 | 323.6 ± 16.9 | 272.9 ± 17.2 | 222.6 ± 29.7** |
| Body weight, g | 22.8 ± 0.3 | 38.2 ± 0.7 | 37.3 ± 1.0 | 37.1 ± 0.5 |
| Food intake, g/mouse | 269.8 ± 17.4 | 268.5 ± 24.9 | 275.8 ± 29.0 | |
| Females | ||||
| Total cholesterol, mg/dl | 265.5 ± 5.8 | 811.1 ± 41.7 | 862.1 ± 49.6 | 718.1 ± 28.1* |
| HDL cholesterol, mg/dl | 96.0 ± 1.9 | 59.2 ± 3.7 | 68.1 ± 5.3 | 68.7 ± 6.2 |
| Triglycerides, mg/dl | 90.7 ± 3.8 | 79.6 ± 6.7 | 78.4 ± 12.0 | 71.2 ± 9.3 |
| Unesterified cholesterol, mg/dl | 73.7 ± 5.3 | 220.1 ± 11.4 | 222.0 ± 14.7 | 185.7 ± 7.5 |
| Body weight, g | 17.0 ± 0.5 | 24.1 ± 1.0 | 23.6 ± 0.3 | 23.3 ± 0.4 |
| Food intake, g/mouse | 288.5 ± 29.9 | 262.3 ± 17.7 | 284.5 ± 45.9 | |
Data expressed as mean ± SEM.
, P < 0.05;
, P < 0.005. N = 9 per group.
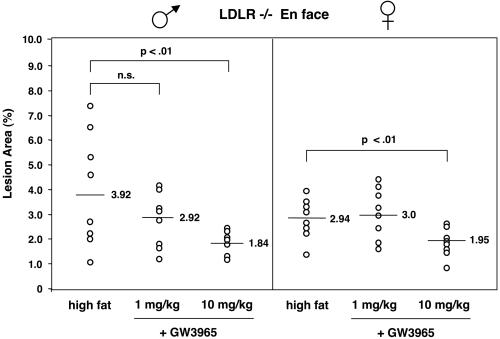
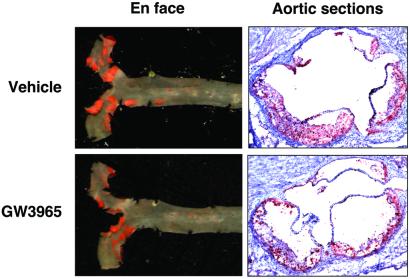
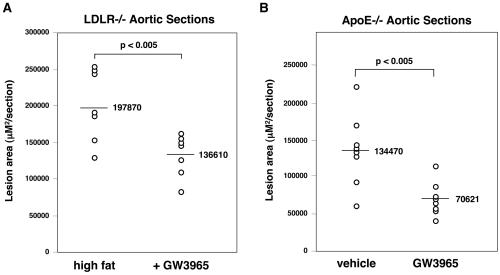
Atherosclerotic lesions in LDLR−/− mice were quantified by en face analysis (21) of aortas after 12 weeks on a high-fat diet in the presence or absence of LXR agonist. Quantification of Sudan IV-stained en face preparations of aortas revealed a dose-dependent reduction in atherosclerosis in mice treated with GW3965. In male mice, the group receiving 1 mpk GW3965 showed a slight decrease in lesion area compared with controls (25%) that did not reach statistical significance (P < 0.28) (Fig. 2). However, male mice receiving 10 mpk GW3965 showed a statistically significant (P < 0.01) 50% reduction in average lesion area (Figs. 2 and 3) compared with control mice. A similar reduction of 35% was observed in female mice receiving 10 mpk GW3965 (Fig. 2, P < 0.01). To document further the effects on atherosclerosis, aortic root sections from male LDLR−/− mice were analyzed. Quantification of lesions after Oil Red O staining again revealed a statistically significant 35% reduction in lesion area in the animals treated with 10 mpk GW3965 compared with controls (P < 0.005, Fig. 4A).
Figure 2.
GW3965 inhibits the development of aortic lesions in LDLR−/− mice. Atherosclerotic lesions were quantitated by en face analysis (see Materials and Methods). n = 9 per group.
Figure 3.
En face and aortic root section analysis of atherosclerosis in LDLR−/− mice.
