Abstract
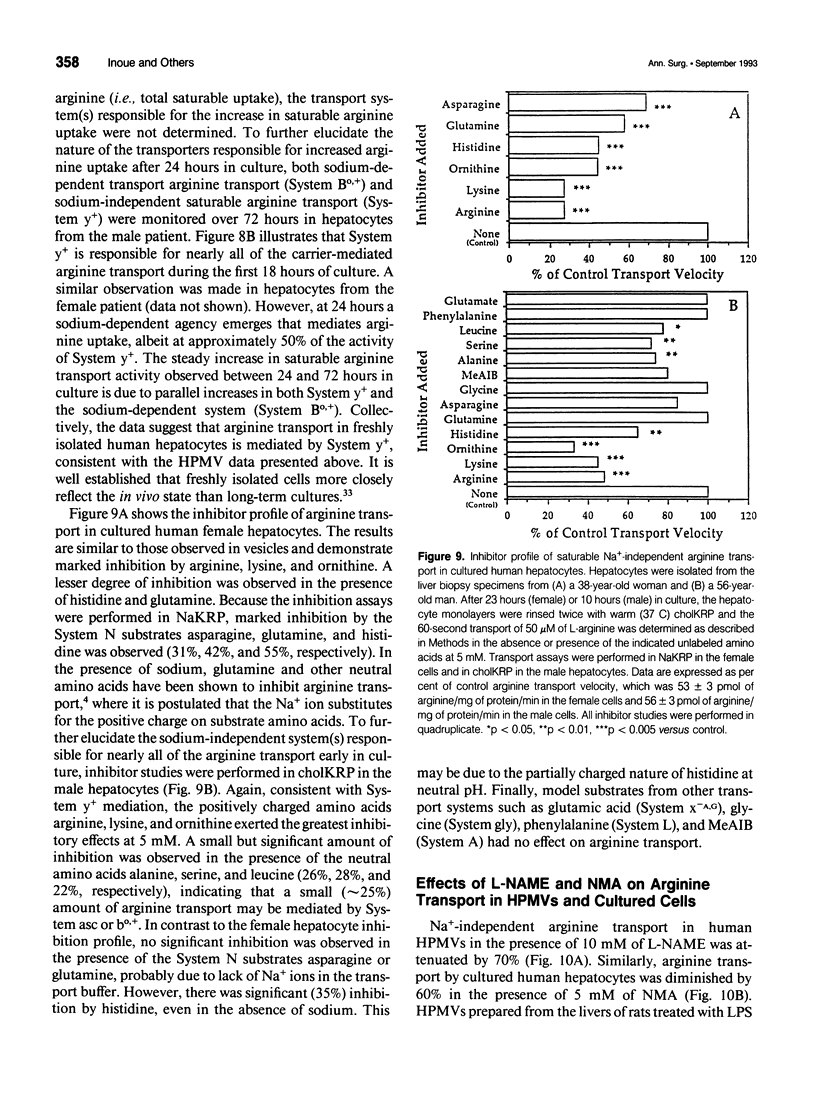
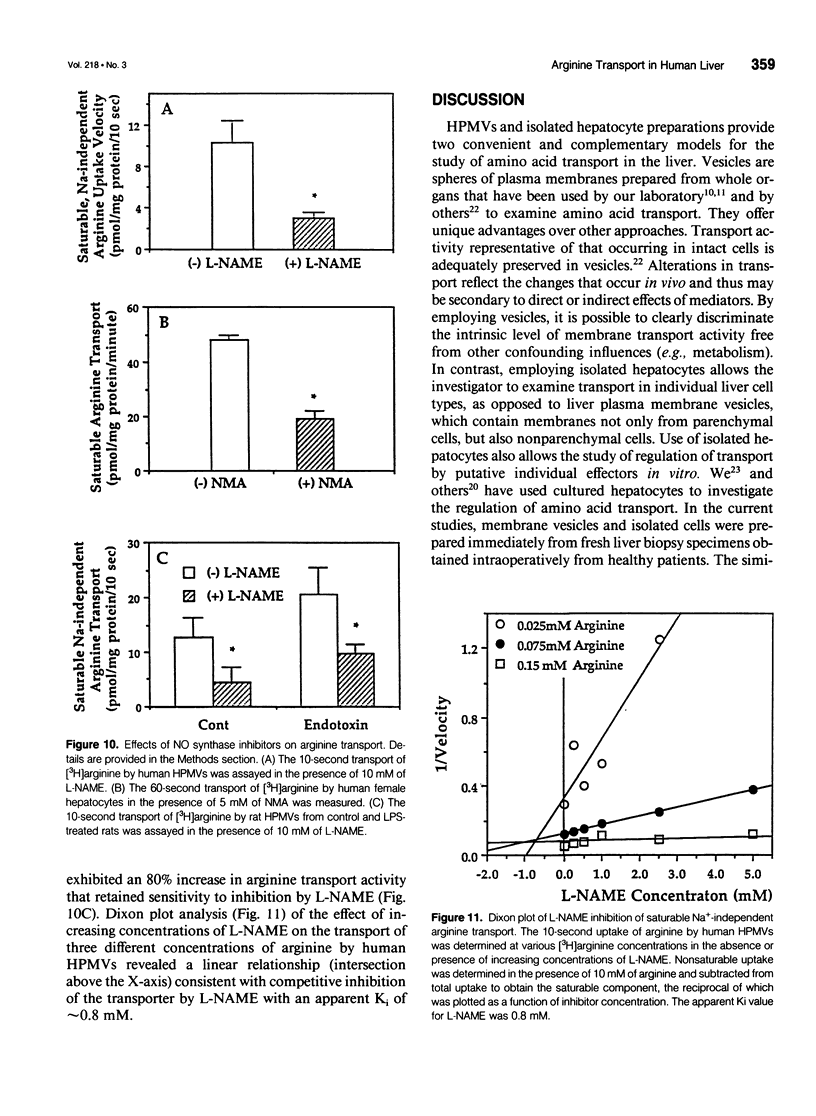
OBJECTIVE: Arginine transport was characterized and studied in human liver. SUMMARY BACKGROUND DATA: Plasma arginine uptake may regulate hepatocyte intracellular availability and the subsequent biosynthesis of nitric oxide (NO), but little is known about arginine transport across the human hepatocyte plasma membrane. METHODS: The authors characterized plasma membrane transport of 3[H]-L-arginine in hepatic plasma membrane vesicles (HPMVs) and in hepatocytes isolated and cultured from human liver biopsy specimens. They also studied the effects of the NO synthase inhibitors omega-nitro-L-arginine methyl ester (L-NAME) and N-methyl-arginine (NMA) on arginine transport in HPMVs and in cultured cells. RESULTS: Arginine transport was saturable, Na(+)-independent, temperature and pH sensitive, and was inhibited by the naturally occurring amino acids lysine, homoarginine, and ornithine (System y+ substrates). Arginine transport by both vesicles and cultured hepatocytes was significantly attenuated by NO synthase inhibitors, suggesting that the arginine transporter and the NO synthase enzyme may share a structurally similar arginine binding site. Dixon plot analysis showed the blockade to occur by competitive, rather than noncompetitive, inhibition. In vivo treatment of rats with lipopolysaccharide (LPS) resulted in a twofold stimulation of saturable arginine transport in the liver. This LPS-induced hepatic arginine transport activity was also inhibited by L-NAME. These data indicate that arginine transport by human hepatocytes is mediated primarily by the Na(+)-independent transport System y+. CONCLUSIONS: Besides inhibition of the NO synthase enzyme, the ability of arginine derivatives to block NO production may also be due to their ability to competitively inhibit arginine transport across the hepatocyte plasma membrane. The use of selective arginine derivatives that compete with arginine at the plasma membrane level may be a metabolic strategy that can be used to modulate the septic response.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbul A. Arginine: biochemistry, physiology, and therapeutic implications. JPEN J Parenter Enteral Nutr. 1986 Mar-Apr;10(2):227–238. doi: 10.1177/0148607186010002227. [DOI] [PubMed] [Google Scholar]
