Abstract
OBJECTIVE: This study investigated the influence of the fetal environment on the healing characteristics of adult skin. SUMMARY BACKGROUND DATA: The remarkable ability of the fetus to heal without scarring is poorly understood. The unique qualities of fetal wound healing may be caused by the fetal environment, the fetal tissues, or a combination of both. There are numerous differences between the prenatal and postnatal environments that may play a role in the unique fetal response to injury. METHODS: Full-thickness adult sheep skin was transplanted onto the backs of 60-day-gestation fetal lambs (term, 145 days of gestation). The adult skin grafts were thus perfused by fetal blood and bathed in amniotic fluid. Previous work has demonstrated that, before midgestation, fetal lambs do not reject allogenic skin grafts. Forty days later (100 days of gestation), incisional wounds were made on both the adult skin graft and the adjacent fetal skin. The wounds were harvested 14 days postwounding and analyzed by both light microscopy and immunohistochemical testing using antibodies to collagen types I, III, and VI. RESULTS: The wounds in the adult skin grafts healed with scar formation. This observation contrasts strongly with the scarless healing of the incisional fetal skin wounds. CONCLUSIONS: This study suggests that scarless fetal skin healing properties are intrinsic to fetal skin and are not primarily the result of the fetal environment.
Full text
PDF

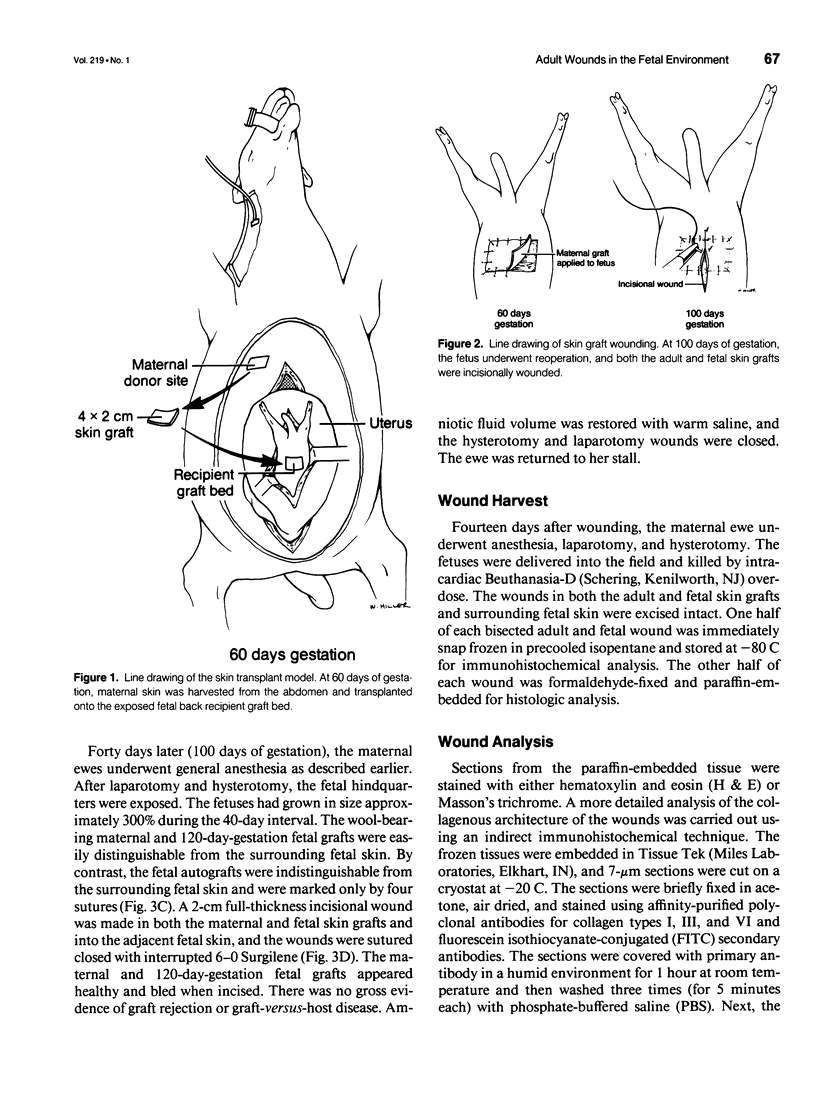
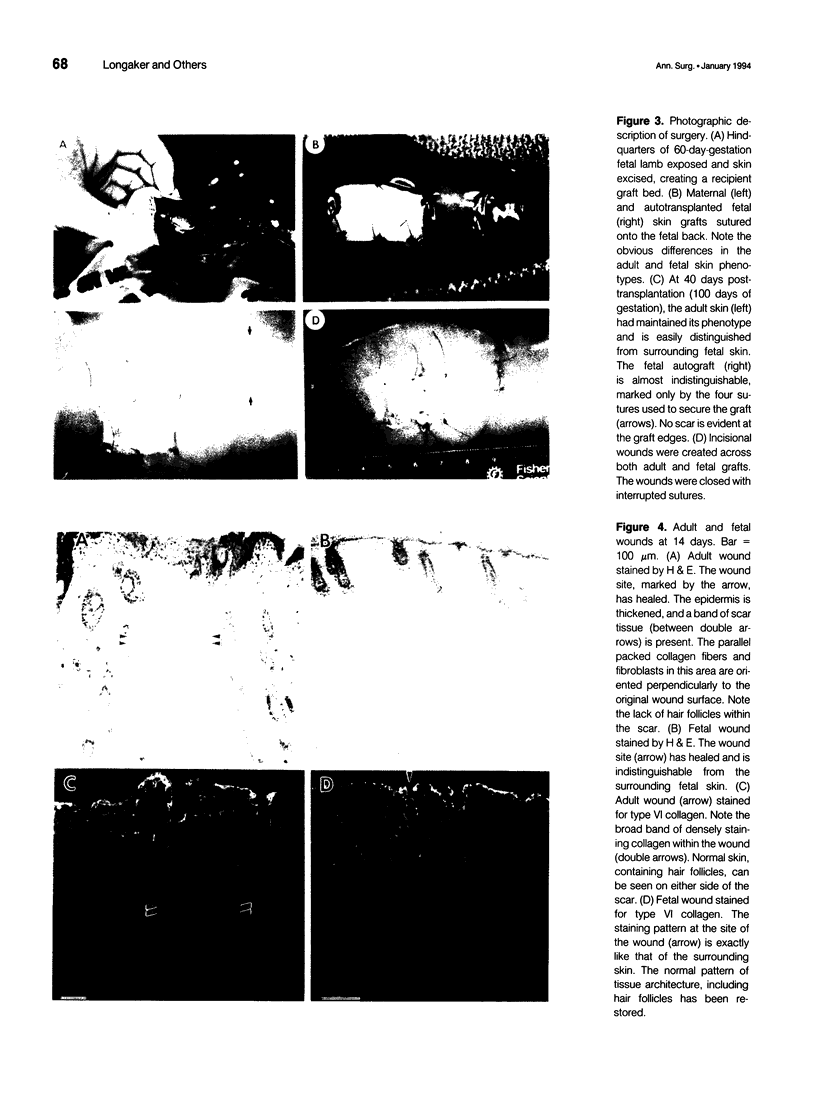




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adzick N. S., Harrison M. R., Flake A. W., Howell L. J., Golbus M. S., Filly R. A. Fetal surgery for cystic adenomatoid malformation of the lung. J Pediatr Surg. 1993 Jun;28(6):806–812. doi: 10.1016/0022-3468(93)90332-f. [DOI] [PubMed] [Google Scholar]
- Adzick N. S., Longaker M. T. Scarless fetal healing. Therapeutic implications. Ann Surg. 1992 Jan;215(1):3–7. doi: 10.1097/00000658-199201000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adzick N. S., Outwater K. M., Harrison M. R., Davies P., Glick P. L., deLorimier A. A., Reid L. M. Correction of congenital diaphragmatic hernia in utero. IV. An early gestational fetal lamb model for pulmonary vascular morphometric analysis. J Pediatr Surg. 1985 Dec;20(6):673–680. doi: 10.1016/s0022-3468(85)80022-1. [DOI] [PubMed] [Google Scholar]
