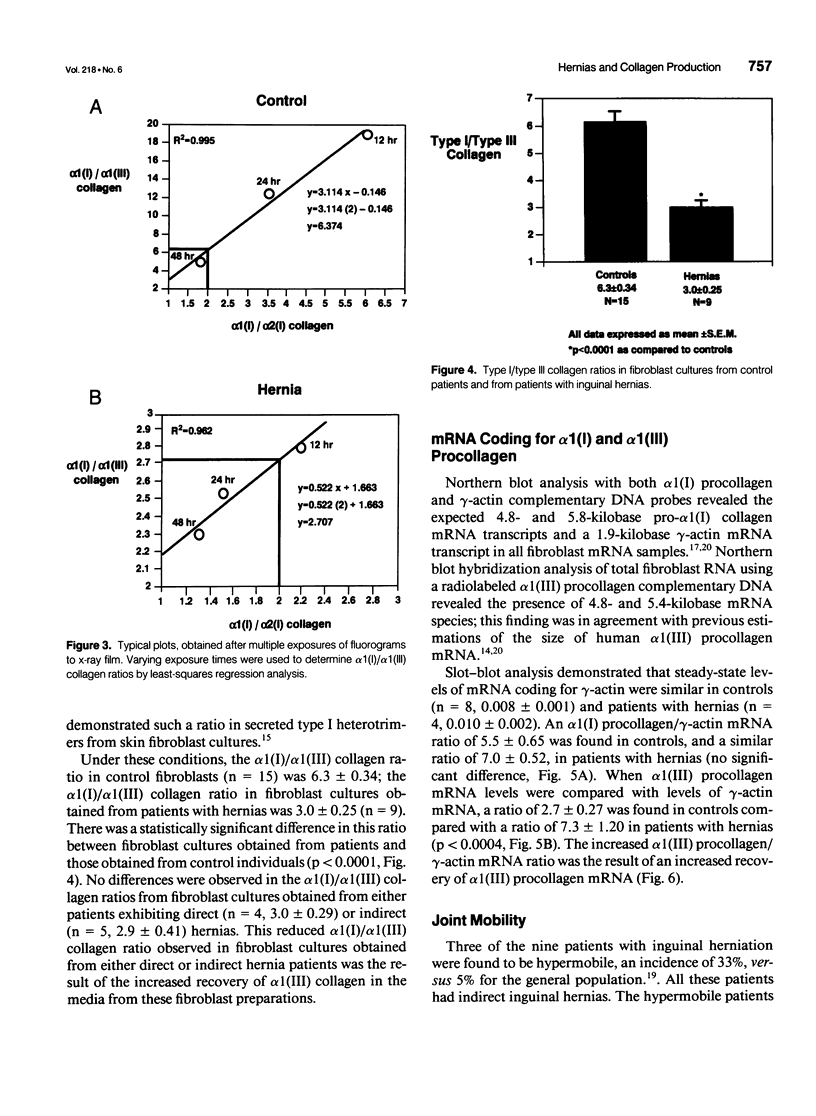
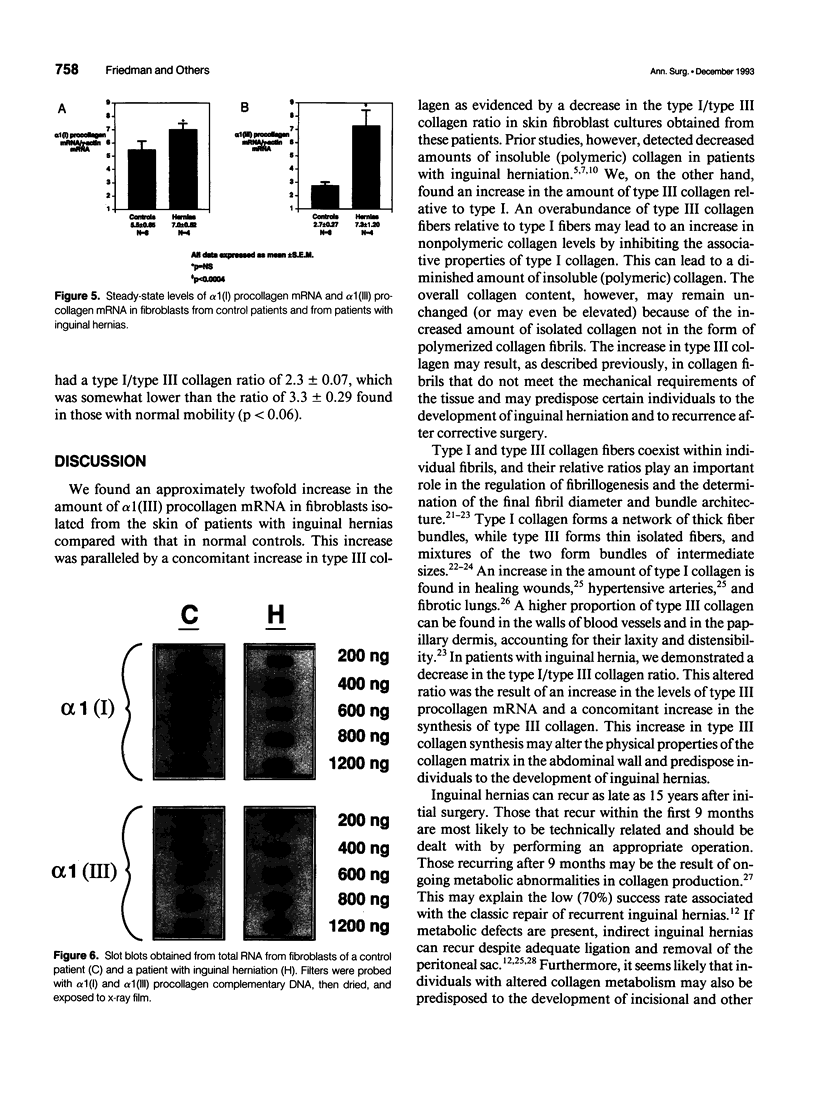
Abstract
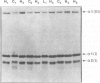
OBJECTIVE: The aim of this study was to determine if alterations in fibrillar collagen synthesis were associated with the development of inguinal hernias. SUMMARY BACKGROUND DATA: Previous work has suggested that alterations in connective tissue accumulation may play a functional role in the development of inguinal hernias. In particular, several investigators have suggested that alterations in collagen synthesis, causally related to connective disorders such as osteogenesis imperfecta, may also be responsible for the inguinal herniation that is markedly increased in such patients. This study was undertaken therefore to study collagen synthesis in patients with inguinal hernia in the absence of any other connective tissue disease. METHODS: Skin fibroblasts from 9 patients with hernias and 15 control individuals were radiolabeled with 3H-proline. Trypsin-chymotrypsin-resistant type I and III collagens were isolated and analyzed by sodium dodecyl sulfate polyacrylamide gel electrophoresis, and recovery was quantified by laser densitometry. Steady-state levels of alpha 1(I) and alpha 1(III) procollagen mRNAs were also determined by northern and slot-blot hybridization analysis. RESULTS: The alpha 1(I)/alpha 1(III) collagen ratios were shown to be 6.3 +/- 0.34 in fibroblasts from control individuals and 3.0 +/- 0.25 in fibroblasts from patients with inguinal hernias. This statistically significant difference (p < 0.0001) was caused by an increase in the secretion of alpha 1(III) procollagen from the fibroblasts of patients with hernias. A concomitant increase in the steady-state levels of alpha 1(III) procollagen mRNA was observed in total RNA isolated from the patients' fibroblasts. CONCLUSIONS: A constitutive and systemic increase in type III collagen synthesis may result in reduced collagen fibril assembly in the abdominal wall, eventually leading to the development of herniation. Although it is not yet clear what genetic factors are responsible for the elevation in type III collagen synthesis in patients with hernias, this study represents the first attempt to define individuals with an abnormality in collagen production that may be specifically related to herniation. A clearer understanding of the possible genetic factors that influence the pathophysiology of this disease will be important to improve the treatment of patients in whom inguinal hernias develop.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Conner W. T., Peacock E. E., Jr The etiology of inguinal hernia. Surg Forum. 1971;22:69–71. [PubMed] [Google Scholar]
