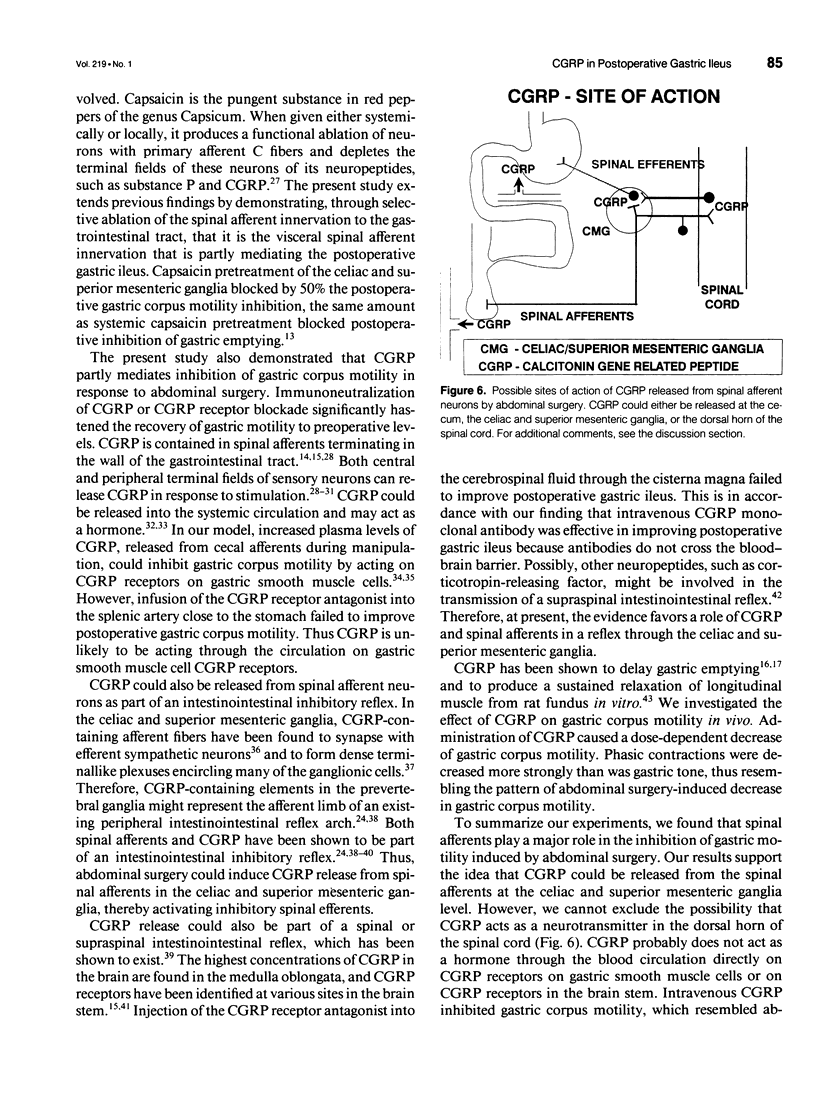
Abstract
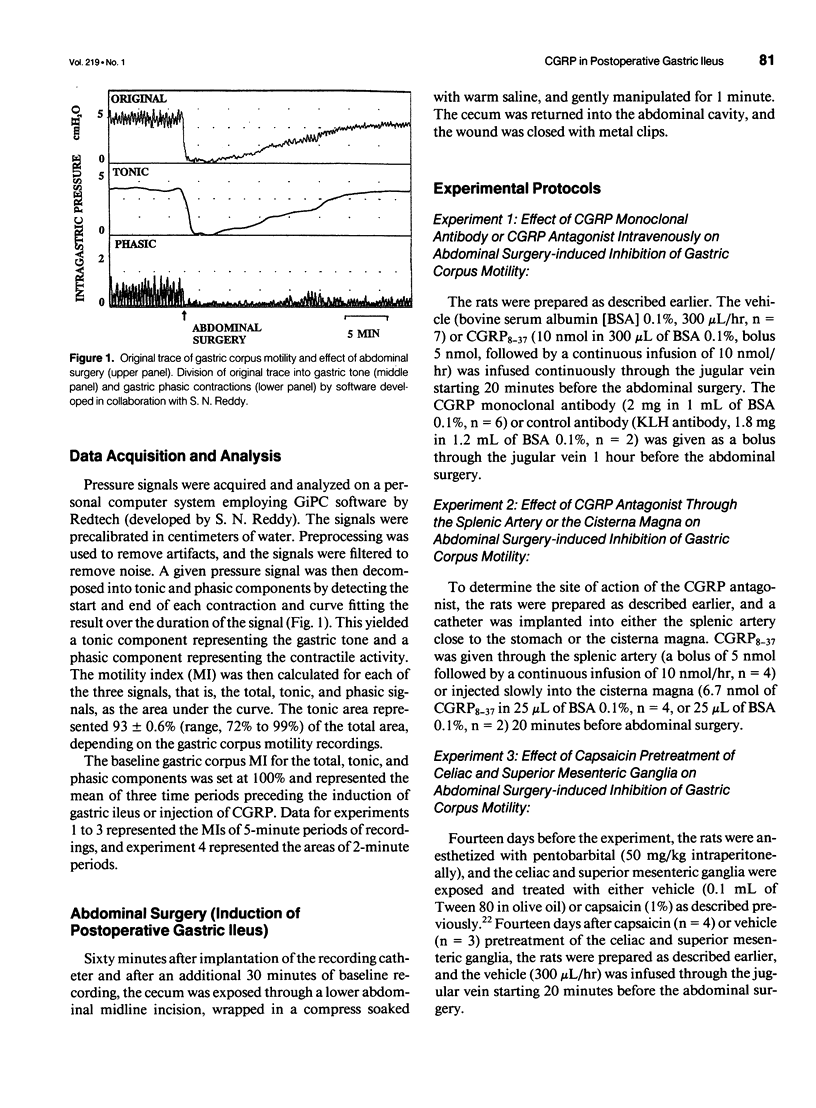
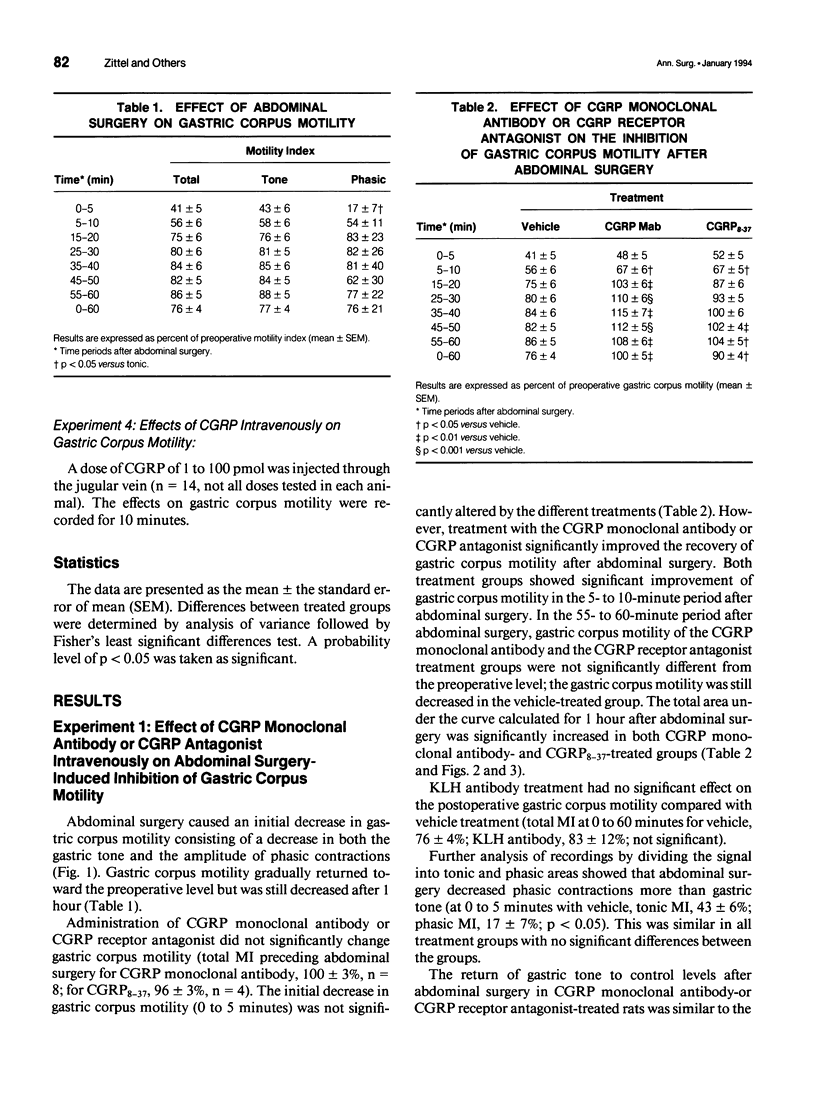
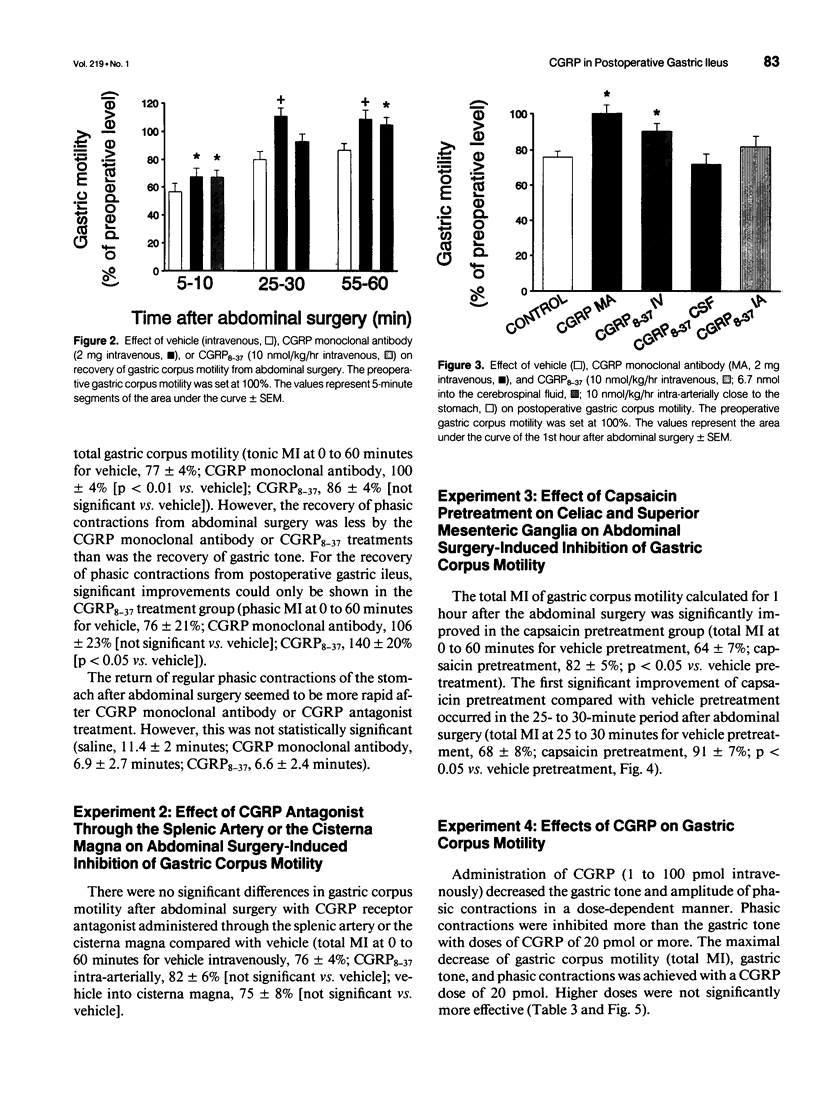
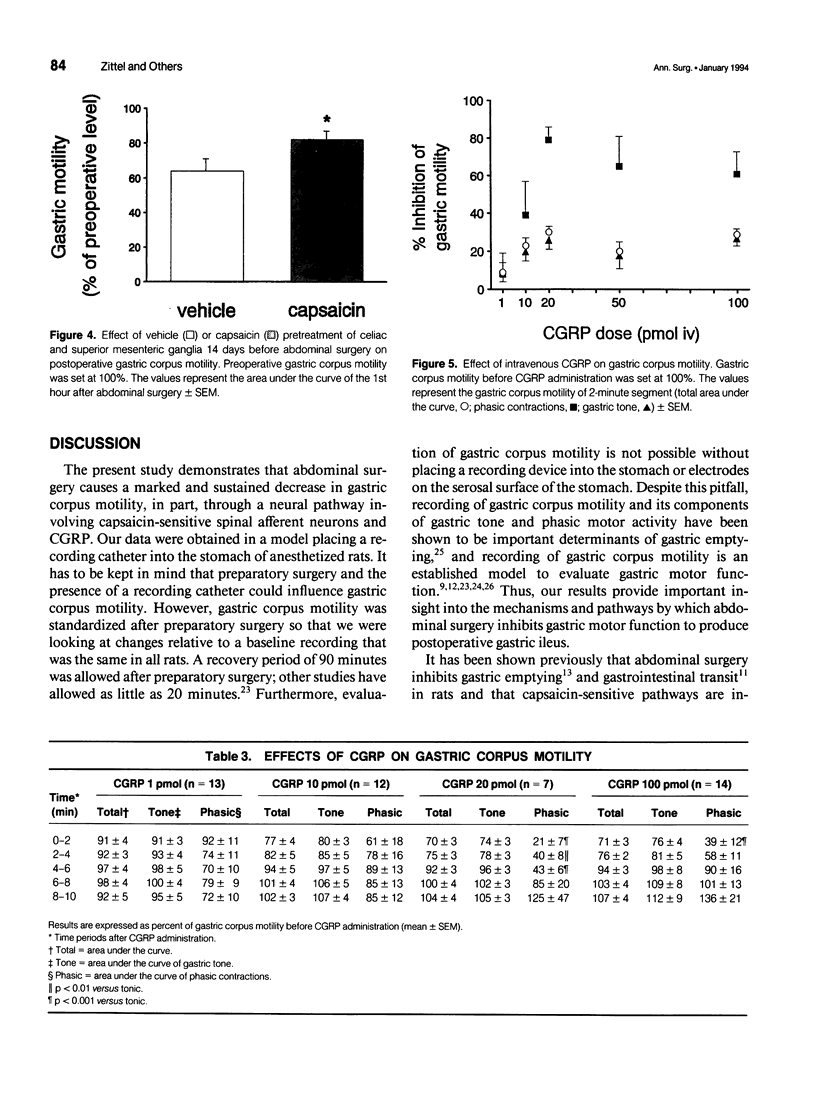
OBJECTIVE: The object of this study was to investigate the mechanisms of postoperative gastric ileus in an experimental model of abdominal surgery in anesthetized rats. SUMMARY BACKGROUND DATA: Sensory neurons partly mediate postoperative gastric ileus. Among other neuropeptides, sensory neurons contain calcitonin gene-related peptide (CGRP) and release CGRP in response to noxious stimulation. Because CGRP inhibits gastric motility, it was hypothesized that abdominal surgery stimulates sensory neurons, which then releases CGRP, thereby inhibiting gastric motility. METHODS: Postoperative ileus was induced by abdominal surgery. Gastric corpus motility was measured by an intragastric catheter. CGRP action was blocked by CGRP immunoneutralization or by a CGRP receptor antagonist. Spinal sensory neurons were ablated by application of a sensory neurotoxin (capsaicin) to the celiac and superior mesenteric ganglia. RESULTS: Abdominal surgery decreased gastric corpus motility in the first 5 minutes after abdominal surgery by 59 +/- 5% and by 24 +/- 4% during the 1st postoperative hour. Capsaicin pretreatment of the celiac and superior mesenteric ganglia, CGRP immunoneutralization, or CGRP receptor antagonism reversed the postoperative decrease in gastric corpus motility during the 1st postoperative hour by 50%, 100%, and 