Abstract
Gastric infection with Helicobacter pylori is a cosmopolitan problem, and is especially common in developing regions where there is also a high prevalence of gastric cancer. These infections are known to cause gastritis and peptic ulcers, and dramatically enhance the risk of gastric cancer. Eradication of this organism is an important medical goal that is complicated by the development of resistance to conventional antimicrobial agents and by the persistence of a low level reservoir of H. pylori within gastric epithelial cells. Moreover, economic and practical problems preclude widespread and intensive use of antibiotics in most developing regions. We have found that sulforaphane [(−)-1-isothiocyanato-(4R)-(methylsulfinyl)butane], an isothiocyanate abundant as its glucosinolate precursor in certain varieties of broccoli and broccoli sprouts, is a potent bacteriostatic agent against 3 reference strains and 45 clinical isolates of H. pylori [minimal inhibitory concentration (MIC) for 90% of the strains is ≤4 μg/ml], irrespective of their resistance to conventional antibiotics. Further, brief exposure to sulforaphane was bactericidal, and eliminated intracellular H. pylori from a human epithelial cell line (HEp-2). In complementary experiments, sulforaphane blocked benzo[a]pyrene-evoked forestomach tumors in ICR mice. This protection resulted from induction of phase 2 detoxication and antioxidant enzymes, and was abrogated in mice lacking the nrf2 gene, which regulates phase 2 enzymes. Thus, the dual actions of sulforaphane in inhibiting Helicobacter infections and blocking gastric tumor formation offer hope that these mechanisms might function synergistically to provide diet-based protection against gastric cancer in humans.
Adenocarcinoma of the stomach is the second most common malignancy in the world, and the principal cause of mortality from cancer in the developing regions of Asia, Africa, and South America (1, 2). The annual incidence approaches 100 per 100,000 in Japan (3), where it is the leading cause of cancer mortality. Much evidence points to the major importance of extrinsic factors in the etiology of this disease: (i) the dramatic decline of stomach cancer in the 20th century, frequently attributed to the advent of refrigeration and improved methods of food preservation (4); (ii) large geographic differences in stomach cancer mortality, and the observation that migration of populations results in cancer incidences that resemble those of the new locations (5); and (iii) the inverse association between high consumption of vegetables and fruits and gastric cancer incidence in developed countries (6). But the most striking advance in our understanding of the etiology of gastric cancer was the identification of the role of infection with Helicobacter pylori in the development of peptic ulcers and their progression to gastric dysplasia and gastric malignancy (reviewed in ref. 7). Gastric cancer is therefore a highly preventable disease. The challenge is to devise methods for the eradication of Helicobacter by practical, implementable methods and to determine whether this will protect medically underserved populations from the ravages of this disease.
The relation of H. pylori to gastric pathology was first described 20 years ago (8). H. pylori is a Gram-negative, bacilliform, motile, microaerophilic bacterium that colonizes the gastric mucosa in humans, causes gastritis and peptic ulcer disease, and is also an important risk factor for development of gastric cancer and mucosa-associated lymphoid tissue (MALT) lymphoma (9–11). The risks for developing gastric adenocarcinoma and MALT lymphoma are 3 to 6 times higher among carriers of H. pylori infections than in uninfected subjects (7, 11, 12). Strong epidemiological evidence supports the correlation of age and markers of poverty with both H. pylori prevalence and stomach cancer (13–15), as well as involvement of H. pylori in iron deficiency anemia (16, 17), a serious public health problem in many developing countries. As much as 40% of the population in developed countries and 70% in developing countries are carriers of this organism (18). Numerous retrospective studies and a recent long-term prospective epidemiological study demonstrated development of gastric cancer in persons infected with H. pylori but not in uninfected persons (19, 20). Several large multicenter intervention trials are currently assessing the potential of H. pylori eradication to prevent gastric cancer, and results should soon be available (21–23). Recent animal studies support the notion that antibiotic therapy targeted at eliminating the microbe may also contribute to the prevention of stomach cancer in humans (24).
Although H. pylori is now recognized as one of the most prevalent human pathogens in the world (25), it is difficult to eradicate in 15–20% of individuals by antibiotic therapy, which is now recommended for infected patients with gastric or duodenal ulcers or gastric mucosa-associated lymphoid tissue lymphoma (26, 27). Multidrug therapies consisting of combinations of two or more antibiotics (e.g., amoxicillin, clarithromycin, or metronidazole) with an inhibitor of acid secretion (histamine H2 antagonist or proton pump inhibitor) are required. But even by the most optimistic estimates, these treatments are not universally successful (26, 28). This lack of efficacy appears to be due, at least in part, to the development of resistance of H. pylori strains to these antibiotics (29, 30) and the persistence of organisms within gastric epithelial cells (31, 32). Moreover, the widespread use of antibiotics to eradicate H. pylori infections in developing countries is both logistically and economically impractical. It is therefore imperative to identify new antimicrobial agents that are effective against both intra- and extracellular forms of H. pylori (especially those that are resistant to conventional antibiotics), and that can be delivered simply and economically by the oral route.
Our studies on the effects of [(−)-1-isothiocyanato-(4R)-(methylsulfinyl)butane] (sulforaphane) on Helicobacter began when one author was told of several individuals who were afflicted with persistent peptic ulcer disease and quite unexpectedly experienced dramatic relief of their symptoms after consuming 3-day-old broccoli sprouts. These cruciferous sprouts are an exceptionally rich source of the isothiocyanate sulforaphane, or more precisely glucoraphanin (33), its naturally occurring glucosinolate precursor (34). Substantial quantities of isothiocyanates (up to 100 mg daily) and even greater quantities of their glucosinolate precursors are widely consumed by humans (34–36). They may act locally within the gastrointestinal tract (37), or may distribute systemically after conversion to their cognate isothiocyanates (34, 38). Sulforaphane was originally isolated on the basis of its antimicrobial activity from hoary cress (Cardaria draba; white top), a widely distributed weed belonging to the plant family Cruciferae (39). Sulforaphane was subsequently shown to inhibit the growth of a variety of microorganisms, including some human pathogens (40). We therefore examined the possibility that sulforaphane might inhibit the growth of H. pylori, and thereby could account for the aforementioned amelioration of gastric symptoms.
