Abstract
OBJECTIVE: This overview on glutamine and cancer discusses the importance of glutamine for tumor growth, summarizes the alterations in interorgan glutamine metabolism that develop in the tumor-bearing host, and reviews the potential benefits of glutamine nutrition in the patient with cancer. SUMMARY BACKGROUND DATA: Glutamine is the most abundant amino acid in the blood and tissues. It is essential for tumor growth and marked changes in organ glutamine metabolism are characteristic of the host with cancer. Because host glutamine depletion has adverse effects, it is important to study the regulation of glutamine metabolism in cancer and to evaluate the impact of glutamine nutrition in the tumor-bearing state. METHODS: Data from a variety of investigations on glutamine metabolism and nutrition related to the host with cancer were compiled and summarized. RESULTS: Numerous studies on glutamine metabolism in cancer indicate that many tumors are avid glutamine consumers in vivo and in vitro. As a consequence of progressive tumor growth, host glutamine depletion develops and becomes a hallmark. This glutamine depletion occurs in part because the tumor behaves as a "glutamine trap" but also because of cytokine-mediated alterations in glutamine metabolism in host tissues. Animal and human studies that have investigated the use of glutamine-supplemented nutrition in the host with cancer suggest that pharmacologic doses of dietary glutamine may be beneficial. CONCLUSIONS: Understanding the control of glutamine metabolism in the tumor-bearing host not only improves the knowledge of metabolic regulation in the patient with cancer but also will lead to improved nutritional support regimens targeted to benefit the host.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adibi S. A. Intravenous use of glutamine in peptide form: clinical applications of old and new observations. Metabolism. 1989 Aug;38(8 Suppl 1):89–92. doi: 10.1016/0026-0495(89)90149-2. [DOI] [PubMed] [Google Scholar]
- Ahluwalia G. S., Grem J. L., Hao Z., Cooney D. A. Metabolism and action of amino acid analog anti-cancer agents. Pharmacol Ther. 1990;46(2):243–271. doi: 10.1016/0163-7258(90)90094-i. [DOI] [PubMed] [Google Scholar]