59%, respectively. CONCLUSIONS: These data indicate that spinal sensory neurons and CGRP partly mediate postoperative gastric ileus. CGRP may be released from spinal sensory neuron terminals in the celiac and superior mesenteric ganglia as part of an extraspinal intestinogastric inhibitory reflex activated by abdominal surgery.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barquist E., Zinner M., Rivier J., Taché Y. Abdominal surgery-induced delayed gastric emptying in rats: role of CRF and sensory neurons. Am J Physiol. 1992 Apr;262(4 Pt 1):G616–G620. doi: 10.1152/ajpgi.1992.262.4.G616. [DOI] [PubMed] [Google Scholar]
- Bartho L., Koczan G., Holzer P., Maggi C. A., Szolcsanyi J. Antagonism of the motor effects of CGRP and of capsaicin on the guinea pig ileum by human CGRP8-37. Ann N Y Acad Sci. 1992 Jun 30;657:538–540. doi: 10.1111/j.1749-6632.1992.tb22827.x. [DOI] [PubMed] [Google Scholar]
- Chakder S., Rattan S. Antagonism of calcitonin gene-related peptide (CGRP) by human CGRP-(8-37): role of CGRP in internal anal sphincter relaxation. J Pharmacol Exp Ther. 1991 Mar;256(3):1019–1024. [PubMed] [Google Scholar]
- Del Fiacco M., Floris A., Lai M. L., Montisci R., Quartu M. Calcitonin gene-related peptide in human celiac/superior mesenteric and inferior mesenteric ganglia. Immunohistochemical localization and coexistence with substance P. Ann N Y Acad Sci. 1992 Jun 30;657:473–476. doi: 10.1111/j.1749-6632.1992.tb22804.x. [DOI] [PubMed] [Google Scholar]