Sulforaphane is also of special interest because of its anticarcinogenic activity. It was isolated 10 years ago as one of the most potent inducers of phase 2 proteins (41) and inhibitors of experimental carcinogenesis in animal systems (42). There is now convincing evidence that induction of phase 2 genes (e.g., glutathione transferases, NAD(P)H:quinone reductase, UDP-glucuronosyltransferases) is a highly effective and sufficient condition for protecting animals and their cells against the toxic and neoplastic effects of carcinogens (43, 44). Nevertheless, the effects of sulforaphane on the formation of gastric tumors have not, to our knowledge, been examined.
In this report, we show that sulforaphane is bactericidal to both extracellular and intracellular forms of H. pylori, by mechanisms that are not yet understood. We also show that sulforaphane protects the mouse forestomach against the neoplastic effects of benzo[a]pyrene, and that this effect depends on induction of phase 2 enzymes because it is abolished in mice deficient in the nrf2 gene, which controls these inductions (45, 46). Thus, the dual properties of sulforaphane as an antibiotic and anticancer agent provide a two-tiered, and possibly synergistic, approach to eliminating H. pylori and reducing the incidence of gastric disease.
Experimental Procedures
Chemicals and Materials.
Sulforaphane was isolated from broccoli seeds (33, 38). Seeds were extracted with hexane, boiled in 20 mM potassium phosphate buffer (pH 7.4), filtered through Celite, and hydrolyzed with crude myrosinase, and the hydrolyzate was partitioned against ethyl acetate. The ethyl acetate was then removed under vacuum in a rotary evaporator, and the resultant product was fractionally distilled to yield a chromatographically pure, light yellow, oily liquid boiling at 134–136°C at 5 μm of mercury. The sulforaphane thus obtained was >95% pure, based on cyclocondensation (47), and its characteristic UV absorbance spectrum (molar extinction coefficient A238 = 910 M−1⋅cm−1). The molecular weight of 177 was confirmed by electrospray mass spectroscopy. Amoxicillin was supplied by GlaxoSmithKline and clarithromycin by Sanofi-Synthelabo (Paris). Metronidazole was purchased from Sigma-Aldrich. Stock solutions were prepared in acetonitrile for sulforaphane, and as recommended by their manufacturers for the other antibiotics. Further dilutions of all antibiotics were made with sterile water.
Bacterial Strains.
Three reference strains (26695, J99, and ATCC 43504) and 45 clinical isolates (LBN200 to LBN244) of H. pylori were used. The clinical isolates were obtained in 2000 and 2001 at the University Hospital Center of Nancy, France, from individual patients with gastritis and gastric or duodenal ulcers. These strains were identified as H. pylori by Gram stain, and oxidase, catalase, and urease production. All strains were stored in Brucella broth (Oxoid, Basingstoke, England) containing 15% (wt/vol) glycerol at −80°C until use. Experiments were all performed at 37°C in a microaerophilic (5% O2, 15% CO2, 80% N2) atmosphere, with H. pylori cultures grown on Columbia agar containing 10% horse blood.
Determination of Bacteriostatic Activity and Antibiotic Resistance.
The bacteriostatic activity of sulforaphane, amoxicillin, clarithromycin, and metronidazole was evaluated for all H. pylori strains by determining the minimal inhibitory concentration (MIC) of each drug by using the agar dilution method (pH 7.4) recommended by the National Committee for Clinical Laboratory Standards (NCCLS; ref. 48). MICs of sulforaphane were also determined under the same conditions at pH 5.8, obtained by addition of 0.1 M HCl (to approximate the gastric juxtamucosal pH). Twofold dilutions of each drug ranging from 64 to 0.03 μg/ml were tested. The MIC was defined as the lowest concentration of each compound that resulted in no visible growth after 3 days of incubation at 37°C under microaerophilic conditions. Clarithromycin resistance was defined by an MIC ≥1 μg/ml, as recommended by NCCLS (48). Resistance breakpoints for metronidazole and amoxicillin were defined as >8 μg/ml (49) and >0.5 μg/ml (50), respectively.
Determination of Bactericidal Activity.
The bactericidal activity of sulforaphane against H. pylori strains LBN201 and 26695 was evaluated by using a time-to-kill assay. Tests were performed in Brucella broth supplemented with 10% FCS, inoculated with each isolate to a final concentration of ≈5 × 106 colony forming units (cfu)/ml. The kinetics of the bactericidal effects were determined both at pH 7.4, the initial pH of the broth, and at pH 5.8 (51), which reflects more closely the pH prevailing in the gastric juxtamucosal environment. For each strain, sulforaphane was tested at both 0.25×, 0.5×, 1×, and 5× the MIC. After 0, 2, 4, 6, 8, and 24 h of incubation at 37°C under microaerophilic conditions and gentle shaking, 100-μl samples were serially diluted and plated onto Columbia blood agar. After 5 days of incubation under microaerophilic conditions at 37°C, colonies were counted. The limit of detection was 20 cfu/ml. Tests were performed in duplicate; results are expressed as mean log10 cfu/ml. Bactericidal activity was defined as a greater than 1,000-fold reduction in viable colony forming units.