- Deak S. B., Nicholls A., Pope F. M., Prockop D. J. The molecular defect in a nonlethal variant of osteogenesis imperfecta. Synthesis of pro-alpha 2(I) chains which are not incorporated into trimers of type I procollagen. J Biol Chem. 1983 Dec 25;258(24):15192–15197. [PubMed] [Google Scholar]
- Deak S. B., Ricotta J. J., Mariani T. J., Deak S. T., Zatina M. A., Mackenzie J. W., Boyd C. D. Abnormalities in the biosynthesis of type III procollagen in cultured skin fibroblasts from two patients with multiple aneurysms. Matrix. 1992 Apr;12(2):92–100. doi: 10.1016/s0934-8832(11)80050-8. [DOI] [PubMed] [Google Scholar]
- Deak S. B., Scholz P. M., Amenta P. S., Constantinou C. D., Levi-Minzi S. A., Gonzalez-Lavin L., Mackenzie J. W. The substitution of arginine for glycine 85 of the alpha 1(I) procollagen chain results in mild osteogenesis imperfecta. The mutation provides direct evidence for three discrete domains of cooperative melting of intact type I collagen. J Biol Chem. 1991 Nov 15;266(32):21827–21832. [PubMed] [Google Scholar]
- Fleischmajer R., Perlish J. S., Burgeson R. E., Shaikh-Bahai F., Timpl R. Type I and type III collagen interactions during fibrillogenesis. Ann N Y Acad Sci. 1990;580:161–175. doi: 10.1111/j.1749-6632.1990.tb17927.x. [DOI] [PubMed] [Google Scholar]
- Glisin V., Crkvenjakov R., Byus C. Ribonucleic acid isolated by cesium chloride centrifugation. Biochemistry. 1974 Jun 4;13(12):2633–2637. doi: 10.1021/bi00709a025. [DOI] [PubMed] [Google Scholar]
- Henkel W., Glanville R. W. Covalent crosslinking between molecules of type I and type III collagen. The involvement of the N-terminal, nonhelical regions of the alpha 1 (I) and alpha 1 (III) chains in the formation of intermolecular crosslinks. Eur J Biochem. 1982 Feb;122(1):205–213. doi: 10.1111/j.1432-1033.1982.tb05868.x. [DOI] [PubMed] [Google Scholar]
- Jessee E. F., Owen D. S., Jr, Sagar K. B. The benign hypermobile joint syndrome. Arthritis Rheum. 1980 Sep;23(9):1053–1056. doi: 10.1002/art.1780230914. [DOI] [PubMed] [Google Scholar]
- Lapiere C. M., Nusgens B., Pierard G. E. Interaction between collagen type I and type III in conditioning bundles organization. Connect Tissue Res. 1977;5(1):21–29. doi: 10.3109/03008207709152608. [DOI] [PubMed] [Google Scholar]
- McCullagh K. A., Balian G. Collagen characterisation and cell transformation in human atherosclerosis. Nature. 1975 Nov 6;258(5530):73–75. doi: 10.1038/258073a0. [DOI] [PubMed] [Google Scholar]