Figure 4.
GW3965 inhibits the development of aortic root lesions in LDLR−/− mice and apoE−/− mice. n = 7 for LDLR−/− and n = 8 for apoE−/−.
The influence of LXR agonists on the development of atherosclerosis was also analyzed in apoE−/− mice. In comparison with LDLR−/− mice, apoE−/− mice develop atherosclerosis on a normal chow diet and have a distinct lipoprotein profile (25). The apoE gene itself has also been an LXR target gene in macrophages (10). Male apoE−/− mice of 12 weeks of age were maintained for 12 weeks on either normal chow diet or normal chow diet containing 10 mpk GW3965. Plasma levels of GW3965 were monitored throughout the study and averaged between 200 and 400 nM (data not shown). At the end of the study, plasma total cholesterol levels and HDL cholesterol were not different between groups (Table 2). However, very-low-density lipoprotein cholesterol was reduced, and triglycerides were significantly increased. Atherosclerotic lesion area in aortic root sections was quantitated after Oil Red O staining. As shown in Fig. 4B, apoE−/− mice receiving LXR agonist showed a 47% reduction in lesion area (P < 0.005) compared with control mice. Thus, GW3965 inhibits the development of atherosclerosis in two distinct murine models. These data indicate that apoE expression is not required for the antiatherogenic effects of LXR agonists.
Table 2.
Plasma lipid levels in apoE−/− mice after 12 weeks of treatment with GW3965
| ApoE−/− males | T = 0 Vehicle | 12 weeks
|
|
|---|---|---|---|
| Vehicle | GW3965 (10 mpk) | ||
| Total cholesterol, mg/dl | 300 ± 20 | 354.5 ± 21.6 | 339 ± 16 |
| HDL cholesterol, mg/dl | 61.0 ± 3.7 | 52.1 ± 1.6 | 48.6 ± 4.1 |
| Triglycerides, mg/dl | 128.7 ± 6.5 | 190.8 ± 14.0 | 256.0 ± 21.4* |
| LDL cholesterol, mg/dl | 199.6 ± 14.2 | 229.8 ± 17.2 | 243.2 ± 13.8 |
| VLDL† cholesterol, mg/dl | 39.8 ± 3.0 | 72.5 ± 6.9 | 47.5 ± 4.0** |
| Body weight, g | 31.9 ± 1.8 | 31.3 ± 1.8 | |
| Food intake, g per mouse | 436.2 ± 26.7 | 391.0 ± 15.4 | |
N = 9 per group. Data expressed as mean ± SEM.
, P < 0.05;
, P < 0.01.
VLDL, very-low-density lipoprotein.
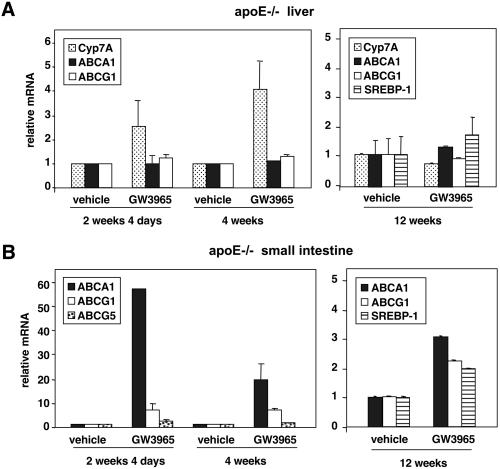
The effect of chronic LXR agonist administration on target gene expression in liver and small intestine of apoE−/− mice was analyzed by real-time quantitative PCR (Taqman) assays. As shown in Fig. 5A, treatment with GW3965 led to a significant induction in hepatic CYP7A expression in liver that persisted after 4 weeks. Consistent with previous work, liver expression of ABCA1 and ABCG1 was minimally affected by LXR agonist treatment. In the intestine, strong induction of ABCA1 and ABCG1 was observed in response to GW3965, although the magnitude of the induction seemed to decline with time (Fig. 5B). Indeed, after 12 weeks, only modest differences in target-gene expression were found between control and treated groups. Similar declines in target-gene expression with time were observed in C57bl/6 and LDLR−/− mice (data not shown). These observations suggest that tolerance or compensation may occur with chronic GW3965 administration.