Determination of Intracellular Bactericidal Activity.
Intracellular activity was studied in HEp-2 cells (ATCC CCL 23), a human epithelial cell line previously used to determine the activity of drugs against intracellular H. pylori (52, 53). HEp-2 cells were routinely cultured in 75-cm2 plastic tissue culture T-flasks containing minimum essential medium supplemented with Eagle's salts, 50 mM l-glutamine, and 0.75% sodium bicarbonate (EMEM), and 10% FCS at 37°C in 5% CO2. The cells were trypsinized, washed in EMEM, seeded into six-well tissue culture plates, and incubated overnight at 37°C in 5% CO2. Monolayers of HEp-2 cells (106 cells per well) were exposed to 108 cfu per well (multiplicity of infection = 100) of H. pylori strains LBN201 or 26659 in EMEM with FCS for 12 h at 37°C in 5% CO2. Cells were gently washed 6× with Hanks' balanced salt solution to remove any nonadherent bacteria, and then incubated with this solution containing 100 μg/ml of gentamicin for 1.5 h to kill extracellular bacteria (54). Monolayers were then washed 6× again with the same solution, and incubated in culture medium containing either sulforaphane at concentrations of 2 and 10 μg/ml (strain LBN201), or 4 and 20 μg/ml (strain 26695), at 37°C in 5% CO2. After 0, 2, 4, 8, and 24 h, replicate plates of cells were washed 6× with Hanks' balanced salt solution, and lysed with distilled water. The number of intracellular viable bacteria (cfu/ml) in the lysate was determined as described above for the extracellular time-to-kill studies. Tests were performed in duplicate; results are expressed as mean log10 cfu/ml. Concentration of sulforaphane and its conjugates in H. pylori-infected HEp-2 cells was determined by using duplicate aliquots of cell lysates obtained as described above. Each of these samples was incubated with 1,2-benzenedithiol to produce a cyclocondensation product (1,3-benzodithiole-2-thione) that could be separated chromatographically and detected at levels as low as 15 pmol (47, 55). Concentrations of sulforaphane and its conjugates were determined as a mean of four determinations.
Carcinogenesis Study.
Inhibition of benzo[a]pyrene-generated gastric tumors by sulforaphane was examined in four groups (20 each) of 9- to 12-week-old female ICR mice; two groups were genotyped as wild-type (+/+) and two groups were nrf2 deficient (−/−) (56). All animals were acclimatized on an AIN-76A diet without ethoxyquin (Teklad, Madison, WI) for 1 wk and maintained on this diet or the diet supplemented with 2.5 mmol (442.5 mg) of sulforaphane per kg of diet, for the control and treated groups, respectively. The estimated daily intake of sulforaphane per mouse was 7.5 μmol. The animals received benzo[a]pyrene (120 mg/kg in 0.2 ml of corn oil) by gavage on four occasions, at 1-wk intervals, beginning after the dietary acclimatization period. The sulforaphane diet was begun 7 days before the first dose of the carcinogen and continued for 2 days after the last dose. The animals were weighed weekly, and were killed by CO2 asphyxiation and cervical dislocation 20 wk after the first dose of carcinogen. The forestomachs were removed and fixed in 10% buffered formalin, and the tumors were counted according to Wattenberg (57). The tumor numbers were analyzed by simple descriptive statistics. Experiments on animals were conducted in compliance with protocols approved by the Animal Care and Use Committee of The Johns Hopkins Medical Institutions.
Results and Discussion
The bacteriostatic potency of sulforaphane was examined for a broad range of clinical isolates, as well as three reference strains of H. pylori. One clinical isolate and one reference strain were then used for subsequent demonstration of both extracellular and intracellular bactericidal potency. In addition, the effect of sulforaphane on gastric tumor formation in the classical benzo[a]pyrene mouse forestomach carcinogenesis model was examined.
Bacteriostatic Potency of Sulforaphane.
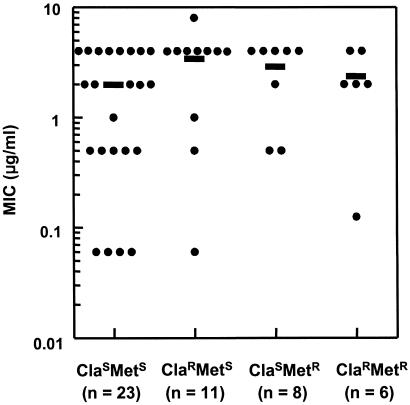
At neutral pH, sulforaphane exhibited high bacteriostatic activity against all 48 strains tested, with MIC values ranging from 0.06 to 8 μg/ml [mean = 2.5 μg/ml; median = 2 μg/ml; MIC90 (MIC at which growth of 90% of strains is inhibited) = 4 μg/ml; Fig. 1]. This activity was not significantly (less than 2-fold) different at pH 5.8, which reflects more closely the gastric juxtamucosal pH (data not shown). Thus, sulforaphane was significantly more potent than other natural compounds that have been tested, such as resveratrol from grape skins and red wine (MIC90 = 25 μg/ml; ref. 58), allixin from garlic bulbs (MIC90 = 25 μg/ml; ref. 59), protolichesterinic acid from the lichen Cetraria islandica (MIC90 = 32 μg/ml; ref. 60), and epigallocatechin gallate from tea (MIC90 = 32 μg/ml; ref. 61). All 48 strains were susceptible to amoxicillin (MIC90 = 0.125 μg/ml, MIC range: 0.06–0.5 μg/ml), and 23 of these strains were also susceptible to both clarithromycin and metronidazole. Of the remaining 25 strains, 11 were resistant to clarithromycin, 8 were resistant to metronidazole, and 6 were resistant to both agents. The inhibitory potency of sulforaphane was not related to the resistance of these strains to the other antibiotics, and there were no significant differences in MIC90 among resistance classes. Moreover, sulforaphane exhibited a high inhibitory activity (MIC90: 4 μg/ml) against both the clarithromycin-resistant strains (n = 17; MIC90 for clarithromycin, 32 μg/ml) and the metronidazole-resistant strains (n = 14; MIC90 for metronidazole, 64 μg/ml) tested.