- Brown R., Brockes J. P. Identification and expression of a regeneration-specific homeobox gene in the newt limb blastema. Development. 1991 Feb;111(2):489–496. doi: 10.1242/dev.111.2.489. [DOI] [PubMed] [Google Scholar]
- Chernoff E. A., Robertson S. Epidermal growth factor and the onset of epithelial epidermal wound healing. Tissue Cell. 1990;22(2):123–135. doi: 10.1016/0040-8166(90)90016-3. [DOI] [PubMed] [Google Scholar]
- Ffrench-Constant C., Hynes R. O. Alternative splicing of fibronectin is temporally and spatially regulated in the chicken embryo. Development. 1989 Jun;106(2):375–388. doi: 10.1242/dev.106.2.375. [DOI] [PubMed] [Google Scholar]
- Fleischmajer R., Perlish J. S., Burgeson R. E., Shaikh-Bahai F., Timpl R. Type I and type III collagen interactions during fibrillogenesis. Ann N Y Acad Sci. 1990;580:161–175. doi: 10.1111/j.1749-6632.1990.tb17927.x. [DOI] [PubMed] [Google Scholar]
- Goustin A. S., Betsholtz C., Pfeifer-Ohlsson S., Persson H., Rydnert J., Bywater M., Holmgren G., Heldin C. H., Westermark B., Ohlsson R. Coexpression of the sis and myc proto-oncogenes in developing human placenta suggests autocrine control of trophoblast growth. Cell. 1985 May;41(1):301–312. doi: 10.1016/0092-8674(85)90083-2. [DOI] [PubMed] [Google Scholar]
- Grey A. M., Schor A. M., Rushton G., Ellis I., Schor S. L. Purification of the migration stimulating factor produced by fetal and breast cancer patient fibroblasts. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2438–2442. doi: 10.1073/pnas.86.7.2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison M. R., Adzick N. S. The fetus as a patient. Surgical considerations. Ann Surg. 1991 Apr;213(4):279–278. doi: 10.1097/00000658-199104000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertle M. D., Adams J. C., Watt F. M. Integrin expression during human epidermal development in vivo and in vitro. Development. 1991 May;112(1):193–206. doi: 10.1242/dev.112.1.193. [DOI] [PubMed] [Google Scholar]
- Hill D. J., Strain A. J., Elstow S. F., Swenne I., Milner R. D. Bi-functional action of transforming growth factor-beta on DNA synthesis in early passage human fetal fibroblasts. J Cell Physiol. 1986 Aug;128(2):322–328. doi: 10.1002/jcp.1041280226. [DOI] [PubMed] [Google Scholar]
- Klein G., Ekblom M., Fecker L., Timpl R., Ekblom P. Differential expression of laminin A and B chains during development of embryonic mouse organs. Development. 1990 Nov;110(3):823–837. doi: 10.1242/dev.110.3.823. [DOI] [PubMed] [Google Scholar]
