Abstract
The physiologic actions of retinoic acids (RAs) are mediated through RA receptors (RARs) and retinoid X receptors (RXRs). The RARα gene has drawn particular attention because it is the common target in all chromosomal translocations in acute promyelocytic leukemia (APL), a unique model in cancer research that responds to the effect of RA. In the great majority of patients with APL, RARα is fused to the PML gene as a result of the t(15;17) translocation. Three distinct types of PML-RARα transcripts, long (L), short (S), and variant (V), were identified. The V-type is characterized by truncation of exon 6 of PML and in some cases by the insertion of a variable “spacer” sequence between the truncated PML and RARα mRNA fusion partners, although the precise mechanisms underlying formation of the V-type transcript remain unclear. To get further insights into the molecular basis of the t(15;17), we sequenced the entire genomic DNA region of RARα. Of note, all previously reported “spacer” sequences in V-type transcripts were found in intron 2 of the RARα gene and most of these sequences were flanked by gt splice donor sites. In most cases, these “cryptic” coding sequences maintained the ORF of the chimeric transcript. Interestingly, two cases with a relatively long spacer sequence showed APL cellular and clinical resistance to RA treatment. In these cases, the aberrant V-type PML-RARα protein displayed increased affinity to the nuclear corepressor protein SMRT, providing further evidence that RA exerts the therapeutic effect on APL through modulation of the RAR–corepressor interaction. Finally, among patients with the L- or S-type PML-RARα fusion transcript, some consensus motifs were identified at the hotspots of the chromosome 17q breakpoints within intron 2 of RARα, strengthening the importance of this intron in the molecular pathogenesis of APL.
Retinoic acids (RAs) play a wide variety of physiological roles during embryogenesis and adult life. The effects of RAs are mediated through two families of nuclear receptors, RA receptors (RARs) and retinoid X receptors (RXRs), each including three members, α, β, and γ. Whereas RXRs can form homodimers, RARs need to heterodimerize with RXRs to constitute a functional receptor. These receptors bind to DNA motifs known as RAREs (RA response elements), located in the regulatory sequences of RA target genes (1). The transregulatory properties of RAR/RXR are determined, in a ligand-dependent manner, by their interaction with two protein complexes, nuclear receptor corepressor (CoR) and coactivator (2). Each member of the RAR and RXR families is encoded by a unique gene, which may generate a number of isoforms with distinct N-terminal sequences through alternative use of 5′ exons (3). The combinatorial expression of both gene families, as well as their isoforms, varies across different tissue/cell types at distinct stages of development, contributing to the biological complexity of RA signaling (1–4).
Over the last decade, the biomedical importance of RARα has been highlighted by its involvement in the pathogenesis of acute promyelocytic leukemia (APL) (2, 5–8). The chromosome translocation t(15;17), a hallmark for APL (9), disrupts the PML gene on chromosome 15 and the RARα gene on chromosome 17 (10–12), resulting in chimeric PML-RARα and RARα-PML fusion genes. On the other hand, the ability of all-trans RA (ATRA) to induce neutrophilic differentiation of APL cells is often cited as a triumph of molecular understanding of leukemia (6). Extensive studies have shown that the breakpoints on chromosome 17 are consistently located in the second intron of the RARα gene, but on chromosome 15, there are different breakpoint cluster regions, namely bcr1, bcr2, and bcr3 located in intron 6, exon 6, and intron 3, respectively (13–21). As a result, three distinct types of PML-RARα transcripts can be generated in different patients. About 70% of patients with APL have bcr-1 or long (L)-type isoform, resulting from the fusion between PML exon 6 (P6) and RARα exon 3 (R3), and 20% of patients with APL have bcr-3 or short (S)-type isoform, resulting from fusion between PML exon 3 (P3) and R3. The third type, bcr-2 or variant (V)-type isoform, accounting for about 10% in APL, is formed through a fusion between the truncated P6(ΔP6) and the R3, frequently with insertion of a sequence into the ΔP6-R3 joining (spacer) (2). Formation of L- and S-type isoforms of fusion transcripts is easily understood, whereas the molecular mechanisms responsible for the V-type isoform remain obscure. Some groups have observed clinical and biological features related to these three PML-RARα isoforms. For example, cells harboring V-type transcripts were found to display decreased sensitivity to ATRA (21) and the incidence of V-type isoform is higher in the pediatric population than in adults (22–24). In addition, in a small subset of patients with APL with variant chromosomal translocations, t(11, 17)(q23;q21), t(5;17)(q35;q21), t(11;17)(q13;q21), and dup17q21–23, the RARα gene is the common target and is fused to corresponding partner genes PLZF, NPM, NuMA, and STAT5b. All these data emphasize the importance of the RARα gene in the pathogenesis of APL (2).
To get a better understanding of the structural organization of the RARα gene and to study the molecular mechanism of the genomic breakpoints and mRNA splicing of the PML-RARα gene, particularly the V-type isoform, we sequenced the full genomic RARα gene. We show that the use of cryptic coding sequences in RARα intron 2 is critical for maintaining the ORF of the fusion gene and that a subset of V-type isoforms mediates RA resistance because of its aberrant binding affinity to the CoR protein complex.
Materials and Methods
Shotgun Sequencing Strategy and Computer Analysis of Sequence Data.