- Peacock E. E., Jr, Madden J. W. Studies on the biology and treatment of recurrent inguinal hernia. II. Morphological changes. Ann Surg. 1974 May;179(5):567–571. doi: 10.1097/00000658-197405000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peacock E. E., Jr Subcutaneous extraperitoneal repair of ventral hernias: a biological basis for fascial transplantation. Ann Surg. 1975 May;181(5):722–727. doi: 10.1097/00000658-197505000-00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce R. A., Glaug M. R., Greco R. S., Mackenzie J. W., Boyd C. D., Deak S. B. Increased procollagen mRNA levels in carbon tetrachloride-induced liver fibrosis in rats. J Biol Chem. 1987 Feb 5;262(4):1652–1658. [PubMed] [Google Scholar]
- Postlethwait R. W. Causes of recurrence after inguinal herniorrhaphy. Surgery. 1971 May;69(5):772–775. [PubMed] [Google Scholar]
- Poth E. J. Inguinal hernia repair using the rectus-pyramidalis sheath. Am J Surg. 1971 Nov;122(5):699–702. doi: 10.1016/0002-9610(71)90304-7. [DOI] [PubMed] [Google Scholar]
- Read R. C. Attenuation of the rectus sheath in inguinal herniation. Am J Surg. 1970 Nov;120(5):610–614. doi: 10.1016/s0002-9610(70)80178-7. [DOI] [PubMed] [Google Scholar]
- Read R. C. Pre-extraperitoneal approach to inguinofemoral herniorrhaphy. Am J Surg. 1967 Nov;114(5):672–678. doi: 10.1016/0002-9610(67)90127-4. [DOI] [PubMed] [Google Scholar]
- Seyer J. M., Hutcheson E. T., Kang A. H. Collagen polymorphism in idiopathic chronic pulmonary fibrosis. J Clin Invest. 1976 Jun;57(6):1498–1507. doi: 10.1172/JCI108420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuttleworth C. A., Forrest L., Jackson D. S. Comparison of the cyanogen bromide peptides of insoluble guinea-pig skin and scar collagen. Biochim Biophys Acta. 1975 Jan 30;379(1):207–216. doi: 10.1016/0005-2795(75)90024-0. [DOI] [PubMed] [Google Scholar]
- Thieme E. T. Recurrent inguinal hernia. Arch Surg. 1971 Aug;103(2):238–241. doi: 10.1001/archsurg.1971.01350080154023. [DOI] [PubMed] [Google Scholar]
- Udén A., Lindhagen T. Inguinal hernia in patients with congenital dislocation of the hip. A sign of general connective tissue disorder. Acta Orthop Scand. 1988 Dec;59(6):667–668. doi: 10.3109/17453678809149421. [DOI] [PubMed] [Google Scholar]
- Uitto J., Perejda A. J., Abergel R. P., Chu M. L., Ramirez F. Altered steady-state ratio of type I/III procollagen mRNAs correlates with selectively increased type I procollagen biosynthesis in cultured keloid fibroblasts. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5935–5939. doi: 10.1073/pnas.82.17.5935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagh P. V., Leverich A. P., Sun C. N., White H. J., Read R. C. Direct inguinal herniation in men: a disease of collagen. J Surg Res. 1974 Dec;17(6):425–433. doi: 10.1016/0022-4804(74)90155-3. [DOI] [PubMed] [Google Scholar]
- Wagh P. V., Read R. C. Defective collagen synthesis in inguinal herniation. Am J Surg. 1972 Dec;124(6):819–822. doi: 10.1016/0002-9610(72)90148-1. [DOI] [PubMed] [Google Scholar]