Figure 5.
Regulation of LXR target gene expression by GW3965 in liver and intestine in apoE−/− mice. ApoE−/− mice (five animals per group) were treated for the indicated time with either vehicle or 10 mpk GW3965. Gene expression was measured by real-time quantitative PCR (Taqman) assays. Data are presented as mRNA expression relative to vehicle control. SREBP-1, sterol regulatory element binding protein-1.
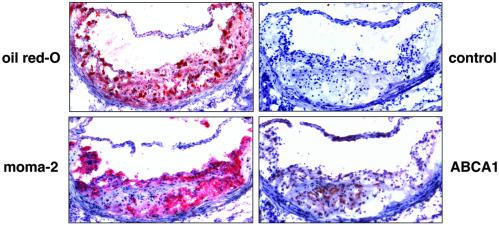
The marked reduction in atherosclerosis observed in mice treated with GW3965 despite modest changes in plasma lipoprotein levels suggests that the mechanism of this effect may involve direct effects on cells of the artery wall in addition to effects in liver and intestine. Although previous work has documented the expression of ABCA1 mRNA in macrophages, expression of this protein in cells of the artery wall has not been examined. We therefore analyzed expression of ABCA1 protein in atherosclerotic lesions. Immunostaining of aortic root sections from LDLR−/− mice with purified anti-mouse ABCA1 antibody (24) revealed significant protein expression that largely colocalized with expression of the macrophage marker MOMA-2 (Fig. 6). In contrast, no macrophage staining was observed with control rabbit antibody.
Figure 6.
Expression of ABCA1 protein in macrophages within aortic lesions from LDLR−/− mice. Aortic root sections from LDLR−/− mice after 12 weeks on a high-fat diet were analyzed for ABCA1 and MOMA-2 protein expression by immunostaining.
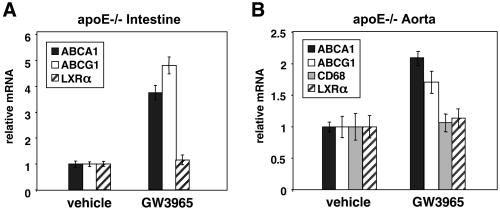
Finally, we investigated the ability of GW3965 to regulate expression of cholesterol efflux genes in the aortas of apoE−/− mice. As shown in Fig. 7A, treatment of apoE−/− mice for 4 days with 10 mpk GW3965 led to an induction of ABCA1 and ABCG1 expression in the small intestine, but had no effect on expression of LXRα itself. A significant induction of ABCA1 and ABCG1 was also observed in the atherosclerotic aortas of apoE−/− mice (Fig. 7B). In contrast, expression of LXRα and CD68 was not different between control and treated mice. Thus, LXR agonist was able to induce aortic expression of ABCA1 and ABCG1 further despite the presence of baseline hyperlipidemia and atherosclerosis. The ability of GW3965 to induce ABCA1 expression in lipid-loaded macrophages in vitro, the expression of LXRα and ABCA1 protein in macrophage-derived foam cells, and the induction of ABCA1 expression in the aortas of treated mice collectively suggest that direct effects of LXR agonists on cells of the artery wall may contribute to their antiatherogenic effects.
Figure 7.
GW3965 induces expression of ABCA1 and ABCG1 in the atherosclerotic aortas of apoE−/− mice. Aorta and small intestines (n = 3) were isolated from apoE−/− mice of 8 months of age after 4 days of treatment with 10 mpk vehicle or GW3965. Gene expression was measured by real-time quantitative PCR (Taqman) assays. Data are presented as mRNA expression relative to vehicle control.
Discussion
The recent characterization of LXRα and LXRβ as regulators of cholesterol absorption and reverse cholesterol transport has generated widespread interest in the development of synthetic LXR ligands as therapeutics. However, the observation that short-term treatment with an LXR ligand stimulates hepatic lipogenesis and raises triglyceride levels has raised questions as to whether LXR agonists or antagonists might be the more useful drug. The effects of chronic LXR agonist administration on lipid metabolism and the development of atherosclerosis have not been addressed previously. We have shown here that the synthetic nonsteroidal LXR agonist GW3965 inhibits the development of atherosclerotic lesions in two distinct murine models. A reduction in lesion area of approximately 50% was observed in both LDLR−/− and apoE−/− male mice treated with GW3965. These observations provide direct evidence for an atheroprotective effect of LXR agonists.