BAC clone RP11–205M17 was kindly provided by J. Murray (University of Iowa). This BAC clone contains the full-length RARα gene (25). BAC DNA was sonicated and size-fractionated for shotgun sequencing as described (26). With sufficient coverage, sequences were assembled and finished with the help of the phred/phrap/consed package (http://www.phrap.org). Several algorithms and databases were used to analyze the genomic sequence of RARα. (i) Repetitive sequences were determined with the software repeatmasker (http://repeatmasker.genome.washington.edu/cgi-bin/RepeatMasker). (ii) GC content was sought with GRAIL 1.3, which was also used to identify the exons with GENESCAN, GENIE, GENID, FGENES (these five programs are from http://rgd.mcw.edu/METAGENE). (iii) cDNA was aligned with genomic sequence of RARα with SIM4 and blast to give a view of the actual splicing sites. (iv) For the analysis of regulatory sequences in the putative promoter region, nnpp (Promoter Prediction by Neural Network), promoter scan, spliceview(webgene), tess, and transfac were applied together to make a comprehensive prediction. (v) Sequences flanking breakpoints in intron 2 were analyzed with the online software meme.
Patient Data.
V-type isoform PML-RARα.
Published data from 18 patients with APL with V-type isoforms were collected (16, 24). In this work, reverse transcription (RT)-PCR and sequence analysis were carried out on three patients. Briefly, total RNA was extracted from the APL samples by the acid-guanidinium-phenol-chloroform method (27), whereas reverse transcription and subsequent PCR were carried out by using reported conditions (28). RT-PCR products were purified with the QIAquick Gel Extraction kit (Qiagen, Hilden, Germany), subcloned into pGEM-T-Easy vector (Promega), and then sequenced.
Protein Interaction Assay.
Plasmids.
The RARα and L-type PML-RARα (in pSG5 vector) expression vectors were as described (29). The V-type PML-RARα (cases F1 and G-S448) expression vectors were constructed by replacing the fusion region of L-type PML-RARα with the fusion region by using appropriate restriction endonucleases or fusion-PCR technology. A glutathione S-transferase (GST)-SMRT peptide (residues 983-1172) containing the receptor interaction domain was kindly provided by R. Evans (The Salk Institute). The protein secondary structure prediction of the spacer sequence of F1 case was analyzed with the online software phdsec (http://cubic.bioc.columbia.edu/predictprotein). F1-Δ1 and F1-Δ2 PML-RARα expression vectors were constructed, respectively, by deleting a short motif of 5 amino acids, or the total sequence, of the spacer of the F1 case (Fig. 2C).
Figure 2.
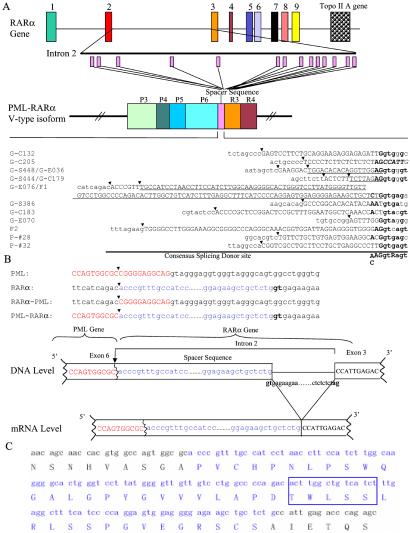
(A) Spacer sequences in PML-RARα V-type isoform from the intron 2 of the RARα gene. G-cases are from the study by Slack et al. (24); P-cases are from the study by Pandolfi et al. (16), and F-cases are from this work. Some spacers were used in more than one case, as underlined. Note the consensus splicing sites (bold) at the 3′ end (with uppercase letters) and downstream of the “cryptic” coding region (with lowercase letters). Black arrows indicate the deduced genomic breakpoints in the RARα gene. (B) Relationship between spacer insertion site in mRNA and genomic breakpoint (showed by black arrow) in RARα gene in the F1 case. (C) Nucleic acid and protein sequences of the fusion region of F1 PML-RARα. The spacer sequence is indicated in blue, containing a possible protein–protein interaction motif (5 amino acids in the box).
In vitro protein-binding assay.
The GST pull-down assay for interaction between different RARs and SMRT protein was performed as described (29).
Gel-shift DNA-binding assay.
The RARE probe DR5G was used in this experiment (29). The in vitro-translated proteins were preincubated for 15 min at room temperature in the following buffer: 20 mM Hepes, pH 7.4/50 mM KCl/1 mM 2-mercaptoethanol/10% glycerol/1 μg poly(dI-dC) (Amersham Pharmacia Biotech, Piscataway, NJ)/100 μg BSA. The 32P-labeled DR5G probe was then added, and the samples were incubated further at room temperature for 30 min and at 4°C for 30 min. Protein–DNA complexes were resolved on 6% polyacrylamide gels equilibrated in 0.5% TBE buffer (90 mM Tris/64.6 mM boric acid/2.5 mM EDTA, pH 8.3). Gels were dried, exposed, and analyzed by a Molecular Imager FX system (Bio-Rad).
Sequences Encompassing the Joining Between RARα and PML Genes in bcr-1 and bcr-2 Patients with APL.
Genomic sequences flanking the PML and RARα gene-joining sites were obtained from 16 described patients, 15 with bcr1 rearrangement and 1 with bcr3 rearrangement (16, 30–33). In this work, sequences from one patient presenting bcr-2 were also obtained by PCR of genomic DNA with appropriate primers.
Results
Features of the RARα Gene.
