Abstract
OBJECTIVE: Accessory adhesion molecules are thought to influence the first interaction between host leukocytes and graft vascular endothelial cells. Their role in transplantation is reviewed. SUMMARY: Adhesion molecules have been divided into three major families: the selectins, the integrins, and the immunoglobulin superfamily. Selectins are small proteins that mediate the first contact between stimulated endothelial cells and leukocytes. Integrins interact with cytoskeletal components of cells, presumably coordinating extracellular stimuli with cytoskeleton dependent actions, such as motility, shape change, and phagocytic responses. Members of the immunoglobulin superfamily are structurally homologous, although they do not necessarily share similar functions. They are involved in T-cell proliferation and intracellular events. METHODS: Various groups of investigators have studied the influence and expression of adhesion molecules following transplantation. The authors of this article have reviewed and summarized the available literature. RESULTS: Many different adhesion molecules are up-regulated during the rejection event. Treatment of transplant recipients with monoclonal antibodies against accessory molecules, such as leukocyte function associated antigen 1 (LFA-1) and intercellular adhesion molecule 1 (ICAM-1), has resulted in either a prolongation of transplant survival or the induction of tolerance in some models. Other interventions are under study. CONCLUSION: By mediating the initial leukocyte/endothelial cell interactions, adhesion molecules may play an important role in graft rejection, mediation of infiltration into the graft, and dissemination of the antigenic message to the lymphoid tissues of the host. Future studies will have to deal not only with conceptualizing their function and mechanisms of action, but also with manipulating their interrelationships to the benefit of the graft recipient.
Full text
PDF

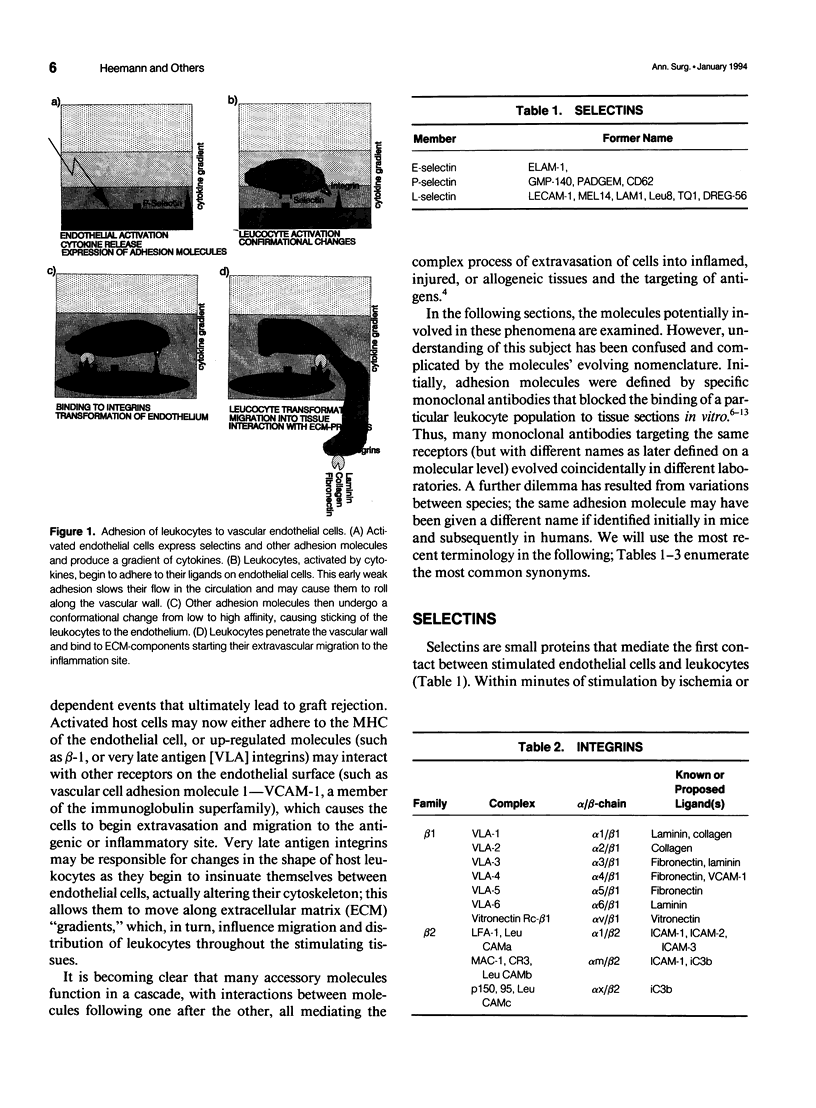

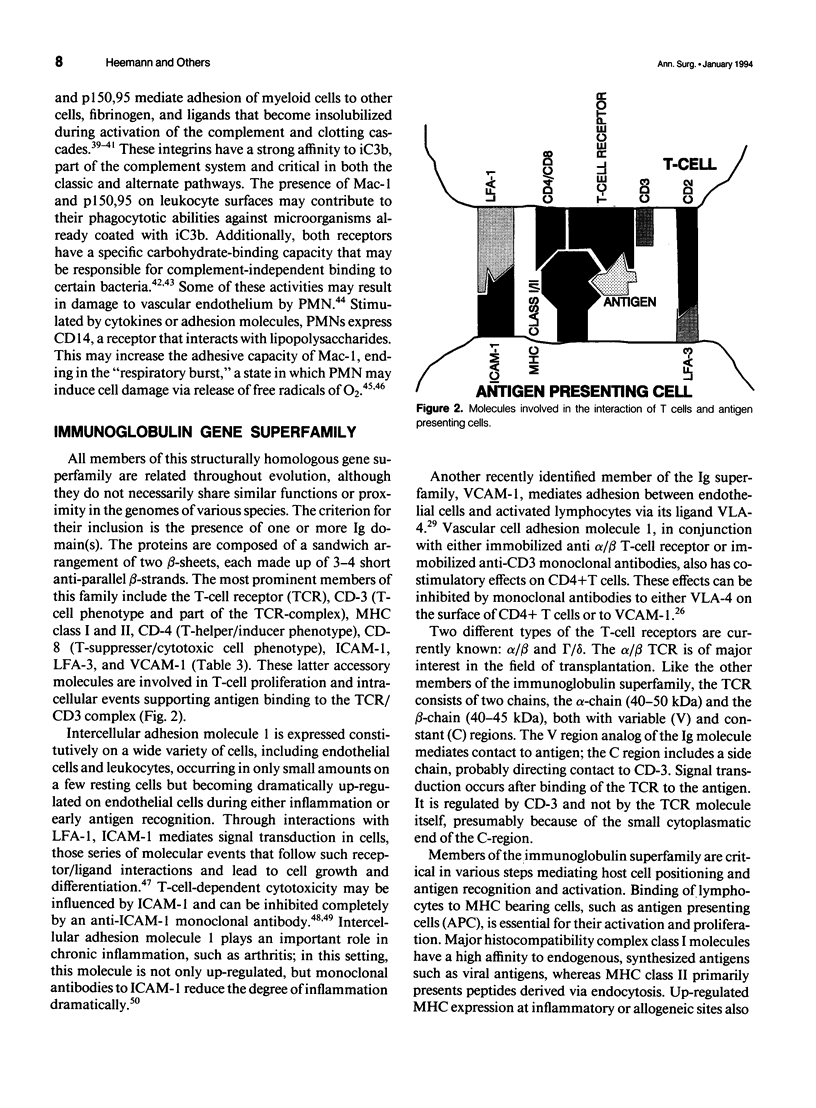




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbassi O., Lane C. L., Krater S., Kishimoto T. K., Anderson D. C., McIntire L. V., Smith C. W. Canine neutrophil margination mediated by lectin adhesion molecule-1 in vitro. J Immunol. 1991 Oct 1;147(7):2107–2115. [PubMed] [Google Scholar]
- Adams D. H., Hubscher S. G., Shaw J., Rothlein R., Neuberger J. M. Intercellular adhesion molecule 1 on liver allografts during rejection. Lancet. 1989 Nov 11;2(8672):1122–1125. doi: 10.1016/s0140-6736(89)91489-x. [DOI] [PubMed] [Google Scholar]
- Adams D. H., Mainolfi E., Elias E., Neuberger J. M., Rothlein R. Detection of circulating intercellular adhesion molecule-1 after liver transplantation--evidence of local release within the liver during graft rejection. Transplantation. 1993 Jan;55(1):83–87. doi: 10.1097/00007890-199301000-00016. [DOI] [PubMed] [Google Scholar]