- Delbro D. S., Lisander B. Inhibition of gastric motility via an extraspinal pathway, by afferent mesenteric nerve stimulation in the pitched rat. Acta Physiol Scand. 1991 Jan;141(1):125–126. doi: 10.1111/j.1748-1716.1991.tb09052.x. [DOI] [PubMed] [Google Scholar]
- Delbro D. Spinovagal reflex modulation of gastric motility in response to mucosal nociceptive stimulation in the anaesthetized rat. Scand J Gastroenterol. 1989 Oct;24(8):933–938. doi: 10.3109/00365528909089237. [DOI] [PubMed] [Google Scholar]
- Diez Guerra F. J., Zaidi M., Bevis P., MacIntyre I., Emson P. C. Evidence for release of calcitonin gene-related peptide and neurokinin A from sensory nerve endings in vivo. Neuroscience. 1988 Jun;25(3):839–846. doi: 10.1016/0306-4522(88)90039-5. [DOI] [PubMed] [Google Scholar]
- Dubois A., Henry D. P., Kopin I. J. Plasma catecholamines and postoperative gastric emptying and small intestinal propulsion in the rat. Gastroenterology. 1975 Mar;68(3):466–469. [PubMed] [Google Scholar]
- Dubois A., Kopin I. J., Pettigrew K. D., Jacobowitz D. M. Chemical and histochemical studies of postoperative sympathetic activity in the digestive tract in rats. Gastroenterology. 1974 Mar;66(3):403–407. [PubMed] [Google Scholar]
- Dubois A., Weise V. K., Kopin I. J. Postoperative ileus in the rat: physiopathology, etiology and treatment. Ann Surg. 1973 Dec;178(6):781–786. doi: 10.1097/00000658-197312000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emson P. C., Zaidi M. Further evidence for the origin of circulating calcitonin gene-related peptide in the rat. J Physiol. 