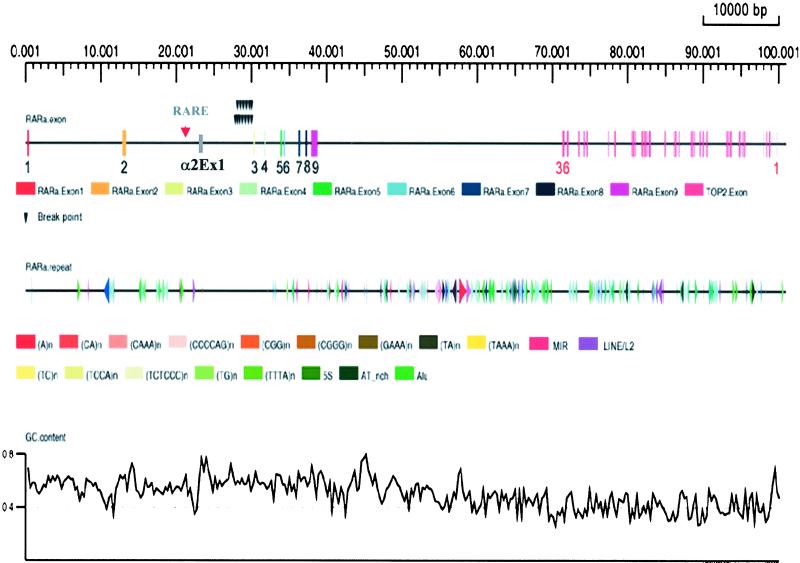
With the shotgun strategy, we obtained a high-quality complete genomic DNA sequence of the RARα gene in the BAC RP11-205M17 with a length of 138,142 bp. By repeatmasker analysis, several repeated elements in human DNA were detected. In total, there are 27 short interspersed elements (SINEs) (16 Alu, 3 MIR, 8 simple repeats) and 3 long interspersed elements (LINEs) over this region (Fig. 1). Considering the determined transcription start site (TSS) as the 5′end (34) and the AATAAA listed in the 3′ untranslated region (UTR) of several described RARα cDNA sequences as the 3′ end, the complete genomic DNA sequence of the RARα gene was 39,398 bp (GenBank accession no. AC090426). This sequence of RARα was subjected to comparison with published cDNA and expressed sequence tags (ESTs) available from dbEST, which confirmed the described exon-intron boundaries (25) and established the exact lengths of each exon and intron. Intron 2, which separates the “classical” exon 2 and exon 3, is 16,919 bp long, occupying about 43% of the RARα gene. Within this large intron, there is a stretch of sequence corresponding to the 5′ end (UTR and coding sequence) of the α2 isoform. Although this alternatively used exon was named as “exon 3” in an early report (3), recent literature prefers not to number this exon (25). To avoid confusion in nomenclature, we designated this exon as α2Ex1 in the present work (Fig. 1). Hence, the RARα gene should have two alternatively used promoters for the classical exon 1 and the α2Ex1, respectively. Examination of a 10-kb region upstream of the classical exon 1 revealed two CpG islands (nucleotides −463 to −1 and nucleotides −1600 to −1104), but no CCAAT or TATA boxes were observed. This region showed no typical RARE, although some other transcription factor binding sites have been found, such as PEBP2, a homolog of AML-1, known to play a role in granulocytic differentiation (35–37) and ELP, a transcriptional repressor (38, 39). The presence of a typical DR5 RARE motif at −49 bp with regard to the TSS of α2Ex1 is in agreement with the observation that the α2 isoform is induced by RAs and that there is a dramatic up-regulation of the α2 isoform during granulocytic differentiation.†† In addition, as a “by product” of this work, we obtained the entire genomic region of the topoisomerase IIA (Topo IIA) gene (accession no. AF071738), which is situated 32,533 bp downstream of the 3′ end of the RARα gene and composed of 35 exons and 34 introns (Fig. 1). These results thus extended the partial sequence data of Lang et al. (40).
Figure 1.
Bioinformatics analysis of the genomic sequences of the RARα and TopoII A genes. Exons are shown as boxes and black arrows indicate the breakpoints of the RARα gene in patients with PML-RARα L-type isoform. Note a typical RARE motif of DR5 (red arrow) is located upstream of the α2Ex1. The sizes of introns 1–8 of the RARα gene (not considering the α1Ex1) are 12,408, 16,919, 1,319, 1,984, 260, 1,794, 756, and 587 bp, respectively.
Mechanism Involved in V-Type PML-RARα Isoform Formation.
It was speculated that the sequence recognized as a spacer between PML and RARα in V-type fusion transcripts may have originated from intron 2 of the RARα gene (16, 17, 24). To address this issue, we analyzed all 18 cases with V-type transcripts in the literature (16, 17, 24) and 3 cases recently studied in our lab. Fourteen cases were selected for further study with spacer sequences at least 8 bp in length, because shorter spacer sequences could be found at more than one site in the large intron 2, making the analysis difficult. Some reported spacer sequences were different from our genomic RARα gene sequence by 1 or 2 bp, probably because of an error in published sequences or of the existence of single nucleotide polymorphisms (SNPs). Indeed, we found that all 14 spacer sequences of V-type PML-RARα transcripts were located in the second intron of the RARα gene (Fig. 2A). Of note, three of the intronic spacer sequences were used twice in distinct patient cells, suggesting a selective effect. As a result, in 13 of 14 patients, the ORFs were maintained although the 3′ moiety of P6 was lost at the mRNA level. Moreover, at the genomic sequence level, immediately downstream of the spacer, there were splice donor signals observing the “gt” rule in 13 of 14 cases (Fig. 2A). To confirm the use of these mechanisms in generating the fusion between the ΔP6 and the intact R3, we analyzed the genomic joining site in two cases, patients F1 and G-E076. As shown in Fig. 2B for the F1 case, in line with a previous report (16), the genomic sequence alternations were exactly the same as deduced from the spacer sequence at the mRNA level. The breakpoint in PML was located between nucleotides 248–249 of P6 and the breakpoint of RARα was situated between nucleotides 12581–12582 of intron 2, just upstream of a spacer sequence. The sequence of the reciprocal chromosome breakpoint in case G-E076 also perfectly matched the use of spacer sequence in ΔP6–R3 fusion in mRNA (data not shown). Interestingly, in case G-C205, the spacer sequence was a stretch of 19 bp immediately upstream of R3 (Fig. 2A). The most probable explanation for this situation is that RARα gene could be disrupted in a position upstream of this 19-bp sequence and then fused to a ΔP6. The distance between the ΔP6-derived sequence and the 5′ end of R3 was too close for a functional splicesome to be formed. Of note, the insertion of these 19 bp allowed a “read-through” of the ORF.