- Alverdy J. A., Aoys E., Weiss-Carrington P., Burke D. A. The effect of glutamine-enriched TPN on gut immune cellularity. J Surg Res. 1992 Jan;52(1):34–38. doi: 10.1016/0022-4804(92)90275-5. [DOI] [PubMed] [Google Scholar]
- Alverdy J. C. Effects of glutamine-supplemented diets on immunology of the gut. JPEN J Parenter Enteral Nutr. 1990 Jul-Aug;14(4 Suppl):109S–113S. doi: 10.1177/014860719001400415. [DOI] [PubMed] [Google Scholar]
- Austgen T. R., Chakrabarti R., Chen M. K., Souba W. W. Adaptive regulation in skeletal muscle glutamine metabolism in endotoxin-treated rats. J Trauma. 1992 May;32(5):600–607. doi: 10.1097/00005373-199205000-00011. [DOI] [PubMed] [Google Scholar]
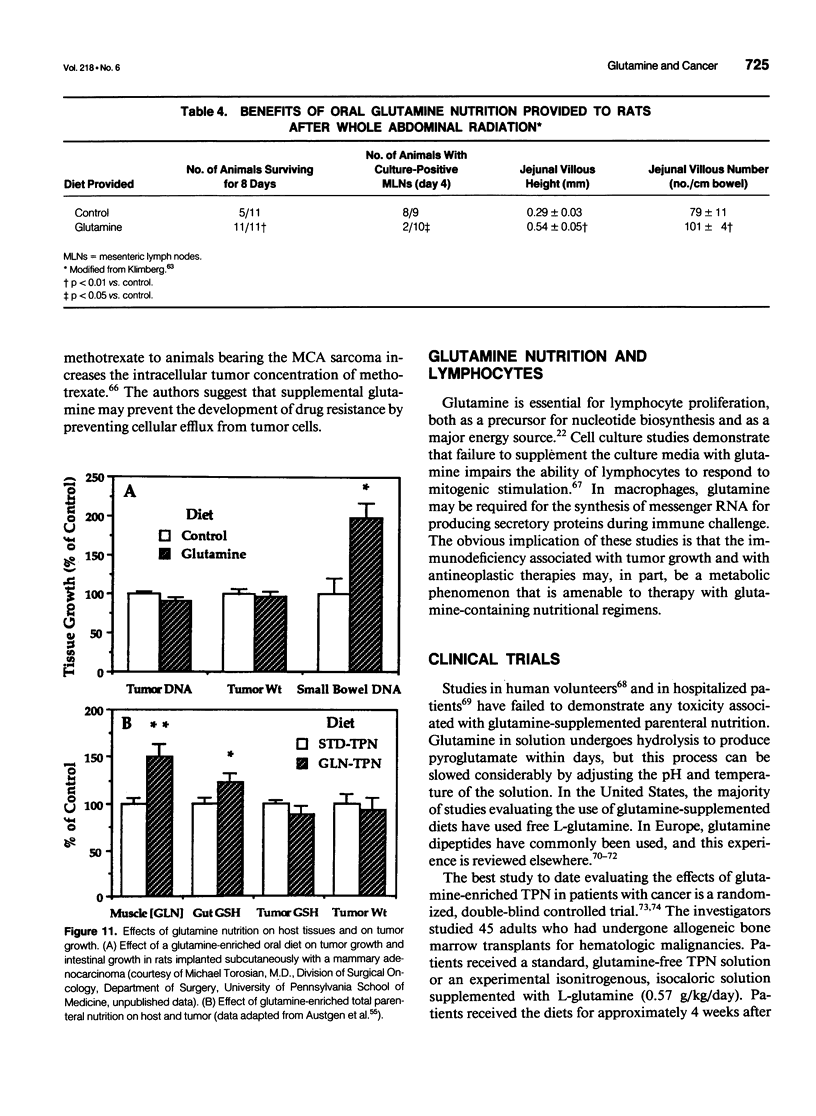
- Austgen T. R., Dudrick P. S., Sitren H., Bland K. I., Copeland E., Souba W. W. The effects of glutamine-enriched total parenteral nutrition on tumor growth and host tissues. Ann Surg. 1992 Feb;215(2):107–113. doi: 10.1097/00000658-199202000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baskerville A., Hambleton P., Benbough J. E. Pathological features of glutaminase toxicity. Br J Exp Pathol. 1980 Apr;61(2):132–138. [PMC free article] [PubMed] [Google Scholar]
- Bennegård K., Lindmark L., Edén E., Svaninger G., Lundholm K. Flux of amino acids across the leg in weight-losing cancer patients. Cancer Res. 1984 Jan;44(1):386–393. [PubMed] [Google Scholar]
- Bergström J., Fürst P., Norée L. O., Vinnars E. Intracellular free amino acid concentration in human muscle tissue. J Appl Physiol. 1974 Jun;36(6):693–697. doi: 10.1152/jappl.1974.36.6.693. [DOI] [PubMed] [Google Scholar]
- Boerner P., Saier M. H., Jr Adaptive regulatory control of System A transport activity in a kidney epithelial cell line (MDCK) and in a transformed variant (MDCK-T1). J Cell Physiol. 1985 Feb;122(2):308–315. doi: 10.1002/jcp.1041220221. [DOI] [PubMed] [Google Scholar]
- Brand K., Fekl W., von Hintzenstern J., Langer K., Luppa P., Schoerner C. Metabolism of glutamine in lymphocytes. Metabolism. 1989 Aug;38(8 Suppl 1):29–33. doi: 10.1016/0026-0495(89)90136-4. [DOI] [PubMed] [Google Scholar]