- Altieri D. C. Occupancy of CD11b/CD18 (Mac-1) divalent ion binding site(s) induces leukocyte adhesion. J Immunol. 1991 Sep 15;147(6):1891–1898. [PubMed] [Google Scholar]
- Berg E. L., Goldstein L. A., Jutila M. A., Nakache M., Picker L. J., Streeter P. R., Wu N. W., Zhou D., Butcher E. C. Homing receptors and vascular addressins: cell adhesion molecules that direct lymphocyte traffic. Immunol Rev. 1989 Apr;108:5–18. doi: 10.1111/j.1600-065x.1989.tb00010.x. [DOI] [PubMed] [Google Scholar]
- Bevilacqua M. P., Pober J. S., Mendrick D. L., Cotran R. S., Gimbrone M. A., Jr Identification of an inducible endothelial-leukocyte adhesion molecule. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9238–9242. doi: 10.1073/pnas.84.24.9238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevilacqua M. P., Stengelin S., Gimbrone M. A., Jr, Seed B. Endothelial leukocyte adhesion molecule 1: an inducible receptor for neutrophils related to complement regulatory proteins and lectins. Science. 1989 Mar 3;243(4895):1160–1165. doi: 10.1126/science.2466335. [DOI] [PubMed] [Google Scholar]
- Briscoe D. M., Schoen F. J., Rice G. E., Bevilacqua M. P., Ganz P., Pober J. S. Induced expression of endothelial-leukocyte adhesion molecules in human cardiac allografts. Transplantation. 1991 Feb;51(2):537–539. [PubMed] [Google Scholar]
- Butcher E. C. Warner-Lambert/Parke-Davis Award lecture. Cellular and molecular mechanisms that direct leukocyte traffic. Am J Pathol. 1990 Jan;136(1):3–11. [PMC free article] [PubMed] [Google Scholar]
- Carlos T. M., Schwartz B. R., Kovach N. L., Yee E., Rosa M., Osborn L., Chi-Rosso G., Newman B., Lobb R., Rosso M. Vascular cell adhesion molecule-1 mediates lymphocyte adherence to cytokine-activated cultured human endothelial cells. Blood. 1990 Sep 1;76(5):965–970. [PubMed] [Google Scholar]
- Cheng Y. F., Clyman R. I., Enenstein J., Waleh N., Pytela R., Kramer R. H. The integrin complex alpha v beta 3 participates in the adhesion of microvascular endothelial cells to fibronectin. Exp Cell Res. 1991 May;194(1):69–77. doi: 10.1016/0014-4827(91)90131-d. [DOI] [PubMed] [Google Scholar]
- Cosimi A. B., Conti D., Delmonico F. L., Preffer F. I., Wee S. L., Rothlein R., Faanes R., Colvin R. B. In vivo effects of monoclonal antibody to ICAM-1 (CD54) in nonhuman primates with renal allografts. J Immunol. 1990 Jun 15;144(12):4604–4612. [PubMed] [Google Scholar]