Figure 1.
Bacteriostatic potency (minimal inhibitory concentration, MIC) of sulforaphane against 45 clinical isolates and 3 reference strains of H. pylori. Twenty-three bacterial strains were susceptible to clarithromycin (ClaS) and metronidazole (MetS), and the rest of the strains were resistant to clarithromycin (ClaR), metronidazole (MetR), or both antibiotics (ClaR MetR). Median MIC values for each of the four groups of strains were 2, 4, 4, and 2 μg/ml, respectively, and were not significantly different from each other. Each data point represents an individual strain, and means are indicated by horizontal bars.
Extracellular Bactericidal Potency of Sulforaphane.
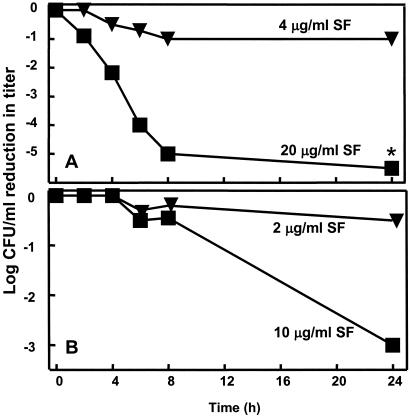
Having established the bacteriostatic activity of sulforaphane against H. pylori, we next evaluated its bactericidal potency by using a time-to-kill assay with one reference strain (26695) and one clinical isolate (LBN201). These experiments were run in parallel at pH 7.4 and 5.8, because most H. pylori colonize the mucous layer and gastric pits of the antrum, where it is assumed that the pH is about 5.5 (62). However, the urease of H. pylori generates ammonia in its immediate surroundings, which thus increases the pH to about neutrality (63, 64). Bactericidal activity was defined in accordance with convention as a reduction in plate counts of ≥1,000 cfu/ml. The effect was nearly always concentration dependent (Fig. 2). For strain LBN201, there was a 1,000-fold reduction within 24 h of exposure to 10 μg/ml (5× MIC) of sulforaphane. There was no effect of changes in pH over the range examined with any of the strains or any of the sulforaphane concentrations tested (0.25×, 0.5×, 1×, or 5× the MIC for those strains; data not shown). At all of the subinhibitory concentrations of sulforaphane tested against strains 26695 and LBN201, no reduction in viable counts was observed at any time during the assays. Although sulforaphane has not been previously tested, the concentration-dependent bactericidal activity of sulforaphane against H. pylori is similar to that of other isothiocyanates against other Gram-positive and Gram-negative bacteria (65, 66).
Figure 2.
Bactericidal potency of sulforaphane (SF) on two strains of H. pylori (A, LBN201, clinical isolate; B, 26695, reference strain) after exposure to 1× and 5× MIC of SF at pH 5.8. (Data points are the means of duplicate determinations; the asterisk indicates a point below the limits of detection.)
Intracellular Bactericidal Potency of Sulforaphane in HEp-2 Cells.
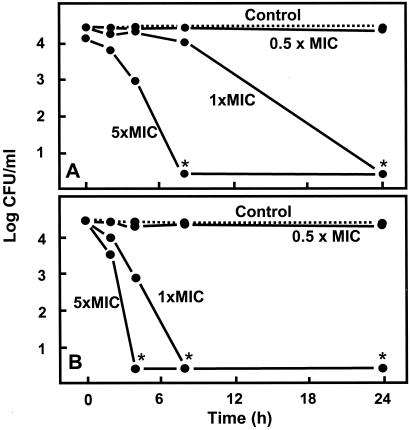
To test the effect of sulforaphane on intracellular forms of H. pylori, reproducible intracellular infections were established, with bacterial titers comparable to those obtained by others with this cell line (67, 68). We found that sulforaphane delivered at a concentration equivalent to its MIC in strains 26695 and LBN201 completely killed intracellular bacteria within 24 h (Fig. 3). Higher, but easily achievable, concentrations (e.g., 5× the MIC) completely killed the intracellular bacteria within 4 to 8 h (Fig. 3), and sulforaphane was well tolerated by the mammalian cells up to concentrations of 110 μM. The cellular uptake of sulforaphane (5 μM initial extracellular concentration) into noninfected HEp-2 cells, as well as ARPE-19, AGS, and Hepa1c1c7 cells, was evaluated, and was very rapid over the first 30 min, reaching levels as high as 500 μM (data not shown) and was followed by rapid decline, presumably by export of sulforaphane as described by Zhang (69–71). Initial accumulation of sulforaphane and its conjugates in H. pylori-infected HEp-2 cells reached levels of between 2- and 5-fold the administered concentration, thus potentially accounting for the rapid 1,000-fold reduction in viable H. pylori titer within 4 h of dosing.
Figure 3.
Eradication of intracellular bacterial infection in cultured HEp-2 cells after treatment with sulforaphane (SF) at 0.5×, 1×, and 5× the MIC for a clinical isolate (A, LBN201; 1, 2, and 10 μg/ml) and a reference strain (B, 26695; 2, 4, and 20 μg/ml) of H. pylori. HEp-2 cells were infected with H. pylori, extracellular bacteria were removed, and the mammalian cells were lysed at each sampling time to measure intracellular sulforaphane concentration and count cfu of H. pylori. Note the sharp demarcation between lack of activity (0.5× MIC) and bactericidal potency (1× MIC). Data points are the means of duplicate determinations; the asterisks indicate points below the limits of detection. Intracellular sulforaphane was calculated to be from 2- to 5-fold greater than initial external concentration, and gradually decreased over time.