1989 May;412:297–308. doi: 10.1113/jphysiol.1989.sp017616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gates T. S., Zimmerman R. P., Mantyh C. R., Vigna S. R., Mantyh P. W. Calcitonin gene-related peptide-alpha receptor binding sites in the gastrointestinal tract. Neuroscience. 1989;31(3):757–770. doi: 10.1016/0306-4522(89)90439-9. [DOI] [PubMed] [Google Scholar]
- Geppetti P., Tramontana M., Evangelista S., Renzi D., Maggi C. A., Fusco B. M., Del Bianco E. Differential effect on neuropeptide release of different concentrations of hydrogen ions on afferent and intrinsic neurons of the rat stomach. Gastroenterology. 1991 Dec;101(6):1505–1511. doi: 10.1016/0016-5085(91)90385-x. [DOI] [PubMed] [Google Scholar]
- Glise H., Abrahamsson H. Reflex inhibition of gastric motility pathophysiological aspects. Scand J Gastroenterol Suppl. 1984;89:77–82. [PubMed] [Google Scholar]
- Glise H., Abrahamsson H. Reflex vagal inhibition of gastric motility by intestinal nociceptive stimulation in the cat. Scand J Gastroenterol. 1980;15(7):769–774. doi: 10.3109/00365528009181528. [DOI] [PubMed] [Google Scholar]
- Holzer P. Capsaicin: cellular targets, mechanisms of action, and selectivity for thin sensory neurons. Pharmacol Rev. 1991 Jun;43(2):143–201. [PubMed] [Google Scholar]
- Holzer P., Lippe I. T., Amann R. Participation of capsaicin-sensitive afferent neurons in gastric motor inhibition caused by laparotomy and intraperitoneal acid. Neuroscience. 1992;48(3):715–722. doi: 10.1016/0306-4522(92)90414-w. [DOI] [PubMed] [Google Scholar]