- Damle N. K., Aruffo A. Vascular cell adhesion molecule 1 induces T-cell antigen receptor-dependent activation of CD4+T lymphocytes. Proc Natl Acad Sci U S A. 1991 Aug 1;88(15):6403–6407. doi: 10.1073/pnas.88.15.6403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David V., Leca G., Corvaia N., Le Deist F., Boumsell L., Bensussan A. Proliferation of resting lymphocytes is induced by triggering T cells through an epitope common to the three CD18/CD11 leukocyte adhesion molecules. Cell Immunol. 1991 Sep;136(2):519–524. doi: 10.1016/0008-8749(91)90372-i. [DOI] [PubMed] [Google Scholar]
- Davis L. S., Oppenheimer-Marks N., Bednarczyk J. L., McIntyre B. W., Lipsky P. E. Fibronectin promotes proliferation of naive and memory T cells by signaling through both the VLA-4 and VLA-5 integrin molecules. J Immunol. 1990 Aug 1;145(3):785–793. [PubMed] [Google Scholar]
- Diamond M. S., Springer T. A. A subpopulation of Mac-1 (CD11b/CD18) molecules mediates neutrophil adhesion to ICAM-1 and fibrinogen. J Cell Biol. 1993 Jan;120(2):545–556. doi: 10.1083/jcb.120.2.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dustin M. L., Springer T. A. Role of lymphocyte adhesion receptors in transient interactions and cell locomotion. Annu Rev Immunol. 1991;9:27–66. doi: 10.1146/annurev.iy.09.040191.000331. [DOI] [PubMed] [Google Scholar]
- Dustin M. L., Springer T. A. T-cell receptor cross-linking transiently stimulates adhesiveness through LFA-1. Nature. 1989 Oct 19;341(6243):619–624. doi: 10.1038/341619a0. [DOI] [PubMed] [Google Scholar]
- Elices M. J., Osborn L., Takada Y., Crouse C., Luhowskyj S., Hemler M. E., Lobb R. R. VCAM-1 on activated endothelium interacts with the leukocyte integrin VLA-4 at a site distinct from the VLA-4/fibronectin binding site. Cell. 1990 Feb 23;60(4):577–584. doi: 10.1016/0092-8674(90)90661-w. [DOI] [PubMed] [Google Scholar]
- Faull R. J., Russ G. R. Tubular expression of intercellular adhesion molecule-1 during renal allograft rejection. Transplantation. 1989 Aug;48(2):226–230. doi: 10.1097/00007890-198908000-00009. [DOI] [PubMed] [Google Scholar]
- Freedman A. S., Munro J. M., Rice G. E., Bevilacqua M. P., Morimoto C., McIntyre B. W., Rhynhart K., Pober J. S., Nadler L. M. Adhesion of human B cells to germinal centers in vitro involves VLA-4 and INCAM-110. Science. 1990 Aug 31;249(4972):1030–1033. doi: 10.1126/science.1697696. [DOI] [PubMed] [Google Scholar]
- Fuggle S. V., Sanderson J. B., Gray D. W., Richardson A., Morris P. J. Variation in expression of endothelial adhesion molecules in pretransplant and transplanted kidneys--correlation with intragraft events. Transplantation. 1993 Jan;55(1):117–123. doi: 10.1097/00007890-199301000-00022. [DOI] [PubMed] [Google Scholar]
- Fujita H., Morita I., Murota S. Involvement of adhesion molecules (CD11a-ICAM-1) in vascular endothelial cell injury elicited by PMA-stimulated neutrophils. Biochem Biophys Res Commun. 1991 Jun 14;177(2):664–672. doi: 10.1016/0006-291x(91)91840-9. [DOI] [PubMed] [Google Scholar]