The reduction in atherosclerosis observed in LDLR−/− mice was accompanied by modest (≈20%) reductions in total cholesterol, but no significant change in HDL levels. Although it is likely that alterations in plasma lipid profiles may contribute to the beneficial effects of LXR agonist in this study, the magnitude of the changes would seem to be insufficient to explain a 50% reduction in lesions. In apoE−/− mice, total cholesterol levels and HDL cholesterol were unchanged, whereas triglycerides were mildly increased. Despite this less favorable lipid profile, apoE−/− mice also showed a substantial reduction in atherosclerosis in response to GW3965. Although previous work has shown that LXR ligands reduce cholesterol absorption (6), the mechanism behind this effect is not entirely clear. Studies on ABCA1 knockout mice have reported that loss of this protein does not alter cholesterol or bile acid excretion (26). Other studies have shown that the lipoproteins primarily affected by loss of ABCA1 expression on Western diet are HDL and very-low-density lipoprotein (27). Thus, the effects of LXR ligands on cholesterol absorption and lipoprotein metabolism are likely to involve additional target genes, perhaps including ABCG1, ABCG5, or ABCG8 (28).
Our observations suggest that direct effects of LXR ligands on macrophages within the artery wall contribute to their anitatherogenic effects. Consistent with this hypothesis, we have shown that reduction of atherosclerosis in hyperlipidemic mice correlates with the induction of genes involved in reverse cholesterol transport in multiple tissues. In addition to inducing ABCA1 expression in intestine, GW3965 also induced ABCA1 and ABCG1 expression in the atherosclerotic aortas of apoE−/− mice. These results suggest that in addition to altering cholesterol absorption from the diet, LXR agonists may promote cholesterol efflux from lipid-loaded macrophages within atherosclerotic lesions. This hypothesis is supported by data showing that GW3965 can stimulate ABCA1 expression in macrophages preloaded with modified LDL in vitro. The studies in apoE knockout mice demonstrate that the ability of LXR agonists to reduce lesion formation does not depend on their ability to regulate macrophage apoE expression. However, recent studies indicate that LXR ligands induce expression of multiple other apolipoproteins in macrophages that may also function as cholesterol acceptors in the context of an atherosclerotic lesion (A. Mak, B.A.L., S.B.J., P.T., and P. Edwards, manuscript in preparation). Further investigation will be required to define precisely the mechanism of action of LXR agonists on atherosclerosis in the apoE−/− and LDLR−/− models.
Ligands for two other nuclear receptors, PPARγ and RXR, have also been shown to inhibit the development of atherosclerosis in LDLR−/− and apoE−/− mice (21, 29, 30). Recent work suggests that the mechanisms of action of PPARγ, RXR, and LXR agonists may overlap. Studies have shown that RXR ligands are capable of activating LXR/RXR heterodimers both in vitro and in vivo. Accordingly, RXR agonists are effective inducers of ABCA1 and ABCG1 expression in macrophages and intestine (6, 8, 10). In addition, the LXRα gene has been shown to be a direct transcriptional target of PPARγ. Studies suggest that PPARγ ligands can modulate ABCA1 expression and cholesterol efflux in macrophages indirectly by increasing LXRα expression (31, 32). However, our results differ from previous studies with PPARγ agonists in that atherosclerosis was reduced in both male and female mice with GW3965. PPARγ agonists are also likely to have antiatherogenic effects that are independent of LXR, such as the ability to improve insulin resistance (29). Thus, it is tempting to speculate that the combination of a PPARγ agonist and an LXR agonist may be particularly effective in reducing the risk for atherosclerosis.
Acknowledgments
We thank Peter Edwards for discussions and Omar Francone (Pfizer) for murine ABCA1 antibody and Brenda Mueller for administrative support. P.T. is an Assistant Investigator of the Howard Hughes Medical Institute at the University of California, Los Angeles. This work was supported by National Institutes of Health Grants HL 66088 (to P.T.), HL 58328 (to W.A.H.), HL30568 and HL60030 (to A.J.L.) and by Human Frontier Science Project Grant RGY-021 (to P.T.).