- Burke D. J., Alverdy J. C., Aoys E., Moss G. S. Glutamine-supplemented total parenteral nutrition improves gut immune function. Arch Surg. 1989 Dec;124(12):1396–1399. doi: 10.1001/archsurg.1989.01410120042009. [DOI] [PubMed] [Google Scholar]
- Chance W. T., Cao L. Q., Fischer J. E. Response of tumor and host to hyperalimentation and antiglutamine treatments. JPEN J Parenter Enteral Nutr. 1990 Mar-Apr;14(2):122–128. doi: 10.1177/0148607190014002122. [DOI] [PubMed] [Google Scholar]
- Chance W. T., Cao L., Fischer J. E. Insulin and acivicin improve host nutrition and prevent tumor growth during total parenteral nutrition. Ann Surg. 1988 Oct;208(4):524–531. doi: 10.1097/00000658-198810000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
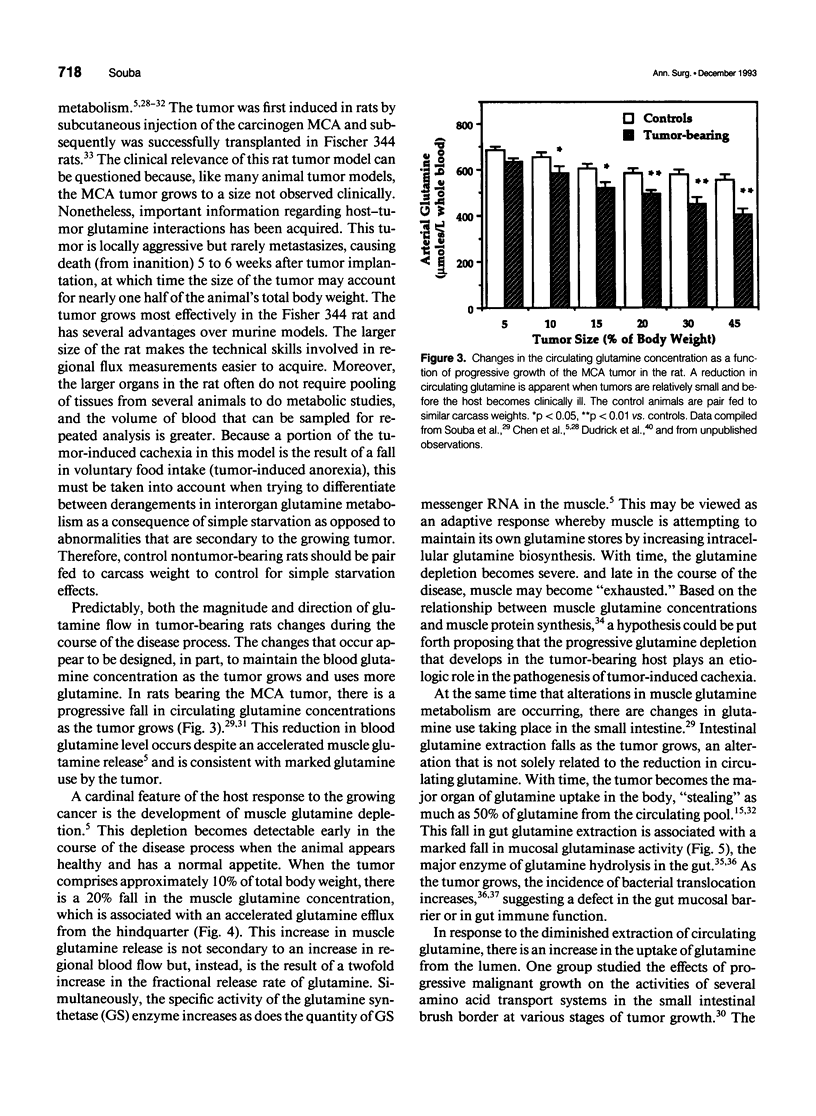
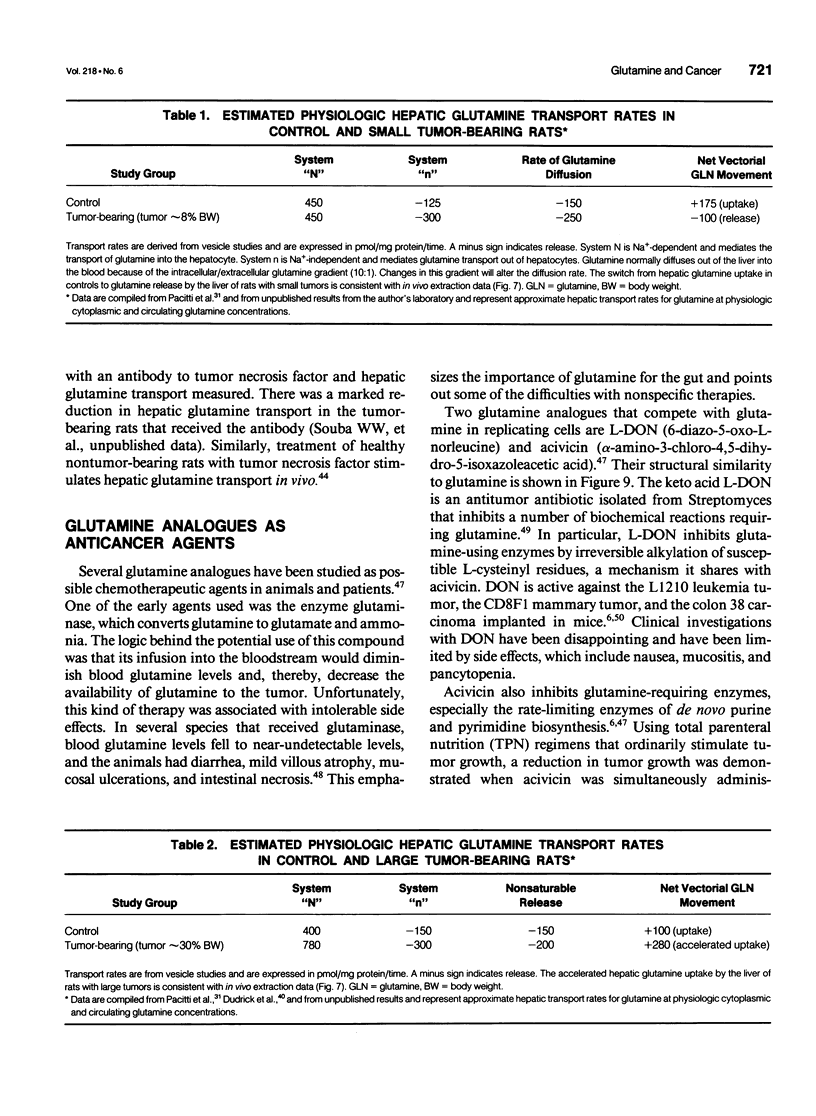
- Chen M. K., Espat N. J., Bland K. I., Copeland E. M., 3rd, Souba W. W. Influence of progressive tumor growth on glutamine metabolism in skeletal muscle and kidney. Ann Surg. 1993 Jun;217(6):655–667. doi: 10.1097/00000658-199306000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M. K., Salloum R. M., Austgen T. R., Bland J. B., Bland K. I., Copeland E. M., 3rd, Souba W. W. Tumor regulation of hepatic glutamine metabolism. JPEN J Parenter Enteral Nutr. 1991 Mar-Apr;15(2):159–164. doi: 10.1177/0148607191015002159. [DOI] [PubMed] [Google Scholar]
- Christensen H. N. Role of amino acid transport and countertransport in nutrition and metabolism. Physiol Rev. 1990 Jan;70(1):43–77. doi: 10.1152/physrev.1990.70.1.43. [DOI] [PubMed] [Google Scholar]
- Dudrick P. S., Inoue Y., Espat N. J., Souba W. W. Na(+)-dependent glutamine transport in the liver of tumour-bearing rats. Surg Oncol. 1993;2(3):205–215. doi: 10.1016/0960-7404(93)90008-m. [DOI] [PubMed] [Google Scholar]
- EAGLE H. Nutrition needs of mammalian cells in tissue culture. Science. 1955 Sep 16;122(3168):501–514. doi: 10.1126/science.122.3168.501. [DOI] [PubMed] [Google Scholar]
- Fischer J. E., Chance W. T. Total parenteral nutrition, glutamine, and tumor growth. JPEN J Parenter Enteral Nutr. 1990 Jul-Aug;14(4 Suppl):86S–89S. doi: 10.1177/0148607190014004101. [DOI] [PubMed] [Google Scholar]