- GOWANS J. L. The recirculation of lymphocytes from blood to lymph in the rat. J Physiol. 1959 Apr 23;146(1):54–69. doi: 10.1113/jphysiol.1959.sp006177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guymer R. H., Mandel T. E. Monoclonal antibody to ICAM-1 prolongs murine heterotopic corneal allograft survival. Aust N Z J Ophthalmol. 1991 May;19(2):141–144. doi: 10.1111/j.1442-9071.1991.tb00642.x. [DOI] [PubMed] [Google Scholar]
- Hallmann R., Jutila M. A., Smith C. W., Anderson D. C., Kishimoto T. K., Butcher E. C. The peripheral lymph node homing receptor, LECAM-1, is involved in CD18-independent adhesion of human neutrophils to endothelium. Biochem Biophys Res Commun. 1991 Jan 15;174(1):236–243. doi: 10.1016/0006-291x(91)90511-5. [DOI] [PubMed] [Google Scholar]
- Hamburger S. A., McEver R. P. GMP-140 mediates adhesion of stimulated platelets to neutrophils. Blood. 1990 Feb 1;75(3):550–554. [PubMed] [Google Scholar]
- Hancock W. W., DiStefano R., Braun P., Schweizer R. T., Tilney N. L., Kupiec-Weglinski J. W. Cyclosporine and anti-interleukin 2 receptor monoclonal antibody therapy suppress accelerated rejection of rat cardiac allografts through different effector mechanisms. Transplantation. 1990 Feb;49(2):416–421. doi: 10.1097/00007890-199002000-00037. [DOI] [PubMed] [Google Scholar]
- Heemann U. W., Tullius S. G., Schumann V., Tilney N. L. Neutrophils and macrophages are prominent in the pathophysiology of chronic rejection of rat kidney allografts. Transplant Proc. 1993 Feb;25(1 Pt 2):937–938. [PubMed] [Google Scholar]
- Hemler M. E. VLA proteins in the integrin family: structures, functions, and their role on leukocytes. Annu Rev Immunol. 1990;8:365–400. doi: 10.1146/annurev.iy.08.040190.002053. [DOI] [PubMed] [Google Scholar]
- Hibbs M. L., Xu H., Stacker S. A., Springer T. A. Regulation of adhesion of ICAM-1 by the cytoplasmic domain of LFA-1 integrin beta subunit. Science. 1991 Mar 29;251(5001):1611–1613. doi: 10.1126/science.1672776. [DOI] [PubMed] [Google Scholar]
- Iigo Y., Takashi T., Tamatani T., Miyasaka M., Higashida T., Yagita H., Okumura K., Tsukada W. ICAM-1-dependent pathway is critically involved in the pathogenesis of adjuvant arthritis in rats. J Immunol. 1991 Dec 15;147(12):4167–4171. [PubMed] [Google Scholar]
- Isobe M., Huebner K., Maddon P. J., Littman D. R., Axel R., Croce C. M. The gene encoding the T-cell surface protein T4 is located on human chromosome 12. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4399–4402. doi: 10.1073/pnas.83.12.4399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isobe M., Yagita H., Okumura K., Ihara A. Specific acceptance of cardiac allograft after treatment with antibodies to ICAM-1 and LFA-1. Science. 1992 Feb 28;255(5048):1125–1127. doi: 10.1126/science.1347662. [DOI] [PubMed] [Google Scholar]