- Holzer P., Lippe I. T., Holzer-Petsche U. Inhibition of gastrointestinal transit due to surgical trauma or peritoneal irritation is reduced in capsaicin-treated rats. Gastroenterology. 1986 Aug;91(2):360–363. doi: 10.1016/0016-5085(86)90569-x. [DOI] [PubMed] [Google Scholar]
- Hölzer H. H., Raybould H. E. Vagal and splanchnic sensory pathways mediate inhibition of gastric motility induced by duodenal distension. Am J Physiol. 1992 Apr;262(4 Pt 1):G603–G608. doi: 10.1152/ajpgi.1992.262.4.G603. [DOI] [PubMed] [Google Scholar]
- Ingram D. M., Sheiner H. J. Postoperative gastric emptying. Br J Surg. 1981 Aug;68(8):572–576. doi: 10.1002/bjs.1800680816. [DOI] [PubMed] [Google Scholar]
- Itano N., Neya T. The effect of cecal volume change on gastric motility in rats. Acta Med Okayama. 1985 Apr;39(2):91–98. doi: 10.18926/AMO/31516. [DOI] [PubMed] [Google Scholar]
- Jürgen Lenz H. Calcitonin and CGRP inhibit gastrointestinal transit via distinct neuronal pathways. Am J Physiol. 1988 Jun;254(6 Pt 1):G920–G924. doi: 10.1152/ajpgi.1988.254.6.G920. [DOI] [PubMed] [Google Scholar]
- Katsoulis S., Conlon J. M. Calcitonin gene-related peptides relax guinea pig and rat gastric smooth muscle. Eur J Pharmacol. 1989 Mar 14;162(1):129–134. doi: 10.1016/0014-2999(89)90612-2. [DOI] [PubMed] [Google Scholar]
- Lee Y., Hayashi N., Hillyard C. J., Girgis S. I., MacIntyre I., Emson P. C., Tohyama M. Calcitonin gene-related peptide-like immunoreactive sensory fibers form synaptic contact with sympathetic neurons in the rat celiac ganglion. Brain Res. 1987 Mar 24;407(1):149–151. doi: 10.1016/0006-8993(87)91229-7. [DOI] [PubMed] [Google Scholar]