Abbreviations
- ABC
ATP-binding cassette
- apoE
apolipoprotein E
- LDL
low-density lipoprotein
- HDL
high-density lipoprotein
- mpk
milligrams per kilogram of body weight
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Janowski B A, Willy P J, Devi T R, Falck J R, Mangelsdorf D J. Nature (London) 1996;383:728–731. doi: 10.1038/383728a0. [DOI] [PubMed] [Google Scholar]
- 2.Janowski B A, Grogan M J, Jones S A, Wisely G B, Kliewer S A, Corey E J, Mangelsdorf D J. Proc Natl Acad Sci USA. 1999;96:266–271. doi: 10.1073/pnas.96.1.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lehmann J M, Kliewer S A, Moore L B, Smith-Oliver T A, Oliver B B, Su J L, Sundseth S S, Winegar D A, Blanchard D E, Spencer T A, Willson T M. J Biol Chem. 1997;272:3137–3140. doi: 10.1074/jbc.272.6.3137. [DOI] [PubMed] [Google Scholar]
- 4.Repa J J, Mangelsdorf D J. Annu Rev Cell Dev Biol. 2000;16:459–481. doi: 10.1146/annurev.cellbio.16.1.459. [DOI] [PubMed] [Google Scholar]
- 5.Costet P, Luo Y, Wang N, Tall A R. J Biol Chem. 2000;275:28240–28245. doi: 10.1074/jbc.M003337200. [DOI] [PubMed] [Google Scholar]
- 6.Repa J J, Turley S D, Lobaccaro J A, Medina J, Li L, Lustig K, Shan B, Heyman R A, Dietschy J M, Mangelsdorf D J. Science. 2000;289:1524–1529. doi: 10.1126/science.289.5484.1524. [DOI] [PubMed] [Google Scholar]
- 7.Schwartz K, Lawn R M, Wade D P. Biochem Biophys Res Commun. 2000;274:794–802. doi: 10.1006/bbrc.2000.3243. [DOI] [PubMed] [Google Scholar]
- 8.Venkateswaran A, Laffitte B A, Joseph S B, Mak P A, Wilpitz D C, Edwards P A, Tontonoz P. Proc Natl Acad Sci USA. 2000;97:12097–12102. doi: 10.1073/pnas.200367697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Venkateswaran A, Repa J J, Lobaccaro J-M A, Bronson A, Mangelsdorf D J, Edwards P A. J Biol Chem. 2000;275:14700–14707. doi: 10.1074/jbc.275.19.14700. [DOI] [PubMed] [Google Scholar]
- 10.Laffitte B A, Repa J J, Joseph S B, Wilpitz D C, Kast H R, Mangelsdorf D J, Tontonoz P. Proc Natl Acad Sci USA. 2001;98:507–512. doi: 10.1073/pnas.021488798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peet D J, Turley S D, Ma W, Janowski B A, Lobaccaro J M, Hammer R E, Mangelsdorf D J. Cell. 1998;93:693–704. doi: 10.1016/s0092-8674(00)81432-4. [DOI] [PubMed] [Google Scholar]
- 12.Schultz J R, Tu H, Luk A, Repa J J, Medina J C, Li L, Schwendner S, Wang S, Thoolen M, Mangelsdorf D J, et al. Genes Dev. 2000;14:2831–2838. doi: 10.1101/gad.850400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Repa J J, Liang G, Ou J, Bashmakov Y, Lobaccaro J M, Shimomura I, Shan B, Brown M S, Goldstein J L, Mangelsdorf D J. Genes Dev. 2000;14:2819–2830. doi: 10.1101/gad.844900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yoshikawa T, Shimano H, Amemiya-Kudo M, Yahagi N, Hasty A H, Matsuzaka T, Okazaki H, Tamura Y, Iizuka Y, Ohashi K, et al. Mol Cell Biol. 2001;21:2991–3000. doi: 10.1128/MCB.21.9.2991-3000.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Joseph S B, Laffitte B A, Patel P H, Watson M A, Matsukuma K E, Walczak R, Collins J L, Osborne T F, Tontonoz P. J Biol Chem. 2002;277:11019–11025. doi: 10.1074/jbc.M111041200. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Y, Repa J J, Gauthier K, Mangelsdorf D J. J Biol Chem. 2001;276:43018–43024. doi: 10.1074/jbc.M107823200. [DOI] [PubMed] [Google Scholar]