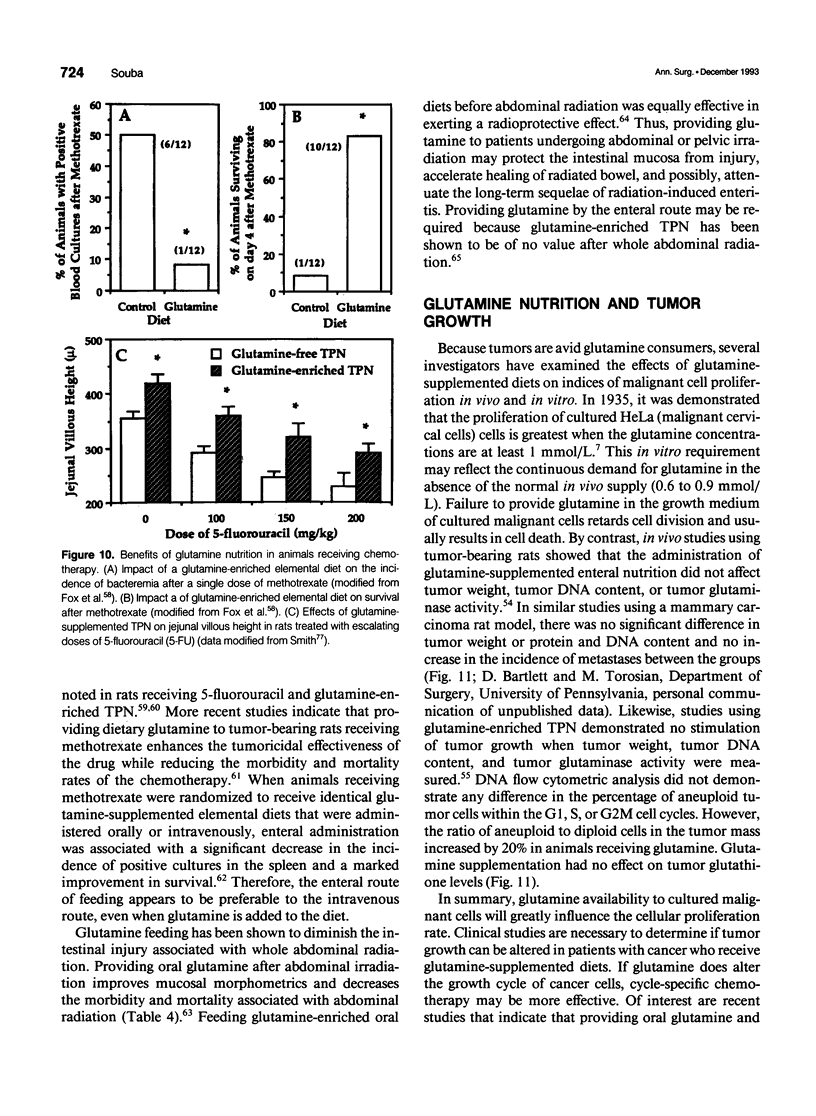
- Fox A. D., Kripke S. A., De Paula J., Berman J. M., Settle R. G., Rombeau J. L. Effect of a glutamine-supplemented enteral diet on methotrexate-induced enterocolitis. JPEN J Parenter Enteral Nutr. 1988 Jul-Aug;12(4):325–331. doi: 10.1177/0148607188012004325. [DOI] [PubMed] [Google Scholar]
- Fürst P., Albers S., Stehle P. Glutamine-containing dipeptides in parenteral nutrition. JPEN J Parenter Enteral Nutr. 1990 Jul-Aug;14(4 Suppl):118S–124S. doi: 10.1177/014860719001400417. [DOI] [PubMed] [Google Scholar]
- Grant J. P., Wells S. A., Jr Tumor resistance in rats immunized to fetal tissues. J Surg Res. 1974 May;16(5):533–540. doi: 10.1016/0022-4804(74)90080-8. [DOI] [PubMed] [Google Scholar]
- Hammarqvist F., Wernerman J., Ali R., von der Decken A., Vinnars E. Addition of glutamine to total parenteral nutrition after elective abdominal surgery spares free glutamine in muscle, counteracts the fall in muscle protein synthesis, and improves nitrogen balance. Ann Surg. 1989 Apr;209(4):455–461. doi: 10.1097/00000658-198904000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinshaw D. B., Burger J. M. Protective effect of glutamine on endothelial cell ATP in oxidant injury. J Surg Res. 1990 Sep;49(3):222–227. doi: 10.1016/0022-4804(90)90123-j. [DOI] [PubMed] [Google Scholar]
- Kisner D. L., Catane R., Muggia F. M. The rediscovery of DON (6-diazo-5-oxo-L-norleucine). Recent Results Cancer Res. 1980;74:258–263. doi: 10.1007/978-3-642-81488-4_30. [DOI] [PubMed] [Google Scholar]
- Klimberg V. S., Nwokedi E., Hutchins L. F., Pappas A. A., Lang N. P., Broadwater J. R., Read R. C., Westbrook K. C. Glutamine facilitates chemotherapy while reducing toxicity. JPEN J Parenter Enteral Nutr. 1992 Nov-Dec;16(6 Suppl):83S–87S. doi: 10.1177/014860719201600609. [DOI] [PubMed] [Google Scholar]
- Klimberg V. S., Pappas A. A., Nwokedi E., Jensen J. C., Broadwater J. R., Lang N. P., Westbrook K. C. Effect of supplemental dietary glutamine on methotrexate concentrations in tumors. Arch Surg. 1992 Nov;127(11):1317–1320. doi: 10.1001/archsurg.1992.01420110063013. [DOI] [PubMed] [Google Scholar]