Effect of Sulforaphane on Gastric Tumor Formation.
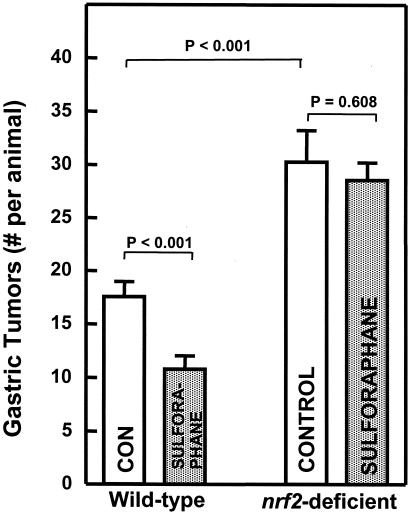
The chemoprotective antitumor effects of sulforaphane have been ascribed to its potent induction of phase 2 detoxication enzymes in rodent tissues. The mouse forestomach responds to sulforaphane by up-regulation of genes controlling glutathione transferases, NAD(P)H:quinone reductase, and other phase 2 proteins (41). To examine the effect of sulforaphane on gastric tumor formation, we used the classical mouse forestomach tumor model, in which administration of four weekly doses of benzo[a]pyrene by gavage results in development of numerous tumors that can be reliably quantified (57). Under standard experimental conditions, the forestomachs of control ICR mice developed 17.6 tumors per mouse 20 wk after the first administration of benzo[a]pyrene. Feeding of sulforaphane (estimated intake 7.5 μmol per day) in the diet for the period extending from 7 days before the first dose to 2 days after the last dose of carcinogen reduced the number of tumors to 10.8 per mouse (a 39% reduction; P < 0.001) (Fig. 4). This observation is consistent with the finding that sulforaphane blocked 7,12-dimethylbenz[a]anthracene (DMBA)-induced mammary tumor formation in rats (42), reduced benzo[a]pyrene-induced aberrant crypt formation in mouse mammary gland explants (72), and suppressed azoxymethane-dependent abnormal colon crypt formation in rats (73).
Figure 4.
Effect of sulforaphane on benzo[a]pyrene-induced neoplasia of the forestomach in female wild-type and nrf2-deficient mice. Female mice (9–12 wk old) were fed sulforaphane at an estimated intake of 7.5 μmol per animal per day, for a period extending from 7 days before, to 2 days after the last dose of carcinogen. Dosing with benzo[a]pyrene (120 mg/kg in 0.2 ml corn oil by gavage), was at four consecutive weekly intervals, and animals were killed 20 wk after the first benzo[a]pyrene treatment. Gastric tumors are reported as follows: (number of gastric tumors in the entire group)/(number of mice at risk at termination of experiment). Two animals in the nrf2-deficient control group had tumors too numerous to count. Open bar, vehicle-treated; filled bar, sulforaphane-treated. Error bars are ± SEM.
The induction of phase 2 proteins is regulated by the Nrf2 transcription factor (45, 46). The recent availability of mice in which the gene coding for the Nrf2 has been disrupted (45, 46) has made it possible to validate the importance of phase 2 induction in the protective response to inducers. Consequently, in the experiment described above, we also included two groups of ICR mice in which the nrf2 gene had been deleted. These animals developed a significantly larger number of forestomach tumors (30.2 per mouse) in comparison with controls (17.6 tumors per mouse; P < 0.001), and an identical regime of sulforaphane administration did not significantly reduce the number of tumors (28.5 per mouse; Fig. 4). The results parallel those obtained with the 1,2-dithiole-3-thione oltipraz in the same experimental system (56).
Conclusions
The present study was undertaken for several reasons. Helicobacter infections are geographically widespread and extremely common, especially in developing countries where there is also a high prevalence of gastric cancer. There is powerful evidence for an etiological connection between these conditions. Helicobacter infections are difficult to eradicate for biological, logistic, sociologic, and economic reasons. H. pylori is able to penetrate the gastric epithelium and occupy a protected “sanctuary” from which it can repopulate the gastric lumen, and many clinical isolates have acquired resistance to one or more conventional antibiotics. These factors pose difficult problems, and the routine and intensive use of modern antibiotics in many developing regions of the world is impractical.
Sulforaphane, a phytochemical, appears to overcome all of these problems. It can be delivered in high concentrations in the diet in the form of edible cruciferous vegetables (33, 38), and it eradicates both intracellular and resistant strains of H. pylori. Although higher concentrations are required to achieve bactericidal activity for the intracellular forms, sulforaphane accumulates intracellularly to high levels, as its glutathione conjugate (69–71). It is present in edible cruciferous plants, can be safely administered to humans (38, 74), and can be directly delivered to the stomach.
Because sulforaphane is highly effective in blocking mammary and colon tumors in animal models (33, 42, 73), it was gratifying—although not surprising—to find that it also blocks stomach tumors in mice, as shown in this paper. The recent discovery that H. pylori eradication in patients with H. pylori-associated gastritis elevated or restored glutathione S-transferase activity and glutathione levels in the antral mucosa (75) further supports the concept that sulforaphane could have a similar direct tumor-preventive effect in humans. This duality of action of sulforaphane in eradicating Helicobacter infections by a direct but unknown mechanism and blocking gastric tumor formation by phase 2 enzyme induction thus offers promise that these effects could operate synergistically and could provide highly effective protection against gastric cancer in humans.