- 17.Luo Y, Tall A R. J Clin Invest. 2000;105:513–520. doi: 10.1172/JCI8573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tontonoz P, Hu E, Graves R A, Budavari A I, Spiegelman B M. Genes Dev. 1994;8:1224–1234. doi: 10.1101/gad.8.10.1224. [DOI] [PubMed] [Google Scholar]
- 19.Laffitte B A, Joseph S B, Walczak R, Pei L, Wilpitz D C, Collins J L, Tontonoz P. Mol Cell Biol. 2001;21:7558–7568. doi: 10.1128/MCB.21.22.7558-7568.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Collins, J. L., Fivush, A. M., Watson, M. A., Galardi, C. M., Lewis, M. C., Moore, L. B., Parks, D. J., Plunket, K. D., Tippin, T. K., Morgan, D. G., et al. (2002) J. Med. Chem., in press. [DOI] [PubMed]
- 21.Collins A R, Meehan W P, Kintscher U, Jackson S, Wakino S, Noh G, Palinski W, Hsueh W A, Law R E. Arterioscler Thromb Vasc Biol. 2001;21:365–371. doi: 10.1161/01.atv.21.3.365. [DOI] [PubMed] [Google Scholar]
- 22.Shih D M, Gu L, Xia Y R, Navab M, Li W F, Hama S, Castellani L W, Furlong C E, Costa L G, Fogelman A M, Lusis A J. Nature (London) 1998;394:284–287. doi: 10.1038/28406. [DOI] [PubMed] [Google Scholar]
- 23.Tontonoz P, Nagy L, Alvarez J G, Thomazy V A, Evans R M. Cell. 1998;93:241–252. doi: 10.1016/s0092-8674(00)81575-5. [DOI] [PubMed] [Google Scholar]
- 24.Bortnick A E, Rothblat G H, Stoudt G, Hoppe K L, Royer L J, McNeish J, Francone O L. J Biol Chem. 2000;275:28634–28640. doi: 10.1074/jbc.M003407200. [DOI] [PubMed] [Google Scholar]
- 25.Zhang S H, Reddick R L, Piedrahita J A, Maeda N. Science. 1992;258:468–471. doi: 10.1126/science.1411543. [DOI] [PubMed] [Google Scholar]
- 26.Groen A K, Bloks V W, Bandsma R H, Ottenhoff R, Chimini G, Kuipers F. J Clin Invest. 2001;108:843–850. doi: 10.1172/JCI12473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McNeish J, Aiello R J, Guyot D, Turi T, Gabel C, Aldinger C, Hoppe K L, Roach M L, Royer L J, de Wet J, et al. Proc Natl Acad Sci USA. 2000;97:4245–4250. doi: 10.1073/pnas.97.8.4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Berge K E, Tian H, Graf G A, Yu L, Grishin N V, Schultz J, Kwiterovich P, Shan B, Barnes R, Hobbs H H. Science. 2000;290:1771–1775. doi: 10.1126/science.290.5497.1771. [DOI] [PubMed] [Google Scholar]
- 29.Li A C, Brown K K, Silvestre M J, Willson T M, Palinski W, Glass C K. J Clin Invest. 2000;106:523–531. doi: 10.1172/JCI10370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Claudel T, Leibowitz M D, Fievet C, Tailleux A, Wagner B, Repa J J, Torpier G, Lobaccaro J M, Paterniti J R, Mangelsdorf D J, et al. Proc Natl Acad Sci USA. 2001;98:2610–2615. doi: 10.1073/pnas.041609298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chawla A, Boisvert W A, Lee C, Laffitte B A, Barak Y, Joseph S B, Liao D, Nagy L, Edwards P A, Curtiss L K, et al. Mol Cell. 2001;7:161–171. doi: 10.1016/s1097-2765(01)00164-2. [DOI] [PubMed] [Google Scholar]
- 32.Chinetti G, Lestavel S, Bocher V, Remaley A T, Neve B, Torra I P, Teissier E, Minnich A, Jaye M, Duverger N, et al. Nat Med. 2001;7:53–58. doi: 10.1038/83348. [DOI] [PubMed] [Google Scholar]