- Lee Y., Takami K., Kawai Y., Girgis S., Hillyard C. J., MacIntyre I., Emson P. C., Tohyama M. Distribution of calcitonin gene-related peptide in the rat peripheral nervous system with reference to its coexistence with substance P. Neuroscience. 1985 Aug;15(4):1227–1237. doi: 10.1016/0306-4522(85)90265-9. [DOI] [PubMed] [Google Scholar]
- Livingston E. H., Passaro E. P., Jr Postoperative ileus. Dig Dis Sci. 1990 Jan;35(1):121–132. doi: 10.1007/BF01537233. [DOI] [PubMed] [Google Scholar]
- Lundberg J. M., Franco-Cereceda A., Alving K., Delay-Goyet P., Lou Y. P. Release of calcitonin gene-related peptide from sensory neurons. Ann N Y Acad Sci. 1992 Jun 30;657:187–193. doi: 10.1111/j.1749-6632.1992.tb22767.x. [DOI] [PubMed] [Google Scholar]
- Maton P. N., Sutliff V. E., Zhou Z. C., Collins S. M., Gardner J. D., Jensen R. T. Characterization of receptors for calcitonin gene-related peptide on gastric smooth muscle cells. Am J Physiol. 1988 Jun;254(6 Pt 1):G789–G794. doi: 10.1152/ajpgi.1988.254.6.G789. [DOI] [PubMed] [Google Scholar]
- Mizutani M., Neya T., Nakayama S. Capsaicin-sensitive afferents activate a sympathetic intestinointestinal inhibitory reflex in dogs. J Physiol. 1990 Jun;425:133–144. doi: 10.1113/jphysiol.1990.sp018096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton C. R., Hutchison W. D. Release of sensory neuropeptides in the spinal cord: studies with calcitonin gene-related peptide and galanin. Neuroscience. 1989;31(3):807–815. doi: 10.1016/0306-4522(89)90443-0. [DOI] [PubMed] [Google Scholar]