Although the ORF in most V-type PML-RARα transcripts was maintained, in one case (F2), the spacer sequence was not “in-frame,” which could result in expression of a 3′ truncated PML protein but not a functional PML-RARα protein. However, in this case, another fusion gene transcript, an S-type isoform, was detected by appropriate reverse transcription (RT)-PCR conditions. Sequence analysis of this RT-PCR product confirmed its origin as a P3–R3 fusion.
A Subset of V-Type PML-RARα with Aberrant Binding to SMRT Protein Correlates with APL Cellular and Clinical Resistance to ATRA.
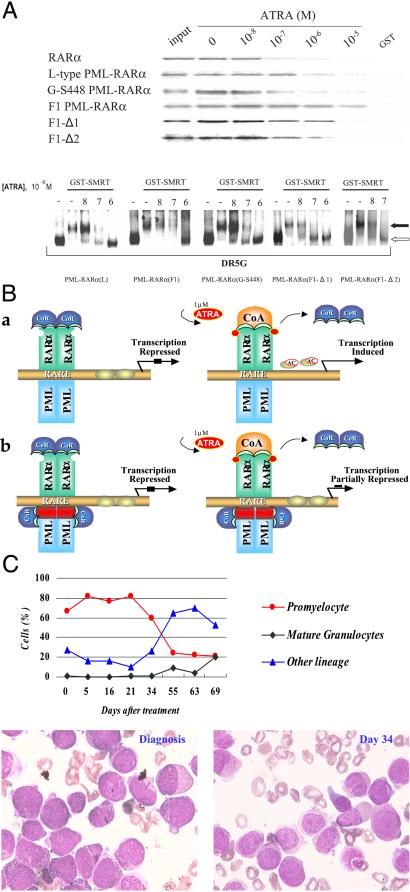
To investigate possible differences between the L- and V-types of PML-RARα in terms of RA sensitivity, we examined the stability of the binding of in vitro-translated L- and V-type PML-RARα proteins to GST-SMRT in a GST pull-down assay and gel-shift assay under different concentrations of ATRA (Fig. 3A). In the GST pull-down experiment, wild-type RARα and L-type PML-RARα began to dissociate from GST-SMRT at 10−7 to 10−6 M ATRA. However, the V-type fusion receptors from two patients showed distinct binding properties to SMRT. Whereas the V-type protein from case G-S448 showed ATRA sensitivity similar to that of the L-type one, the PML-RARα from the F1 case did not dissociate from GST-SMRT even at 10−5 M ATRA. Sequencing of the full-length cDNA ruled out a point mutation in the major functional domains of the protein in F1 (data not shown). That the aberrant binding to SMRT was mediated by the “spacer” from RARα intron 2 and not by the truncated PML exon 6 was confirmed by the fact that F1 protein with deletion of the spacer (F1-Δ2) restored the sensitivity to the effect of ATRA (Fig. 3A Upper). Moreover, when a 5-aa stretch of the spacer, predicted to be a protein–protein interaction motif, was deleted from F1 V-type protein (F1-Δ1; Fig. 2C), the SMRT binding ability of the fusion receptor was significantly reduced at 10−5 M ATRA (Fig. 3A Upper). The gel-shift assay, however, may be more sensitive to detect the dissociation of SMRT from PML-RARα and generated concordant data. The dissociation of SMRT from both L-type PML-RARα and G-S448 V-type fusion receptor homodimers could be observed at 10−7 M ATRA, whereas all F1-type PML-RARα remained in the complex with SMRT at the same concentration of ATRA, and a smear of bands suggestive of reduced SMRT binding was seen only at 10−6 M ATRA. However, the F1-Δ1 and F1-Δ2 (Fig. 3A Lower) PML-RARα proteins could dissociate from SMRT at 10−7 M ATRA.
Figure 3.
(A Upper) Ligand-dependent dissociation of SMRT from wild-type RARα and different PML-RARα proteins. (A Lower) Gel-shift analysis of the interaction between GST-SMRT and the L-type, F1, F1-Δ1, F1-Δ2, and G-S448 PML-RARα on DR5G and the effect of ATRA. The filled and open arrows indicate the bands of PML-RARα/SMRT complex and PML-RARα homodimer, respectively. (B) A molecular model of APL pathogenesis and differentiation therapy. Under physiologic concentrations of ATRA, the CoR complex still binds with typical (a) or V-type (b) PML-RARα, thereby repressing target gene transcription. When APL cells are treated with 10−6 M of ATRA, this repression can be relieved in typical PML-RARα (a) but not in F1 V-type, because of a second CoR binding site in the spacer (red). (C) Clinical response of the F1 case to ATRA. (Upper) The changes of cellular components in bone marrow (BM). (Lower Left and Right) BM picture at diagnosis and on day 34 of ATRA therapy, respectively.