- Kücherer-Ehret A., Pottgiesser J., Kreutzberg G. W., Thoenen H., Edgar D. Developmental loss of laminin from the interstitial extracellular matrix correlates with decreased laminin gene expression. Development. 1990 Dec;110(4):1285–1293. doi: 10.1242/dev.110.4.1285. [DOI] [PubMed] [Google Scholar]
- LEVENSON S. M., GEEVER E. F., CROWLEY L. V., OATES J. F., 3rd, BERARD C. W., ROSEN H. THE HEALING OF RAT SKIN WOUNDS. Ann Surg. 1965 Feb;161:293–308. doi: 10.1097/00000658-196502000-00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longaker M. T., Adzick N. S. The biology of fetal wound healing: a review. Plast Reconstr Surg. 1991 Apr;87(4):788–798. doi: 10.1097/00006534-199104000-00032. [DOI] [PubMed] [Google Scholar]
- Longaker M. T., Chiu E. S., Adzick N. S., Stern M., Harrison M. R., Stern R. Studies in fetal wound healing. V. A prolonged presence of hyaluronic acid characterizes fetal wound fluid. Ann Surg. 1991 Apr;213(4):292–296. doi: 10.1097/00000658-199104000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longaker M. T., Whitby D. J., Adzick N. S., Crombleholme T. M., Langer J. C., Duncan B. W., Bradley S. M., Stern R., Ferguson M. W., Harrison M. R. Studies in fetal wound healing, VI. Second and early third trimester fetal wounds demonstrate rapid collagen deposition without scar formation. J Pediatr Surg. 1990 Jan;25(1):63–69. doi: 10.1016/s0022-3468(05)80165-4. [DOI] [PubMed] [Google Scholar]
- Lorenz H. P., Longaker M. T., Perkocha L. A., Jennings R. W., Harrison M. R., Adzick N. S. Scarless wound repair: a human fetal skin model. Development. 1992 Jan;114(1):253–259. doi: 10.1242/dev.114.1.253. [DOI] [PubMed] [Google Scholar]
- Lorenz H. P., Whitby D. J., Longaker M. T., Adzick N. S. Fetal wound healing. The ontogeny of scar formation in the non-human primate. Ann Surg. 1993 Apr;217(4):391–396. doi: 10.1097/00000658-199304000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie A., Leeming G. L., Jowett A. K., Ferguson M. W., Sharpe P. T. The homeobox gene Hox 7.1 has specific regional and temporal expression patterns during early murine craniofacial embryogenesis, especially tooth development in vivo and in vitro. Development. 1991 Feb;111(2):269–285. doi: 10.1242/dev.111.2.269. [DOI] [PubMed] [Google Scholar]
- McCullagh P. Immunological tolerance of sheep to skin allografts. Transplantation. 1988 Aug;46(2):280–285. doi: 10.1097/00007890-198808000-00018. [DOI] [PubMed] [Google Scholar]