- Klimberg V. S., Salloum R. M., Kasper M., Plumley D. A., Dolson D. J., Hautamaki R. D., Mendenhall W. R., Bova F. C., Bland K. I., Copeland E. M., 3rd Oral glutamine accelerates healing of the small intestine and improves outcome after whole abdominal radiation. Arch Surg. 1990 Aug;125(8):1040–1045. doi: 10.1001/archsurg.1990.01410200104017. [DOI] [PubMed] [Google Scholar]
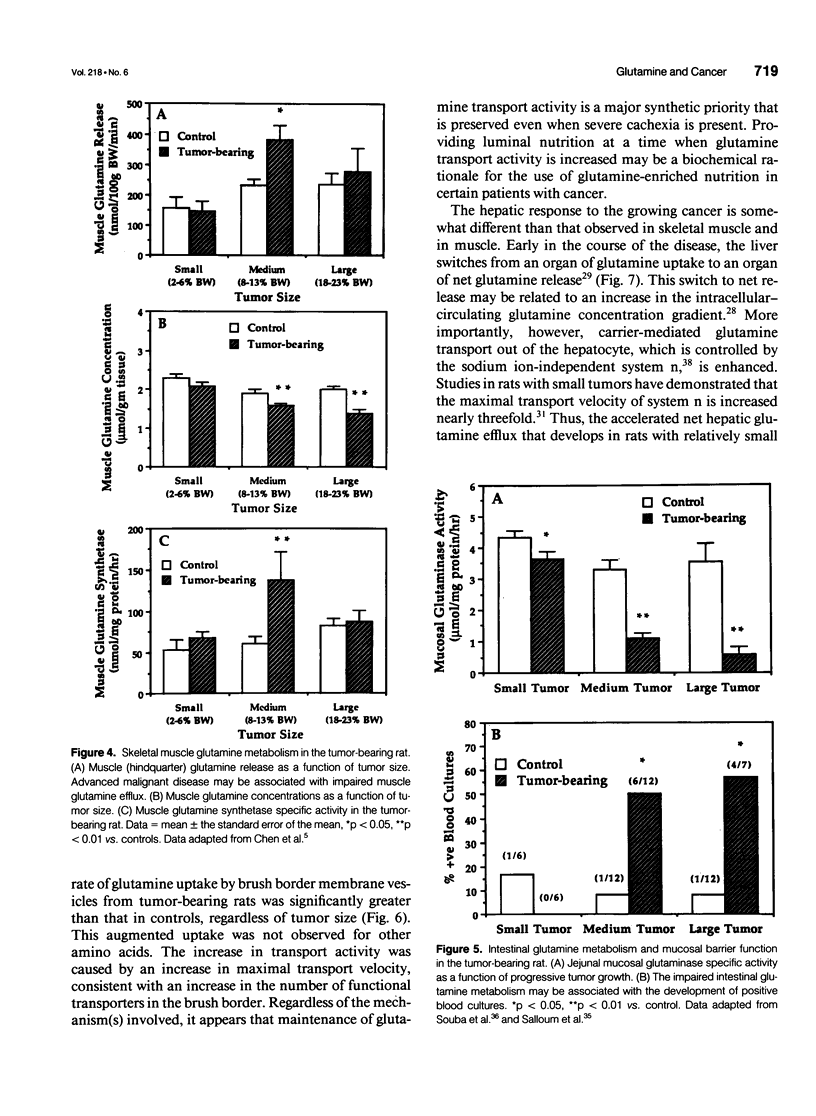
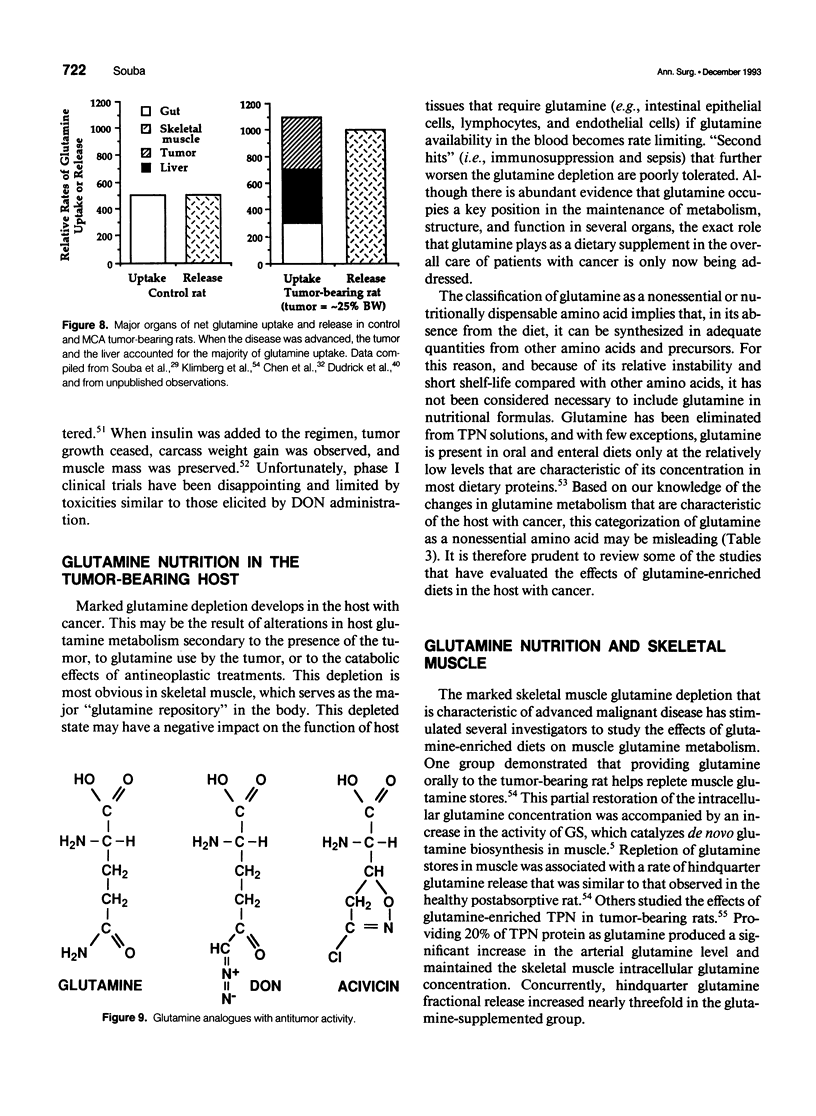
- Klimberg V. S., Souba W. W., Salloum R. M., Plumley D. A., Cohen F. S., Dolson D. J., Bland K. I., Copeland E. M., 3rd Glutamine-enriched diets support muscle glutamine metabolism without stimulating tumor growth. J Surg Res. 1990 Apr;48(4):319–323. doi: 10.1016/0022-4804(90)90066-b. [DOI] [PubMed] [Google Scholar]
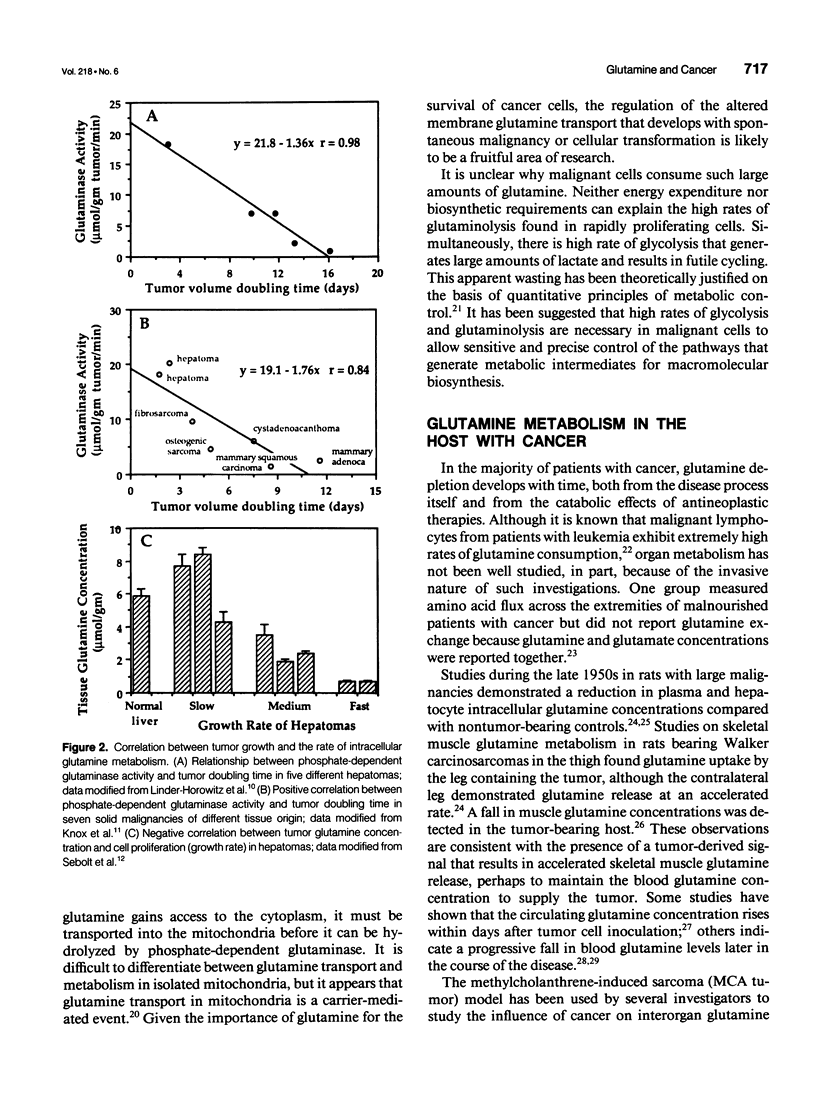
- Knox W. E., Horowitz M. L., Friedell G. H. The proportionality of glutaminase content to growth rate and morphology of rat neoplasms. Cancer Res. 1969 Mar;29(3):669–680. [PubMed] [Google Scholar]
- Kovacević Z., Morris H. P. The role of glutamine in the oxidative metabolism of malignant cells. Cancer Res. 1972 Feb;32(2):326–333. [PubMed] [Google Scholar]
- Lacey J. M., Wilmore D. W. Is glutamine a conditionally essential amino acid? Nutr Rev. 1990 Aug;48(8):297–309. doi: 10.1111/j.1753-4887.1990.tb02967.x. [DOI] [PubMed] [Google Scholar]
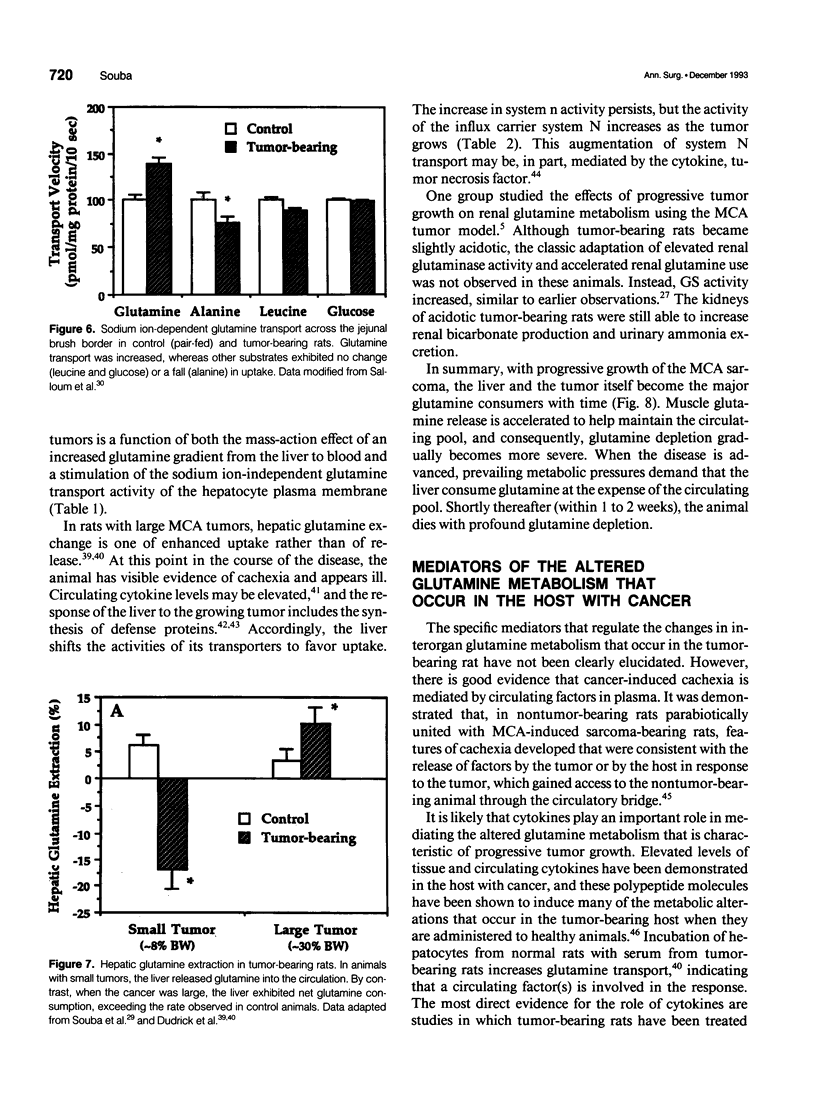
- Langstein H. N., Norton J. A. Mechanisms of cancer cachexia. Hematol Oncol Clin North Am. 1991 Feb;5(1):103–123. [PubMed] [Google Scholar]
- Linder-Horowitz M., Knox W. E., Morris H. P. Glutaminase activities and growth rates of rat hepatomas. Cancer Res. 1969 Jun;29(6):1195–1199. [PubMed] [Google Scholar]
- Medina M. A., Sánchez-Jiménez F., Márquez J., Rodríguez Quesada A., Núez de Castro I. Relevance of glutamine metabolism to tumor cell growth. Mol Cell Biochem. 1992 Jul 6;113(1):1–15. doi: 10.1007/BF00230880. [DOI] [PubMed] [Google Scholar]
- Norton J. A., Moley J. F., Green M. V., Carson R. E., Morrison S. D. Parabiotic transfer of cancer anorexia/cachexia in male rats. Cancer Res. 1985 Nov;45(11 Pt 1):5547–5552. [PubMed] [Google Scholar]