Acknowledgments
The French and American groups contributed equally to these collaborative studies. We gratefully acknowledge the contributions of Traci Testerman and Harry Mobley (Department of Microbiology, University of Maryland Medical Center, Baltimore) and Elizabeth A. Montgomery (Department of Pathology, Johns Hopkins School of Medicine) in initial pilot experiments that are not reported herein, and of Pamela Talalay (Department of Neurology, Johns Hopkins School of Medicine) for her expert editorial advice. Two of the authors (J.W.F. and P.T.) as well as Johns Hopkins University, own stock in Brassica Protection Products (BPP), a company whose mission is to develop chemoprotective food products and which sells broccoli sprouts. No support of these studies was provided by BPP. J.W.F. and P.T. are board members and scientific consultants to BPP, and their stock is subject to certain restrictions under University policy. The terms of this arrangement are being managed by Johns Hopkins University in accordance with its conflict of interest policies. These studies were supported by generous gifts from the Lewis B. and Dorothy Cullman Foundation, the Barbara Lubin Goldsmith Foundation, and the McMullan Family Fund, all of New York, and by National Institutes of Health Grant CA 94076.
Abbreviations
- cfu
colony forming units
- EMEM
Eagle's minimal essential medium
- MIC
minimal inhibitory concentration
- MIC90
MIC at which growth of 90% of strains is inhibited
References
- 1.Pisani P, Parkin D M, Bray F, Ferlay J. Int J Cancer. 1999;83:18–29. doi: 10.1002/(sici)1097-0215(19991210)83:6<870::aid-ijc35>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 2.Parkin D M, Pisani P, Ferlay J. Int J Cancer. 1999;80:827–841. doi: 10.1002/(sici)1097-0215(19990315)80:6<827::aid-ijc6>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 3.Alexander R H, Kelsen D P, Tepper J E. In: Cancer Principles and Practice of Oncology. 4th Ed. DeVita V T Jr, Heilman S, Rosenberg S A, editors. Philadelphia: Lippincott; 1993. pp. 818–848. [Google Scholar]
- 4.Paik D C, Saborio D V, Oropeza R, Freeman H P. Int J Epidemiol. 2001;30:181–182. doi: 10.1093/ije/30.1.181. [DOI] [PubMed] [Google Scholar]
- 5.Galanis D J, Kolonel L N, Lee J, Nomura A. Int J Epidemiol. 1998;27:173–180. doi: 10.1093/ije/27.2.173. [DOI] [PubMed] [Google Scholar]
- 6.World Cancer Research Fund. Food, Nutrition and the Prevention of Cancer: A Global Perspective. Washington: American Institute for Cancer Research; 1997. [DOI] [PubMed] [Google Scholar]
- 7.Forman D. Br Med Bull. 1998;54:71–78. doi: 10.1093/oxfordjournals.bmb.a011682. [DOI] [PubMed] [Google Scholar]
- 8.Warren J R, Marshall B J. Lancet. 1983;1:1273–1275. [PubMed] [Google Scholar]
- 9.Scheiman J M, Cutler A F. Am J Med. 1999;106:224–225. doi: 10.1016/s0002-9343(98)00393-3. [DOI] [PubMed] [Google Scholar]
- 10.Dunn B E, Cohen H, Blaser M J. Clin Microbiol Rev. 1997;10:720–742. doi: 10.1128/cmr.10.4.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Danesh J. In: Cancer Surveys. Newton R, Beral V, Weiss RA, editors. Vol. 33. London: Imperial Cancer Research Fund; 1999. pp. 263–289. [Google Scholar]
- 12.Huang J-Q, Sridhar S, Chen Y, Hunt R. Gastroenterology. 1998;114:1169–1179. doi: 10.1016/s0016-5085(98)70422-6. [DOI] [PubMed] [Google Scholar]
- 13.Veldhuyzen van Zanten S J. Aliment Pharmacol Ther. 1995;9, Suppl. 2:41–44. [PubMed] [Google Scholar]
- 14.Malaty H M, Graham D Y, Wattigney W A, Srinivasan S R, Osato M, Berenson G S. Clin Infect Dis. 1999;28:279–282. doi: 10.1086/515105. [DOI] [PubMed] [Google Scholar]
- 15.Graham D Y. J Gastroenterol Hepatol. 1991;6:105–113. doi: 10.1111/j.1440-1746.1991.tb01448.x. [DOI] [PubMed] [Google Scholar]
- 16.Choe Y H, Lee J E, Kim S K. Acta Paediatr. 2000;89:154–157. doi: 10.1080/080352500750028753. [DOI] [PubMed] [Google Scholar]
- 17. Parkinson, A. J., Gold, B. D., Bulkow, L., Wainwright, R. B., Swaminathan, B., Khanna, B., Petersen, K. M. & Fitzgerald, M. A. (2000) Clin. Diagn. Lab. Immunol. 7885–7888. [DOI] [PMC free article] [PubMed]
- 18.Lacy B E, Rosemore J. J Nutr. 2001;131:2789S–2793S. doi: 10.1093/jn/131.10.2789S. [DOI] [PubMed] [Google Scholar]
- 19.Uemura N, Okamoto S, Yamamoto S, Matsumura N, Yamaguchi S, Yamakido M, Taniyama K, Sasaki N, Schlemper R J. N Engl J Med. 2001;345:784–789. doi: 10.1056/NEJMoa001999. [DOI] [PubMed] [Google Scholar]