- Jutila M. A., Berg E. L., Kishimoto T. K., Picker L. J., Bargatze R. F., Bishop D. K., Orosz C. G., Wu N. W., Butcher E. C. Inflammation-induced endothelial cell adhesion to lymphocytes, neutrophils, and monocytes. Role of homing receptors and other adhesion molecules. Transplantation. 1989 Nov;48(5):727–731. doi: 10.1097/00007890-198911000-00001. [DOI] [PubMed] [Google Scholar]
- Jutila M. A., Kishimoto T. K., Finken M. Low-dose chymotrypsin treatment inhibits neutrophil migration into sites of inflammation in vivo: effects on Mac-1 and MEL-14 adhesion protein expression and function. Cell Immunol. 1991 Jan;132(1):201–214. doi: 10.1016/0008-8749(91)90019-8. [DOI] [PubMed] [Google Scholar]
- Kupiec-Weglinski J. W., De Sousa M. Lymphocyte traffic is modified in vivo by anti-laminin antibody. Immunology. 1991 Feb;72(2):312–313. [PMC free article] [PubMed] [Google Scholar]
- Kupiec-Weglinski J. W., Heemann U. W., Coito A. J., Tullius S. G., Tilney N. L., de Sousa M. Adhesion molecule interaction with extracellular matrix. Exp Nephrol. 1993 Mar-Apr;1(2):78–82. [PubMed] [Google Scholar]
- Kupiec-Weglinski J. W., Tilney N. L. Lymphocyte migration patterns in organ allograft recipients. Immunol Rev. 1989 Apr;108:63–82. doi: 10.1111/j.1600-065x.1989.tb00013.x. [DOI] [PubMed] [Google Scholar]
- Larson R. S., Springer T. A. Structure and function of leukocyte integrins. Immunol Rev. 1990 Apr;114:181–217. doi: 10.1111/j.1600-065x.1990.tb00565.x. [DOI] [PubMed] [Google Scholar]
- Lawrence M. B., Springer T. A. Leukocytes roll on a selectin at physiologic flow rates: distinction from and prerequisite for adhesion through integrins. Cell. 1991 May 31;65(5):859–873. doi: 10.1016/0092-8674(91)90393-d. [DOI] [PubMed] [Google Scholar]
- Le Mauff B., Hourmant M., Rougier J. P., Hirn M., Dantal J., Baatard R., Cantarovich D., Jacques Y., Soulillou J. P. Effect of anti-LFA1 (CD11a) monoclonal antibodies in acute rejection in human kidney transplantation. Transplantation. 1991 Aug;52(2):291–296. doi: 10.1097/00007890-199108000-00020. [DOI] [PubMed] [Google Scholar]
- Lewinsohn D. M., Bargatze R. F., Butcher E. C. Leukocyte-endothelial cell recognition: evidence of a common molecular mechanism shared by neutrophils, lymphocytes, and other leukocytes. J Immunol. 1987 Jun 15;138(12):4313–4321. [PubMed] [Google Scholar]
- Lo S. K., Lee S., Ramos R. A., Lobb R., Rosa M., Chi-Rosso G., Wright S. D. Endothelial-leukocyte adhesion molecule 1 stimulates the adhesive activity of leukocyte integrin CR3 (CD11b/CD18, Mac-1, alpha m beta 2) on human neutrophils. J Exp Med. 1991 Jun 1;173(6):1493–1500. doi: 10.1084/jem.173.6.1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luscinskas F. W., Cybulsky M. I., Kiely J. M., Peckins C. S., Davis V. M., Gimbrone M. A., Jr Cytokine-activated human endothelial monolayers support enhanced neutrophil transmigration via a mechanism involving both endothelial-leukocyte adhesion molecule-1 and intercellular adhesion molecule-1. J Immunol. 1991 Mar 1;146(5):1617–1625. [PubMed] [Google Scholar]