- Ovejera A. A., Houchens D. P., Catane R., Sheridan M. A., Muggia F. M. Efficacy of 6-diazo-5-oxo-L-norleucine and N-[N-gamma-glutamyl-6-diazo-5-oxo-norleucinyl]-6-diazo-5-oxo-norleucine against experimental tumors in conventional and nude mice. Cancer Res. 1979 Aug;39(8):3220–3224. [PubMed] [Google Scholar]
- Pacitti A. J., Chen M. K., Bland K. I., Copeland E. M., Souba W. W. Mechanisms of accelerated hepatic glutamine efflux in the tumour-bearing rat. Surg Oncol. 1992 Apr;1(2):173–182. doi: 10.1016/0960-7404(92)90031-f. [DOI] [PubMed] [Google Scholar]
- Pacitti A. J., Inoue Y., Souba W. W. Tumor necrosis factor stimulates amino acid transport in plasma membrane vesicles from rat liver. J Clin Invest. 1993 Feb;91(2):474–483. doi: 10.1172/JCI116225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penn R. L., Maca R. D., Berg R. D. Increased translocation of bacteria from the gastrointestinal tracts of tumor-bearing mice. Infect Immun. 1985 Mar;47(3):793–798. doi: 10.1128/iai.47.3.793-798.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quesada A. R., Medina M. A., Márquez J., Sánchez-Jiménez F. M., Núez de Castro I. Contribution by host tissues to circulating glutamine in mice inoculated with Ehrlich ascites tumor cells. Cancer Res. 1988 Mar 15;48(6):1551–1553. [PubMed] [Google Scholar]
- Reitzer L. J., Wice B. M., Kennell D. Evidence that glutamine, not sugar, is the major energy source for cultured HeLa cells. J Biol Chem. 1979 Apr 25;254(8):2669–2676. [PubMed] [Google Scholar]
- Rennie M. J., MacLennan P. A., Hundal H. S., Weryk B., Smith K., Taylor P. M., Egan C., Watt P. W. Skeletal muscle glutamine transport, intramuscular glutamine concentration, and muscle-protein turnover. Metabolism. 1989 Aug;38(8 Suppl 1):47–51. doi: 10.1016/0026-0495(89)90140-6. [DOI] [PubMed] [Google Scholar]
- Rivera S., Azcón-Bieto J., López-Soriano F. J., Miralpeix M., Argilés J. M. Amino acid metabolism in tumour-bearing mice. Biochem J. 1988 Jan 15;249(2):443–449. doi: 10.1042/bj2490443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salloum R. M., Copeland E. M., 3rd, Bland K. I., Souba W. W. Selective stimulation of brush border glutamine transport in the tumor-bearing rat. J Surg Res. 1991 Apr;50(4):391–397. doi: 10.1016/0022-4804(91)90208-4. [DOI] [PubMed] [Google Scholar]