- 20.Fox J G, Wang T C. N Engl J Med. 2001;345:829–832. doi: 10.1056/NEJM200109133451111. [DOI] [PubMed] [Google Scholar]
- 21.Ebert M P, Leodolter A, Malfertheiner P. Hepatogastroenterology. 2001;48:1569–1571. [PubMed] [Google Scholar]
- 22.Wong B C, Ching C K, Lam S K. Hong Kong Med J. 1999;5:175–179. [PubMed] [Google Scholar]
- 23.Miehlke S, Kirsch C, Dragosics B, Gschwantler M, Oberhuber G, Antos D, Dite P, Lauter J, Labenz J, Leodolter A, et al. World J Gastroenterol. 2001;7:243–247. doi: 10.3748/wjg.v7.i2.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shimizu N, Ikehara Y, Inada K, Nakanishi H, Tsukamoto T, Nozaki K, Kaminishi M, Kuramoto S, Sugiyama A, Katsuyama T, Tatematsu M. Cancer Res. 2000;60:1512–1514. [PubMed] [Google Scholar]
- 25.Genta R M, Graham D Y. Virchows Arch. 1994;425:339–347. doi: 10.1007/BF00189571. [DOI] [PubMed] [Google Scholar]
- 26.Graham D Y. Gastroenterology. 2000;118:S2–S8. doi: 10.1016/s0016-5085(00)70003-5. [DOI] [PubMed] [Google Scholar]
- 27.Knigge K L. Postgrad Med. 2001;110:71–77. doi: 10.3810/pgm.2001.09.1019. [DOI] [PubMed] [Google Scholar]
- 28.Trust T J, Alm R A, Pappo J. Eur J Surg Suppl. 2001;2001:82–88. doi: 10.1080/110241501317076317. [DOI] [PubMed] [Google Scholar]
- 29.Versalovic J, Fox J G. In: Manual of Clinical Microbiology. Murray P R, Baron E J, Pfaller M A, Tenover F C, Yolken R H, editors. Washington, DC: Am. Soc. Microbiol.; 1999. pp. 727–738. [Google Scholar]
- 30.Graham D Y. Gastroenterology. 1998;115:1272–1277. doi: 10.1016/s0016-5085(98)70100-3. [DOI] [PubMed] [Google Scholar]
- 31.Engstrand L, Graham D, Scheynius A, Genta R M, El-Zaatari F. Am J Clin Pathol. 1997;108:504–509. doi: 10.1093/ajcp/108.5.504. [DOI] [PubMed] [Google Scholar]
- 32.Lozniewski A, Muhale F, Hatier R, Marais A, Conroy M C, Edert D, Le Faou A, Weber M, Duprez A. Infect Immun. 1999;67:1798–1805. doi: 10.1128/iai.67.4.1798-1805.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fahey J W, Zhang Y, Talalay P. Proc Natl Acad Sci USA. 1997;94:10367–10372. doi: 10.1073/pnas.94.19.10367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fahey J W, Zalcmann A T, Talalay P. Phytochemistry. 2001;56:5–51. doi: 10.1016/s0031-9422(00)00316-2. , and correction (2002) 59, 237. [DOI] [PubMed] [Google Scholar]
- 35.Mulin W J, Sahasrabudhe H R. Nutr Rep Int. 1978;18:273–279. [Google Scholar]
- 36.Fenwick G R, Heaney P K, Mullin W J. CRC Crit Rev Food Sci Nutr. 1983;18:123–201. doi: 10.1080/10408398209527361. [DOI] [PubMed] [Google Scholar]
- 37.Halliwell B, Zhao K, Whiteman M. Free Radical Res. 2000;33:819–830. doi: 10.1080/10715760000301341. [DOI] [PubMed] [Google Scholar]
- 38.Shapiro T A, Fahey J W, Wade K L, Stephenson K K, Talalay P. Cancer Epidemiol Biomarkers Prev. 2001;10:501–508. [PubMed] [Google Scholar]
- 39.Procházka Z, Komersová I. Ceskoslov Farm. 1959;8:373–376. [Google Scholar]
- 40.Dornberger K, Böckel V, Heyer J, Schönfeld C, Tonew M, Tonew E. Pharmazie. 1975;30:792–796. [PubMed] [Google Scholar]
- 41.Zhang Y, Talalay P, Cho C G, Posner G H. Proc Natl Acad Sci USA. 1992;89:2399–2403. doi: 10.1073/pnas.89.6.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang Y, Kensler T W, Cho C G, Posner G H, Talalay P. Proc Natl Acad Sci USA. 1994;91:3147–3150. doi: 10.1073/pnas.91.8.3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Talalay P, Fahey J W. J Nutr. 2001;131:3027S–3033S. doi: 10.1093/jn/131.11.3027S. [DOI] [PubMed] [Google Scholar]
- 44.Fahey J W, Talalay P. Food Chem Toxicol. 1999;37:973–979. doi: 10.1016/s0278-6915(99)00082-4. [DOI] [PubMed] [Google Scholar]
- 45.Itoh K, Chiba T, Takahashi S, Ishii T, Igarashi K, Katoh Y, Oyake T, Hayashi N, Satoh K, Hatayama I, et al. Biochem Biophys Res Commun. 1997;236:313–322. doi: 10.1006/bbrc.1997.6943. [DOI] [PubMed] [Google Scholar]
- 46.Itoh K, Wakabayashi N, Katoh Y, Ishii T, Igarashi K, Engel J D, Yamamoto M. Genes Dev. 1999;13:76–86. doi: 10.1101/gad.13.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang Y, Wade K L, Prestera T, Talalay P. Anal Biochem. 1996;239:160–167. doi: 10.1006/abio.1996.0311. [DOI] [PubMed] [Google Scholar]