- Bensadoun A., Weinstein D. Assay of proteins in the presence of interfering materials. Anal Biochem. 1976 Jan;70(1):241–250. doi: 10.1016/s0003-2697(76)80064-4. [DOI] [PubMed] [Google Scholar]
- Billiar T. R., Curran R. D., Harbrecht B. G., Stuehr D. J., Demetris A. J., Simmons R. L. Modulation of nitrogen oxide synthesis in vivo: NG-monomethyl-L-arginine inhibits endotoxin-induced nitrate/nitrate biosynthesis while promoting hepatic damage. J Leukoc Biol. 1990 Dec;48(6):565–569. doi: 10.1002/jlb.48.6.565. [DOI] [PubMed] [Google Scholar]
- Curran R. D., Billiar T. R., Stuehr D. J., Hofmann K., Simmons R. L. Hepatocytes produce nitrogen oxides from L-arginine in response to inflammatory products of Kupffer cells. J Exp Med. 1989 Nov 1;170(5):1769–1774. doi: 10.1084/jem.170.5.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran R. D., Billiar T. R., Stuehr D. J., Ochoa J. B., Harbrecht B. G., Flint S. G., Simmons R. L. Multiple cytokines are required to induce hepatocyte nitric oxide production and inhibit total protein synthesis. Ann Surg. 1990 Oct;212(4):462–471. doi: 10.1097/00000658-199010000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene B., Pacitti A. J., Souba W. W. Characterization of L-arginine transport by pulmonary artery endothelial cells. Am J Physiol. 1993 Apr;264(4 Pt 1):L351–L356. doi: 10.1152/ajplung.1993.264.4.L351. [DOI] [PubMed] [Google Scholar]
- Gurr J. A., Potter V. R. The significance of differences between fresh cell suspensions and fresh or maintained monolayers. Ann N Y Acad Sci. 1980;349:57–66. doi: 10.1111/j.1749-6632.1980.tb29514.x. [DOI] [PubMed] [Google Scholar]
- Ignarro L. J., Buga G. M., Wood K. S., Byrns R. E., Chaudhuri G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9265–9269. doi: 10.1073/pnas.84.24.9265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilberg M. S., Christensen H. N. Electron-transferring enzymes in the plasma membrane of the Ehrlich ascites tumor cell. Biochemistry. 1979 Apr 17;18(8):1525–1530. doi: 10.1021/bi00575a021. [DOI] [PubMed] [Google Scholar]