Of note, the clinical data from patient F1 suggested resistance to the therapeutic effect of ATRA. As shown on Fig. 3C, the bone marrow promyelocytes remained at 20% after 72 days of standard-dose ATRA therapy. A remission was then achieved when chemotherapy was implemented, but the patient relapsed shortly thereafter. Importantly, the previously described G-E076 case had a very similar “spacer” sequence to the F1 case (Fig. 2A) and this patient also showed APL cellular and clinical resistance to ATRA (24). On the other hand, a number of cases with insertion of a short “spacer” responded well to ATRA (24). Hence, V-type PML-RARα patients seem to be a heterogeneous group with regard to the response to differentiation therapy, a small subset of which have a distinct phenotype compared with classical PML-RARα patients.
Analysis of the Breakpoints Clusters in the RARα Gene.
Previous studies showed that the chromosomal breakpoints of RARα were always located in intron 2. Now that the genomic sequence of this intron is available, we tried to address the mechanisms facilitating the translocation through analysis of breakpoint regions in 15 cases with bcr1 rearrangement and in 1 case with bcr3 rearrangement. Although the identification of the breakpoint region was limited because of probe selection, we still found two chromosomal breakage hot spots located near the 3′ end of intron 2 spanning a 258-bp sequence interval. The first hotspot included 2 breaks within 4 bp, whereas the second one contained 6 breaks within 58 bp. At the sequence level, precise reciprocal fusion without the deletion or insertion of nucleotides was found in all patients. To analyze the possible recognition mechanism of the breakpoints further, meme software was used to discover candidate consensus patterns. Three patterns were found and named rarabpm1, rarabpm2, and rarabpm3 (Table 1). Rarabpm2 seems to be a pyrimidine-rich region, rarabpm3 is a “GC”-rich region, whereas rarabpm1 seems to consist of a pyrimidine- and a “GC”-rich region (Table 1). However, these consensus patterns do not correspond to any known specific protein binding sites.
Table 1.
Multi alignments of the consensus patterns found by MEME
| Strand | P value | Alignment | |
|---|---|---|---|
| Rarabpm1 | |||
| Case 15 | + | 2.75e-25 | CTTCAAGCGTTAACTCCTTCCTAACTCGGGGGGAGAACGGGGCCAGGCCGC |
| Case 10 | + | 2.75e-25 | CCTCCCTCCTCTTCAAGCGTTAACTCCTTCCTAACTCGGGGGGAGAACGGGGCCA |
| Case 13 | + | 2.75e-25 | CCTCCCTCCTCTTCAAGCGTTAACTCCTTCCTAACTCGGGGGGAGAACGGGGCC |
| Rarabpm2 | TCTTCCCTCCCTACT | ||
| Consensus | G T C TGACTC | ||
| Sequence | A | ||
| Case 5 | − | 1.07e-08 | GCTTATGTCCTCTTCCCTCCCTCCTCTTCAAGCGT |
| Case 10 | − | 1.07e-08 | TCTTCCCTCCCTCCTCTTCAAGCGT |
| Case 13 | + | 1.07e-08 | CTCTTCCCTCCCTCCTCTTCAAGCGT |
| Case 11 | + | 2.38e-07 | CCCTGGGGACTCTTCTCCCTGTATTCAGGGTATCT |
| Case 12 | + | 2.38e-07 | CCCTGGGGACTCTTCTCCCTGTATTCAGGGTATCT |
| Case 3 | + | 2.60e-07 | GCCCCTCCCCTCTTCTCTCTCTAGCCATTG |
| Case 2 | − | 2.66e-06 | GCTCTGTCTGTCTGGCCTCAATACTATGTCCGGGT |
| Case 8 | − | 3.48e-06 | CGCCGTCTGCTCTTCACTCCAACACCAACTGCCCT |
| Case 9 | − | 3.48e-06 | GTTTCCCTGCTCTGCCCTTTGAACCCAGAGCTCGA |
| Rarabpm3 | GCCCAGGGGA | ||
| Consensus sequence | GGGT C | ||
| Case 7 | + | 1.71e-05 | AGAATCTGCTGGGCTGGGGATGGTGTGGGC |
| Case 9 | + | 1.71e-05 | TGGAGGCCTGGGGAGGTGGGGCAT |
| Case 11 | + | 1.71e-05 | GGCTTTTGGGGCCCTGGGGACTCTTCTCCC |
| Case 12 | + | 1.71e-05 | GGCTTTTGGGGCCCTGGGGACTCTTCTCCC |
| Case 6 | − | 3.41e-05 | AGACTGGTGGGGGGAGGGGAGAT |
| Case 8 | + | 3.41e-05 | AGAGCAGACGGCGGTGGGGAG |
| Case 15 | + | 5.74e-05 | GGGCCAGGCCGCCCAGGGGCA |
| Case 16 | + | 5.74e-05 | GGGCCAGGCCGCCCAGGGGCAGGAGCTTTA |
| Case 5 | − | 6.99e-05 | CATAAGCTGGGGGCAGAGGAAACTGG |
| Case 3 | − | 9.31e-05 | AGAGAGAAGAGGGGAGGGGCAGTTAGAGAC |
Cases 1–8 are from the study by Yoshida et al. (33); case 9 is from the study by de The et al. (11); case 10 is from the study by Alcalay et al. (30); cases 11–13 are from the study by Dong et al. (31); and cases 14–16 are from the study by Satoshi et al. (32). The extracted sequences flanking every RARα t(15;17) breakpoint are shown aligned with each other on the match sites of the patterns found by meme, and the 10 sequence positions preceding and following each site are also shown. “+” means the forward extracted sequence, and “−” means the reverse complement of the extracted sequence. The P value gives the probability of a random string (generated from the background letter frequencies) having the same match score or higher. The multilevel consensus sequence corresponding to the pattern is an aid in remembering and understanding the pattern. The cases are sorted in increasing order by P value separately for each column of the pattern.