- 48.National Committee for Clinical Laboratory Standards. Approved Standard M7–A5: Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically. 5th Ed. Wayne, PA: National Committee for Clinical Laboratory Standards; 2000. [Google Scholar]
- 49.Hirschl A M, Apfalter P, Makristathis A, Rotter M L, Wimmer M. Antimicrob Agents Chemother. 2000;44:1977–1979. doi: 10.1128/aac.44.7.1977-1979.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kato S, Fujimura S, Udagawa H, Shimizu T, Maisawa S, Ozawa K, Iinuma K. J Clin Microbiol. 2002;40:649–653. doi: 10.1128/JCM.40.2.649-653.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Flamm R K, Beyer J, Tanaka S K, Clement J. J Antimicrob Chemother. 1996;38:719–725. doi: 10.1093/jac/38.4.719. [DOI] [PubMed] [Google Scholar]
- 52.Gustafsson I, Engstrand L, Cars O. Antimicrob Agents Chemother. 2001;45:353–355. doi: 10.1128/AAC.45.1.353-355.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Piccolomini R, Di Bonaventura G, Picciani C, Laterza F, Vecchiet J, Neri M. Antimicrob Agents Chemother. 2001;45:1568–1571. doi: 10.1128/AAC.45.5.1568-1571.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wilkinson S M, Uhl J R, Kline B C, Cockerill F R., 3rd J Clin Pathol. 1998;51:127–133. doi: 10.1136/jcp.51.2.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ye L, Dinkova-Kostova A T, Wade K L, Zhang Y, Shapiro T A, Talalay P. Clin Chim Acta. 2002;316:43–53. doi: 10.1016/s0009-8981(01)00727-6. [DOI] [PubMed] [Google Scholar]
- 56.Ramos-Gomez M, Kwak M-K, Dolan P M, Itoh K, Yamamoto M, Talalay P, Kensler T W. Proc Natl Acad Sci USA. 2001;98:3410–3415. doi: 10.1073/pnas.051618798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wattenberg L. J Natl Cancer Inst. 1977;58:395–398. doi: 10.1093/jnci/58.2.395. [DOI] [PubMed] [Google Scholar]
- 58.Mahady G B, Pendland S L. Am J Gastroenterol. 2000;95:1849. doi: 10.1111/j.1572-0241.2000.02146.x. [DOI] [PubMed] [Google Scholar]
- 59.Mahady G B, Matsuura H, Pendland S L. Am J Gastroenterol. 2001;96:3454–3455. doi: 10.1111/j.1572-0241.2001.05351.x. [DOI] [PubMed] [Google Scholar]
- 60.Ingolfsdottir K, Hjalmarsdottir M A, Sigurdsson A, Gudjonsdottir G A, Brynjolfsdottir A, Steingrimsson O. Antimicrob Agents Chemother. 1997;41:215–217. doi: 10.1128/aac.41.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mabe K, Yamada M, Oguni I, Takahashi T. Antimicrob Agents Chemother. 1999;43:1788–1791. doi: 10.1128/aac.43.7.1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Frieri G, De Petris G, Agio A, Santarelli D, Ligas E, Rosoni R, Caprilli R. Digestion. 1995;56:107–110. doi: 10.1159/000201229. [DOI] [PubMed] [Google Scholar]
- 63.Talley N J, Ormand J E, Frie C A, Zinsmeister A. Am J Gastroenterol. 1992;87:590–594. [PubMed] [Google Scholar]
- 64.Kelly S M, Crampton J R, Hunter J O. Dig Dis Sci. 1993;38:129–131. doi: 10.1007/BF01296784. [DOI] [PubMed] [Google Scholar]
- 65.Delaquis P J, Mazza G. Food Technol. 1995;49:73–84. [Google Scholar]
- 66.Lin C M, Kim J, Du W X, Wei C I. J Food Protect. 2000;63:25–30. doi: 10.4315/0362-028x-63.1.25. [DOI] [PubMed] [Google Scholar]
- 67.Evans D G, Evans D J, Graham D Y. Gastroenterology. 1992;102:1557–1567. doi: 10.1016/0016-5085(92)91714-f. [DOI] [PubMed] [Google Scholar]
- 68.Björkholm B, Zhukhovitsky V, Löfman C, Hultén K, Enroth H, Block M, Rigo R, Falk P, Engstrand L. Helicobacter. 2000;5:148–154. doi: 10.1046/j.1523-5378.2000.00023.x. [DOI] [PubMed] [Google Scholar]
- 69.Zhang Y. Carcinogenesis. 2000;21:1175–1182. [PubMed] [Google Scholar]
- 70.Zhang Y. Carcinogenesis. 2001;22:425–431. doi: 10.1093/carcin/22.3.425. [DOI] [PubMed] [Google Scholar]
- 71.Ye L, Zhang Y. Carcinogenesis. 2001;22:1987–1992. doi: 10.1093/carcin/22.12.1987. [DOI] [PubMed] [Google Scholar]
- 72.Gerhäuser C, You M, Liu J, Moriarty R M, Hawthorne M, Mehta R G, Moon R C, Pezzuto J M. Cancer Res. 1997;57:272–278. [PubMed] [Google Scholar]
- 73.Chung F-L, Conaway C C, Rao C V, Reddy B S. Carcinogenesis. 2000;21:2287–2291. doi: 10.1093/carcin/21.12.2287. [DOI] [PubMed] [Google Scholar]
- 74.Shapiro T A, Fahey J W, Wade K L, Stephenson K K, Talalay P. Cancer Epidemiol Biomarkers Prev. 1998;7:1091–1100. [PubMed] [Google Scholar]
- 75.Oijen A H, Verhulst M L, Roelofs H M, Peters W H, de Boer W A, Jansen J B. Jpn J Cancer Res. 2001;92:1329–1334. doi: 10.1111/j.1349-7006.2001.tb02157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]