- Sastrasinh S., Sastrasinh M. Glutamine transport in submitochondrial particles. Am J Physiol. 1989 Dec;257(6 Pt 2):F1050–F1058. doi: 10.1152/ajprenal.1989.257.6.F1050. [DOI] [PubMed] [Google Scholar]
- Sauer L. A., Dauchy R. T. Ketone body, glucose, lactic acid, and amino acid utilization by tumors in vivo in fasted rats. Cancer Res. 1983 Aug;43(8):3497–3503. [PubMed] [Google Scholar]
- Sauer L. A., Nagel W. O., Dauchy R. T., Miceli L. A., Austin J. E. Stimulation of tumor growth in adult rats in vivo during an acute fast. Cancer Res. 1986 Jul;46(7):3469–3475. [PubMed] [Google Scholar]
- Scheltinga M. R., Young L. S., Benfell K., Bye R. L., Ziegler T. R., Santos A. A., Antin J. H., Schloerb P. R., Wilmore D. W. Glutamine-enriched intravenous feedings attenuate extracellular fluid expansion after a standard stress. Ann Surg. 1991 Oct;214(4):385–395. doi: 10.1097/00000658-199110000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott T. E., Moellman J. R. Intravenous glutamine fails to improve gut morphology after radiation injury. JPEN J Parenter Enteral Nutr. 1992 Sep-Oct;16(5):440–444. doi: 10.1177/0148607192016005440. [DOI] [PubMed] [Google Scholar]
- Sebolt J. S., Weber G. Negative correlation of L-glutamine concentration with proliferation rate in rat hepatomas. Life Sci. 1984 Jan 16;34(3):301–306. doi: 10.1016/0024-3205(84)90603-9. [DOI] [PubMed] [Google Scholar]
- Smith R. J. Glutamine metabolism and its physiologic importance. JPEN J Parenter Enteral Nutr. 1990 Jul-Aug;14(4 Suppl):40S–44S. doi: 10.1177/014860719001400402. [DOI] [PubMed] [Google Scholar]
- Souba W. W. Interorgan ammonia metabolism in health and disease: a surgeon's view. JPEN J Parenter Enteral Nutr. 1987 Nov-Dec;11(6):569–579. doi: 10.1177/0148607187011006569. [DOI] [PubMed] [Google Scholar]
- Souba W. W., Pacitti A. J. How amino acids get into cells: mechanisms, models, menus, and mediators. JPEN J Parenter Enteral Nutr. 1992 Nov-Dec;16(6):569–578. doi: 10.1177/0148607192016006569. [DOI] [PubMed] [Google Scholar]