- Maddon P. J., Littman D. R., Godfrey M., Maddon D. E., Chess L., Axel R. The isolation and nucleotide sequence of a cDNA encoding the T cell surface protein T4: a new member of the immunoglobulin gene family. Cell. 1985 Aug;42(1):93–104. doi: 10.1016/s0092-8674(85)80105-7. [DOI] [PubMed] [Google Scholar]
- Moolenaar W., Bruijn J. A., Schrama E., Ferrone S., Daha M. R., Zwinderman A. H., Hoedemaeker P. J., van Es L. A., van der Woude F. J. T-cell receptors and ICAM-1 expression in renal allografts during rejection. Transpl Int. 1991 Sep;4(3):140–145. doi: 10.1007/BF00335334. [DOI] [PubMed] [Google Scholar]
- Morales-Ducret J., Wayner E., Elices M. J., Alvaro-Gracia J. M., Zvaifler N. J., Firestein G. S. Alpha 4/beta 1 integrin (VLA-4) ligands in arthritis. Vascular cell adhesion molecule-1 expression in synovium and on fibroblast-like synoviocytes. J Immunol. 1992 Aug 15;149(4):1424–1431. [PubMed] [Google Scholar]
- Mourad W., Geha R. S., Chatila T. Engagement of major histocompatibility complex class II molecules induces sustained, lymphocyte function-associated molecule 1-dependent cell adhesion. J Exp Med. 1990 Nov 1;172(5):1513–1516. doi: 10.1084/jem.172.5.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan M. S., Warren J. S., Smith C. W., Anderson D. C., Yeh C. G., Rudolph A. R., Ward P. A. Lung injury after deposition of IgA immune complexes. Requirements for CD18 and L-arginine. J Immunol. 1992 May 15;148(10):3086–3092. [PubMed] [Google Scholar]
- Nathan C., Srimal S., Farber C., Sanchez E., Kabbash L., Asch A., Gailit J., Wright S. D. Cytokine-induced respiratory burst of human neutrophils: dependence on extracellular matrix proteins and CD11/CD18 integrins. J Cell Biol. 1989 Sep;109(3):1341–1349. doi: 10.1083/jcb.109.3.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nocera A., Cosimi A. B., Colvin R. B., Gesner M. L., Fuller T. C. Function and surface phenotype of T lymphocytes infiltrating renal allografts in nonhuman primates treated with monoclonal antibodies. Transplantation. 1989 Nov;48(5):732–741. doi: 10.1097/00007890-198911000-00002. [DOI] [PubMed] [Google Scholar]
- Oppenheimer-Marks N., Davis L. S., Lipsky P. E. Human T lymphocyte adhesion to endothelial cells and transendothelial migration. Alteration of receptor use relates to the activation status of both the T cell and the endothelial cell. J Immunol. 1990 Jul 1;145(1):140–148. [PubMed] [Google Scholar]
- Panayotou G., End P., Aumailley M., Timpl R., Engel J. Domains of laminin with growth-factor activity. Cell. 1989 Jan 13;56(1):93–101. doi: 10.1016/0092-8674(89)90987-2. [DOI] [PubMed] [Google Scholar]