Discussion
One of the characteristics of the PML-RARα fusion gene is the presence of distinct types of PML-RARα transcripts. In most patients with the V-type isoform, the ORFs are still maintained because of an inserted spacer sequence between the ΔP6 and R3. To explain the formation of the V-type isoform, we initially proposed the following hypothesis. The chromosomal breakpoints in the PML gene should be located within the P6. As a result, not only is the coding region in this exon disrupted, but also the splicing donor signal downstream of P6 is eliminated, which might cause a problem for the protein-coding ability of the PML-RARα fusion gene. However, as suggested by previous data in a few cases (16, 17, 24), if the ΔP6 sequence is fused to a region of the RARα intron 2 where short coding sequences with “gt” donor sites are present, the use of these coding sequences with activation of the cryptic splicing donor sites could maintain the ORF. This hypothesis is now strongly supported by our finding that the suspected “cryptic” coding sequences in 14 cases are all located in intron 2, 13 of them followed by a gt signal. Nevertheless, to finally prove this hypothesis, the genomic structure of reciprocal chromosomal breaks on both PML and RARα genes should be determined and compared with the corresponding mRNA sequence. Indeed, we have done just this in F1 and G-E076 cases, definitively resolving the sequence origin in these cases. This mechanism likely applies to many other cases, because cryptic splice sites are frequent in the genome (≈1 in 300 bp). However, in V-type PML-RARα genomic fusions, the use of these sites did not seem to be random in that some sites were recurrently used among different patients (Fig. 2A). Two reasons may explain this phenomenon. First, there must be coding sequences upstream of the sites allowing the rescue of the ORF. Second, sequences near these sites should be favorable for chromosomal translocation to occur. Notably, in most described genetic disease models, the splicing of an intronic sequence into mRNA caused by activation of “cryptic” splice sites resulted in the disruption of the ORF and therefore a “loss of function” mutation (41). In contrast, in the V-form cases the use of cryptic splicing signals represents a “gain of function” mechanism. Interestingly, if the use of spacer sequence is not enough to maintain the ORF, there could be “second line” mechanism, as demonstrated by the presence of an S-type isoform in addition to an “out of frame” V-type isoform in case F2 in our work. This mechanism may be critical for APL cells to retain their growth advantage. Of note, in one report ΔP6 and R3 sequences were directly joined without spacer sequences (24). It is likely that potential splicing donor site is located immediately flanking the P6 breakpoint in this case.
Normally, RARα has the ability to interact either with the transcriptional nuclear receptor coactivator apparatus or with the N-CoR/Sin3/HDAC1 CoR complex (2). Under physiologic concentrations of RA, the interaction with the CoR is relieved, and RARα functions as a transcription activator. Fusion of PML to RARα, however, results in an enhanced interaction with the CoR complex, thereby constitutively repressing RARα target genes. This transcriptional repression can be relieved only when APL cells are treated with pharmacological concentrations of ATRA. In contrast, the association of CoR to PLZF-RARα resists the modulating effect of ATRA because of the presence of a second CoR binding site on the PLZF moiety of the fusion protein (42). Of note, APL cells with V-type isoform were reported to be less sensitive to ATRA (21). One of the important findings of this work is the identification of a subset of V-type PML-RARα cases with decreased ATRA sensitivity with regard to SMRT dissociation. This subset is characterized by a relatively long “spacer” with the same cryptic coding sequence (134 and 127 bp in cases F1 and G-E076, respectively) inserted into the joining sites between PML and RARα. Subsequent studies showed that deletion of this spacer restored the sensitivity to ATRA, suggesting it is the spacer sequence but not the truncation of exon 6, which is responsible for this aberrant binding to SMRT. Bioinformatics analysis suggested a possible protein–protein interaction motif in this spacer and deletion of this small motif decreased the binding to SMRT (Figs. 2B and 3A). Interestingly, these two patients had a phenotype of clinical resistance to ATRA. On the other hand, the reported patients with APL of V-type PML-RARα with short spacer sequence responded well to ATRA (24). Our results have thus added more evidence to the model of transcriptional repression as the basal mechanisms of APL leukemogenesis and the relieving of this transcriptional repression as the major mechanism of differentiation therapy (Fig. 3B).
Because nonrandom chromosomal translocations are crucial pathogenic events during leukemic transformation, an understanding of the genesis of these translocations is important. Here, we addressed this issue for RARα gene sequences flanking the breakpoints of a number of previously reported patients. Although the study on breakpoints in RARα intron 2 was limited by the probe selection in patients with t(15;17), at least two breakage hot spots were located within intron 2 of the RARα gene. No heptamer-nanomer sequence reminiscent of V-D-J rearrangement of Ig gene, nor 4-bp duplication suggestive of Topo II effect, or long interspersed element/short interspersed element sequences in favor of homologous recombination were found. However, some consensus patterns were found around the breakpoints (rarabpm1, rarabpm2, and rarabpm3), which may provide a chance for some protein recognition sites to stand out in the double helix of DNA. This hypothesis should be examined in further experiments.