- Souba W. W., Strebel F. R., Bull J. M., Copeland E. M., Teagtmeyer H., Cleary K. Interorgan glutamine metabolism in the tumor-bearing rat. J Surg Res. 1988 Jun;44(6):720–726. doi: 10.1016/0022-4804(88)90106-0. [DOI] [PubMed] [Google Scholar]
- Stovroff M. C., Fraker D. L., Norton J. A. Cachectin activity in the serum of cachectic, tumor-bearing rats. Arch Surg. 1989 Jan;124(1):94–99. doi: 10.1001/archsurg.1989.01410010104021. [DOI] [PubMed] [Google Scholar]
- Taudou G., Wiart J., Panijel J. Influence of amino acid deficiency and tRNA aminoacylation on DNA synthesis and DNA polymerase activity during the secondary immune response in vitro. Mol Immunol. 1983 Mar;20(3):255–261. doi: 10.1016/0161-5890(83)90064-0. [DOI] [PubMed] [Google Scholar]
- WU C., BAUER J. M. A study of free amino acids and of glutamine synthesis in tumor-bearing rats. Cancer Res. 1960 Jul;20:848–857. [PubMed] [Google Scholar]
- Warren R. S., Jeevanandam M., Brennan M. F. Comparison of hepatic protein synthesis in vivo versus in vitro in the tumor-bearing rat. J Surg Res. 1987 Jan;42(1):43–50. doi: 10.1016/0022-4804(87)90063-1. [DOI] [PubMed] [Google Scholar]
- Warren R. S., Jeevanandam M., Brennan M. F. Protein synthesis in the tumor-influenced hepatocyte. Surgery. 1985 Aug;98(2):275–282. [PubMed] [Google Scholar]
- Yamamoto H., Aikawa T., Matsuaka H., Ishikawa E. Relative uptake of plasma amino acids by fetal and tumor tissues. Metabolism. 1974 Nov;23(11):1017–1022. doi: 10.1016/0026-0495(74)90068-7. [DOI] [PubMed] [Google Scholar]
- Ziegler T. R., Benfell K., Smith R. J., Young L. S., Brown E., Ferrari-Baliviera E., Lowe D. K., Wilmore D. W. Safety and metabolic effects of L-glutamine administration in humans. JPEN J Parenter Enteral Nutr. 1990 Jul-Aug;14(4 Suppl):137S–146S. doi: 10.1177/0148607190014004201. [DOI] [PubMed] [Google Scholar]
- Ziegler T. R., Young L. S., Benfell K., Scheltinga M., Hortos K., Bye R., Morrow F. D., Jacobs D. O., Smith R. J., Antin J. H. Clinical and metabolic efficacy of glutamine-supplemented parenteral nutrition after bone marrow transplantation. A randomized, double-blind, controlled study. Ann Intern Med. 1992 May 15;116(10):821–828. doi: 10.7326/0003-4819-116-10-821. [DOI] [PubMed] [Google Scholar]