- Kilberg M. S. Measurement of amino acid transport by hepatocytes in suspension or monolayer culture. Methods Enzymol. 1989;173:564–575. doi: 10.1016/s0076-6879(89)73039-1. [DOI] [PubMed] [Google Scholar]
- Kilbourn R. G., Griffith O. W. Overproduction of nitric oxide in cytokine-mediated and septic shock. J Natl Cancer Inst. 1992 Jun 3;84(11):827–831. doi: 10.1093/jnci/84.11.827. [DOI] [PubMed] [Google Scholar]
- Kilbourn R. G., Jubran A., Gross S. S., Griffith O. W., Levi R., Adams J., Lodato R. F. Reversal of endotoxin-mediated shock by NG-methyl-L-arginine, an inhibitor of nitric oxide synthesis. Biochem Biophys Res Commun. 1990 Nov 15;172(3):1132–1138. doi: 10.1016/0006-291x(90)91565-a. [DOI] [PubMed] [Google Scholar]
- Kim J. W., Closs E. I., Albritton L. M., Cunningham J. M. Transport of cationic amino acids by the mouse ecotropic retrovirus receptor. Nature. 1991 Aug 22;352(6337):725–728. doi: 10.1038/352725a0. [DOI] [PubMed] [Google Scholar]
- Knowles R. G., Merrett M., Salter M., Moncada S. Differential induction of brain, lung and liver nitric oxide synthase by endotoxin in the rat. Biochem J. 1990 Sep 15;270(3):833–836. doi: 10.1042/bj2700833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamas S., Marsden P. A., Li G. K., Tempst P., Michel T. Endothelial nitric oxide synthase: molecular cloning and characterization of a distinct constitutive enzyme isoform. Proc Natl Acad Sci U S A. 1992 Jul 15;89(14):6348–6352. doi: 10.1073/pnas.89.14.6348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochoa J. B., Udekwu A. O., Billiar T. R., Curran R. D., Cerra F. B., Simmons R. L., Peitzman A. B. Nitrogen oxide levels in patients after trauma and during sepsis. Ann Surg. 1991 Nov;214(5):621–626. doi: 10.1097/00000658-199111000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacitti A. J., Austgen T. R., Souba W. W. Adaptive regulation of alanine transport in hepatic plasma membrane vesicles from the endotoxin-treated rat. J Surg Res. 1991 Jul;51(1):46–53. doi: 10.1016/0022-4804(91)90068-w. [DOI] [PubMed] [Google Scholar]