Acknowledgments
The authors thank all members of the Shanghai Institute of Hematology and the Chinese National Human Genome Center at Shanghai for their support. This work was supported in part by the Chinese National Key Basic Research Project (973), the National Natural Science Foundation of China, the Chinese National High Tech Program (863), the Shanghai Commission for Science and Technology, the Shanghai Commission for Education, the Samuel Waxman Cancer Research Foundation, and by the Clyde Wu Foundation of the Shanghai Institute of Hematology.
Abbreviations
- RA
retinoic acid
- RAR
RA receptor
- RXR
retinoid X receptor
- RARE
RA response element
- CoR
nuclear receptor corepressor
- ATRA
all-trans RA
- APL
acute promyelocytic leukemia
- P6
PML exon 6
- R3
RARα exon 3
- L
S, V, long, short, and variant types of PML-RARα transcripts
- GST
glutathione S-transferase
Footnotes
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. AC090426).
Zelent, A., Zhu, J., Lanotte, M., Gallagher, R., Waxman, S., Heyworth, C. M. & Enver, T. (1997) Blood 90, Suppl., 44a–45a (abstr.).
References
- 1.Chambon P, Zelent A, Petkovich M. In: Retinoids: 10 Years On. Saurat J H, editor. Basel: Karger; 1972. pp. 10–17. [Google Scholar]
- 2.Melnick A, Licht J D. Blood. 1999;93:3167–3215. [PubMed] [Google Scholar]
- 3.Leroy P, Krust A, Zelent A, Mendelsohn C, Garnier J M, Kastner P, Dierich A, Chambon P. EMBO J. 1991;10:59–69. doi: 10.1002/j.1460-2075.1991.tb07921.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leid M, Kastner P, Chambon P. Trends Biochem Sci. 1992;17:427–433. doi: 10.1016/0968-0004(92)90014-z. [DOI] [PubMed] [Google Scholar]
- 5.Warrell R P, de The H, Wang Z Y, Degos L. N Engl J Med. 1993;329:177–189. doi: 10.1056/NEJM199307153290307. [DOI] [PubMed] [Google Scholar]
- 6.Wang Z Y, Chen Z. Lancet Oncol. 2000;1:101–106. doi: 10.1016/s1470-2045(00)00017-6. [DOI] [PubMed] [Google Scholar]
- 7.Huang M E, Ye Y C, Chen S R, Chai J R, Lu J X, Zhoa L, Gu L J, Wang Z Y. Blood. 1988;72:567–572. [PubMed] [Google Scholar]
- 8.Tallman M S, Andersen J W, Schiffer C A, Appelbaum F R, Feusner J H, Ogden A, Shepherd L, Willman C, Bloomfield C D, Rowe J M, Wiernik P H. N Engl J Med. 1997;337:1021–1028. doi: 10.1056/NEJM199710093371501. [DOI] [PubMed] [Google Scholar]
- 9.Rowley J D, Golomb H M, Dougherty C. Lancet. 1977;1:549–550. doi: 10.1016/s0140-6736(77)91415-5. (lett.). [DOI] [PubMed] [Google Scholar]
- 10.de The H, Chomienne C, Lanotte M, Degos L, Dejean A. Nature (London) 1990;347:558–561. doi: 10.1038/347558a0. [DOI] [PubMed] [Google Scholar]
- 11.de The H, Lavau C, Marchio A, Chomienne C, Degos L, Dejean A. Cell. 1991;66:675–684. doi: 10.1016/0092-8674(91)90113-d. [DOI] [PubMed] [Google Scholar]
- 12.Kakizuka A, Miller W H, Umesono K, Warrell R P, Frankel S R, Murty V V, Dmitrovsky E, Evans R M. Cell. 1991;66:663–674. doi: 10.1016/0092-8674(91)90112-c. [DOI] [PubMed] [Google Scholar]
- 13.Chen S J, Zhu Y J, Tong J H, Dong S, Huang W, Chen Y, Xiang W M, Zhang L, Li X S, Qian G Q, et al. Blood. 1991;78:2696–2701. [PubMed] [Google Scholar]
- 14.Tong J H, Dong S, Geng J P, Huang W, Wang Z Y, Sun G L, Chen S J, Chen Z, Larsen C J, Berger R. Oncogene. 1992;7:311–316. [PubMed] [Google Scholar]
- 15.Kastner P, Perez A, Lutz Y, Rochette-Egly C, Gaub M P, Durand B, Lanotte M, Berger R, Chambon P. EMBO J. 1992;11:629–642. doi: 10.1002/j.1460-2075.1992.tb05095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pandolfi P P, Alcalay M, Fagioli M, Zangrilli D, Mencarelli A, Diverio D, Biondi A, Lo Coco F, Rambaldi A, Grignani F. EMBO J. 1992;11:1397–1407. doi: 10.1002/j.1460-2075.1992.tb05185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen S J, Chen Z, Chen A, Tong J H, Dong S, Wang Z Y, Waxman S, Zelent A. Oncogene. 1992;7:1223–1232. [PubMed] [Google Scholar]
- 18.Miller W H, Kakizuka A, Frankel S R, Warrell R P, DeBlasio A, Levine K, Evans R M, Dmitrovsky E. Proc Natl Acad Sci USA. 1992;89:2694–2698. doi: 10.1073/pnas.89.7.2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Castaigne S, Balitrand N, de The H, Dejean A, Degos L, Chomienne C. Blood. 1992;79:3110–3115. [PubMed] [Google Scholar]