- Patarroyo M., Makgoba M. W. Leucocyte adhesion to cells in immune and inflammatory responses. Lancet. 1989 Nov 11;2(8672):1139–1142. doi: 10.1016/s0140-6736(89)91498-0. [DOI] [PubMed] [Google Scholar]
- Paul L. C., Davidoff A., Benediktsson H., Issekutz T. B. The efficacy of LFA-1 and VLA-4 antibody treatment in rat vascularized cardiac allograft rejection. Transplantation. 1993 May;55(5):1196–1199. [PubMed] [Google Scholar]
- Pelletier R. P., Ohye R. G., Vanbuskirk A., Sedmak D. D., Kincade P., Ferguson R. M., Orosz C. G. Importance of endothelial VCAM-1 for inflammatory leukocytic infiltration in vivo. J Immunol. 1992 Oct 1;149(7):2473–2481. [PubMed] [Google Scholar]
- Pryzwansky K. B., Wyatt T., Reed W., Ross G. D. Phorbol ester induces transient focal concentrations of functional, newly expressed CR3 in neutrophils at sites of specific granule exocytosis. Eur J Cell Biol. 1991 Feb;54(1):61–75. [PubMed] [Google Scholar]
- Renkonen R., Turunen J. P., Rapola J., Häyry P. Characterization of high endothelial-like properties of peritubular capillary endothelium during acute renal allograft rejection. Am J Pathol. 1990 Sep;137(3):643–651. [PMC free article] [PubMed] [Google Scholar]
- Rose M. L., Page C., Hengstenberg C., Yacoub M. H. Identification of antigen presenting cells in normal and transplanted human heart: importance of endothelial cells. Hum Immunol. 1990 Jun;28(2):179–185. doi: 10.1016/0198-8859(90)90017-j. [DOI] [PubMed] [Google Scholar]
- Rose M., Page C., Hengstenberg C., Yacoub M. Immunocytochemical markers of activation in cardiac transplant rejection. Eur Heart J. 1991 Aug;12 (Suppl 500):147–150. doi: 10.1093/eurheartj/12.suppl_d.147. [DOI] [PubMed] [Google Scholar]
- Roubey R. A., Ross G. D., Merrill J. T., Walton F., Reed W., Winchester R. J., Buyon J. P. Staurosporine inhibits neutrophil phagocytosis but not iC3b binding mediated by CR3 (CD11b/CD18). J Immunol. 1991 May 15;146(10):3557–3562. [PubMed] [Google Scholar]
- Shimizu Y., Shaw S., Graber N., Gopal T. V., Horgan K. J., Van Seventer G. A., Newman W. Activation-independent binding of human memory T cells to adhesion molecule ELAM-1. Nature. 1991 Feb 28;349(6312):799–802. doi: 10.1038/349799a0. [DOI] [PubMed] [Google Scholar]
- Shimizu Y., Van Seventer G. A., Horgan K. J., Shaw S. Regulated expression and binding of three VLA (beta 1) integrin receptors on T cells. Nature. 1990 May 17;345(6272):250–253. doi: 10.1038/345250a0. [DOI] [PubMed] [Google Scholar]
- Shimizu Y., van Seventer G. A., Horgan K. J., Shaw S. Costimulation of proliferative responses of resting CD4+ T cells by the interaction of VLA-4 and VLA-5 with fibronectin or VLA-6 with laminin. J Immunol. 1990 Jul 1;145(1):59–67. [PubMed] [Google Scholar]