- McCullagh P. Inability of fetal skin to induce allograft tolerance in fetal lambs. Immunology. 1989 Aug;67(4):489–495. [PMC free article] [PubMed] [Google Scholar]
- Mulvihill S. J., Stone M. M., Fonkalsrud E. W., Debas H. T. Trophic effect of amniotic fluid on fetal gastrointestinal development. J Surg Res. 1986 Apr;40(4):291–296. doi: 10.1016/0022-4804(86)90189-7. [DOI] [PubMed] [Google Scholar]
- SILVERSTEIN A. M., PRENDERGAST R. A., KRANER K. L. FETAL RESPONSE TO ANTIGENIC STIMULUS. IV. REJECTION OF SKIN HOMOGRAFTS BY THE FETAL LAMB. J Exp Med. 1964 Jan 1;119:955–964. doi: 10.1084/jem.119.6.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schor S. L., Schor A. M., Grey A. M., Chen J., Rushton G., Grant M. E., Ellis I. Mechanism of action of the migration stimulating factor produced by fetal and cancer patient fibroblasts: effect on hyaluronic and synthesis. In Vitro Cell Dev Biol. 1989 Aug;25(8):737–746. doi: 10.1007/BF02623727. [DOI] [PubMed] [Google Scholar]
- Schor S. L., Schor A. M., Rushton G., Smith L. Adult, foetal and transformed fibroblasts display different migratory phenotypes on collagen gels: evidence for an isoformic transition during foetal development. J Cell Sci. 1985 Feb;73:221–234. doi: 10.1242/jcs.73.1.221. [DOI] [PubMed] [Google Scholar]
- Smith L. T., Holbrook K. A. Embryogenesis of the dermis in human skin. Pediatr Dermatol. 1986 Sep;3(4):271–280. doi: 10.1111/j.1525-1470.1986.tb00525.x. [DOI] [PubMed] [Google Scholar]
- Taylor R. N., Williams L. T. Developmental expression of platelet-derived growth factor and its receptor in the human placenta. Mol Endocrinol. 1988 Jul;2(7):627–632. doi: 10.1210/mend-2-7-627. [DOI] [PubMed] [Google Scholar]
- Whitby D. J., Ferguson M. W. Immunohistochemical localization of growth factors in fetal wound healing. Dev Biol. 1991 Sep;147(1):207–215. doi: 10.1016/s0012-1606(05)80018-1. [DOI] [PubMed] [Google Scholar]
- Whitby D. J., Ferguson M. W. The extracellular matrix of lip wounds in fetal, neonatal and adult mice. Development. 1991 Jun;112(2):651–668. doi: 10.1242/dev.112.2.651. [DOI] [PubMed] [Google Scholar]
- Whitby D. J., Longaker M. T., Harrison M. R., Adzick N. S., Ferguson M. W. Rapid epithelialisation of fetal wounds is associated with the early deposition of tenascin. J Cell Sci. 1991 Jul;99(Pt 3):583–586. doi: 10.1242/jcs.99.3.583. [DOI] [PubMed] [Google Scholar]