- 20.Geng J P, Tong J H, Dong S, Wang Z Y, Chen S J, Chen Z, Zelent A, Berger R, Larsen C J. Leukemia. 1993;7:20–26. [PubMed] [Google Scholar]
- 21.Gallagher R E, Li Y P, Rao S, Paietta E, Andersen J, Etkind P, Bennett J M, Tallman M S, Wiernik P H. Blood. 1995;86:1540–1547. [PubMed] [Google Scholar]
- 22.Guglielmi C, Martelli M P, Diverio D, Fenu S, Vegna M L, Cantu-Rajnoldi A, Biondi A, Cocito M G, Del Vecchio L, Tabilio A, et al. Br J Haematol. 1998;102:1035–1041. doi: 10.1046/j.1365-2141.1998.00871.x. [DOI] [PubMed] [Google Scholar]
- 23.Kane J R, Head D R, Balazs L, Hulshof M G, Motroni T A, Raimondi S C, Carroll A J, Behm F G, Krance R A, Shurtleff S A, et al. Leukemia. 1996;10:1296–1302. [PubMed] [Google Scholar]
- 24.Slack J L, Willman C L, Andersen J W, Li Y P, Viswanatha D S, Bloomfield C D, Tallman M S, Gallagher R E. Blood. 2000;95:398–403. [PubMed] [Google Scholar]
- 25.Hjalt T A, Murray J C. Mamm Genome. 1999;10:528–529. doi: 10.1007/s003359901036. [DOI] [PubMed] [Google Scholar]
- 26.Zhang T, Xiong H, Kan L X, Zhang C K, Jiao X F, Fu G, Zhang Q H, Lu L, Tong J H, Gu B W, et al. Proc Natl Acad Sci USA. 1999;96:11422–11427. doi: 10.1073/pnas.96.20.11422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chomczynski P, Sacchi N. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 28.Huang W, Sun G L, Li X S, Cao Q, Lu Y, Jang G S, Zhang F Q, Chai J R, Wang Z Y, Waxman S. Blood. 1993;82:1264–1269. [PubMed] [Google Scholar]
- 29.Dong S, Zhu J, Reid A, Strutt P, Guidez F, Zhong H J, Wang Z Y, Licht J, Waxman S, Chomienne C, et al. Proc Natl Acad Sci USA. 1996;93:3624–3629. doi: 10.1073/pnas.93.8.3624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alcalay M, Zangrilli D, Pandolfi P P, Longo L, Mencarelli A, Giacomucci A, Rocchi M, Biondi A, Rambaldi A, Lo Coco F. Proc Natl Acad Sci USA. 1991;88:1977–1981. doi: 10.1073/pnas.88.5.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dong S, Geng J P, Tong J H, Wu Y, Cai J R, Sun G L, Chen S R, Wang Z Y, Larsen C J, Berger R. Genes Chromosomes Cancer. 1993;6:133–139. doi: 10.1002/gcc.2870060302. [DOI] [PubMed] [Google Scholar]
- 32.Tashiro S, Kotomura N, Tanaka K, Suzuki K, Kyo T, Dohy H, Niwa O, Kamada N. Oncogene. 1994;9:1939–1945. [PubMed] [Google Scholar]
- 33.Yoshida H, Naoe T, Fukutani H, Kiyoi H, Kubo K, Ohno R. Genes Chromosomes Cancer. 1995;12:37–44. doi: 10.1002/gcc.2870120107. [DOI] [PubMed] [Google Scholar]
- 34.Brand N J, Petkovich M, Chambon P. Nucleic Acids Res. 1990;18:6799–6806. doi: 10.1093/nar/18.23.6799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ogawa E, Maruyama M, Kagoshima H, Inuzuka M, Lu J, Satake M, Shigesada K, Ito Y. Proc Natl Acad Sci USA. 1993;90:6859–6863. doi: 10.1073/pnas.90.14.6859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nuchprayoon I, Meyers S, Scott L M, Suzow J, Hiebert S, Friedman A D. Mol Cell Biol. 1994;14:5558–5568. doi: 10.1128/mcb.14.8.5558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zeng C, van Wijnen A J, Stein J L, Meyers S, Sun W, Shopland L, Lawrence J B, Penman S, Lian J B, Stein G S. Proc Natl Acad Sci USA. 1997;94:6746–6751. doi: 10.1073/pnas.94.13.6746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tsukiyama T, Ueda H, Hirose S, Niwa O. Mol Cell Biol. 1992;12:1286–1291. doi: 10.1128/mcb.12.3.1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kotomura N, Okada M, Ninomiya Y, Tsukiyama T, Umesono K, Evans R M, Niwa O. J Biochem (Tokyo) 1994;116:1309–1316. doi: 10.1093/oxfordjournals.jbchem.a124680. [DOI] [PubMed] [Google Scholar]
- 40.Lang A J, Mirski S E, Cummings H J, Yu Q, Gerlach J H, Cole S P. Gene. 1998;221:255–266. doi: 10.1016/s0378-1119(98)00468-5. [DOI] [PubMed] [Google Scholar]
- 41.Thompson M W, Mcinnes R R, Willard H F. Genetics in Medicine. Philadelphia: Saunders; 1991. pp. 247–270. [Google Scholar]
- 42.Guidez F, Ivins S, Zhu J, Soderstrom M, Waxman S, Zelent A. Blood. 1998;91:2634–2642. [PubMed] [Google Scholar]