- Pacitti A. J., Copeland E. M., Souba W. W. Stimulation of hepatocyte System y(+)-mediated L-arginine transport by an inflammatory agent. Surgery. 1992 Aug;112(2):403–411. [PubMed] [Google Scholar]
- Pacitti A. J., Inoue Y., Plumley D. A., Copeland E. M., Souba W. W. Growth hormone regulates amino acid transport in human and rat liver. Ann Surg. 1992 Sep;216(3):353–362. doi: 10.1097/00000658-199209000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacitti A. J., Inoue Y., Souba W. W. Tumor necrosis factor stimulates amino acid transport in plasma membrane vesicles from rat liver. J Clin Invest. 1993 Feb;91(2):474–483. doi: 10.1172/JCI116225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer R. M., Ashton D. S., Moncada S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature. 1988 Jun 16;333(6174):664–666. doi: 10.1038/333664a0. [DOI] [PubMed] [Google Scholar]
- Petros A., Bennett D., Vallance P. Effect of nitric oxide synthase inhibitors on hypotension in patients with septic shock. Lancet. 1991 Dec 21;338(8782-8783):1557–1558. doi: 10.1016/0140-6736(91)92376-d. [DOI] [PubMed] [Google Scholar]
- Schenerman M. A., Kilberg M. S. Maintenance of glucagon-stimulated system A amino acid transport activity in rat liver plasma membrane vesicles. Biochim Biophys Acta. 1986 Apr 25;856(3):428–436. doi: 10.1016/0005-2736(86)90133-1. [DOI] [PubMed] [Google Scholar]
- Souba W. W., Pacitti A. J. How amino acids get into cells: mechanisms, models, menus, and mediators. JPEN J Parenter Enteral Nutr. 1992 Nov-Dec;16(6):569–578. doi: 10.1177/0148607192016006569. [DOI] [PubMed] [Google Scholar]
- Van Winkle L. J., Christensen H. N., Campione A. L. Na+-dependent transport of basic, zwitterionic, and bicyclic amino acids by a broad-scope system in mouse blastocysts. J Biol Chem. 1985 Oct 5;260(22):12118–12123. [PubMed] [Google Scholar]
- White M. F. The transport of cationic amino acids across the plasma membrane of mammalian cells. Biochim Biophys Acta. 1985 Dec 9;822(3-4):355–374. doi: 10.1016/0304-4157(85)90015-2. [DOI] [PubMed] [Google Scholar]