- Shiohara T., Moriya N., Saizawa K., Gotoh C., Yagita H., Nagashima M. Evidence for involvement of lymphocyte function-associated antigen 1 in T cell migration to epidermis. J Immunol. 1991 Feb 1;146(3):840–845. [PubMed] [Google Scholar]
- Smith C. W., Kishimoto T. K., Abbassi O., Hughes B., Rothlein R., McIntire L. V., Butcher E., Anderson D. C., Abbass O. Chemotactic factors regulate lectin adhesion molecule 1 (LECAM-1)-dependent neutrophil adhesion to cytokine-stimulated endothelial cells in vitro. J Clin Invest. 1991 Feb;87(2):609–618. doi: 10.1172/JCI115037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhoff G., Behrend M., Haverich A. Signs of endothelial inflammation in human heart allografts. Eur Heart J. 1991 Aug;12 (Suppl 500):141–143. doi: 10.1093/eurheartj/12.suppl_d.141. [DOI] [PubMed] [Google Scholar]
- Terasaki P. I., Cecka J. M., Lim E., Takemoto S., Cho Y., Gjertson D., Ogura K., Koyama H., Mitsuishi Y., Yuge J. UCLA and UNOS Registries. Overview. Clin Transpl. 1991:409–430. [PubMed] [Google Scholar]
- Van Seventer G. A., Shimizu Y., Horgan K. J., Shaw S. The LFA-1 ligand ICAM-1 provides an important costimulatory signal for T cell receptor-mediated activation of resting T cells. J Immunol. 1990 Jun 15;144(12):4579–4586. [PubMed] [Google Scholar]
- Vermot Desroches C., Rigal D., Andréoni C. Regulation and functional involvement of distinct determinants of leucocyte function-associated antigen 1 (LFA-1) in T-cell activation in vitro. Scand J Immunol. 1991 Mar;33(3):277–286. doi: 10.1111/j.1365-3083.1991.tb01773.x. [DOI] [PubMed] [Google Scholar]
- Williams A. J., Atkins R. C., Fries J. W., Gimbrone M. A., Jr, Cybulsky M. I., Collins T. Nucleotide sequence of rat vascular cell adhesion molecule-1 cDNA. Biochim Biophys Acta. 1992 Jun 15;1131(2):214–216. doi: 10.1016/0167-4781(92)90081-a. [DOI] [PubMed] [Google Scholar]
- Wright S. D., Ramos R. A., Hermanowski-Vosatka A., Rockwell P., Detmers P. A. Activation of the adhesive capacity of CR3 on neutrophils by endotoxin: dependence on lipopolysaccharide binding protein and CD14. J Exp Med. 1991 May 1;173(5):1281–1286. doi: 10.1084/jem.173.5.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wuthrich R. P., Jenkins T. A., Snyder T. L. Regulation of cytokine-stimulated vascular cell adhesion molecule-1 expression in renal tubular epithelial cells. Transplantation. 1993 Jan;55(1):172–177. doi: 10.1097/00007890-199301000-00032. [DOI] [PubMed] [Google Scholar]
- Yong K., Addison I. E., Johnson B., Webster A. D., Linch D. C. Role of leucocyte integrins in phagocyte responses to granulocyte-macrophage colony stimulating factor (GM-CSF): in vitro and in vivo studies on leucocyte adhesion deficiency neutrophils. Br J Haematol. 1991 Feb;77(2):150–157. doi: 10.1111/j.1365-2141.1991.tb07970.x. [DOI] [PubMed] [Google Scholar]