- Mulderry P. K., Ghatei M. A., Bishop A. E., Allen Y. S., Polak J. M., Bloom S. R. Distribution and chromatographic characterisation of CGRP-like immunoreactivity in the brain and gut of the rat. Regul Pept. 1985 Oct;12(2):133–143. doi: 10.1016/0167-0115(85)90194-6. [DOI] [PubMed] [Google Scholar]
- Nilsson F., Jung B. Gastric evacuation and small bowel propulsion after laparotomy. A study with a double isotope technique in rat. Acta Chir Scand. 1973;139(8):724–730. [PubMed] [Google Scholar]
- Raybould H. E., Kolve E., Taché Y. Central nervous system action of calcitonin gene-related peptide to inhibit gastric emptying in the conscious rat. Peptides. 1988 Jul-Aug;9(4):735–737. doi: 10.1016/0196-9781(88)90114-3. [DOI] [PubMed] [Google Scholar]
- Raybould H. E., Sternini C., Eysselein V. E., Yoneda M., Holzer P. Selective ablation of spinal afferent neurons containing CGRP attenuates gastric hyperemic response to acid. Peptides. 1992 Mar-Apr;13(2):249–254. doi: 10.1016/0196-9781(92)90104-b. [DOI] [PubMed] [Google Scholar]
- Ruwart M. J., Klepper M. S., Rush B. D. Carbachol stimulation of gastrointestinal transit in the postoperative lleus rat. J Surg Res. 1979 Jan;26(1):18–26. doi: 10.1016/0022-4804(79)90073-8. [DOI] [PubMed] [Google Scholar]
- Skofitsch G., Jacobowitz D. M. Calcitonin and calcitonin gene-related peptide receptor binding sites in the rat central nervous system. Ann N Y Acad Sci. 1992 Jun 30;657:420–422. doi: 10.1111/j.1749-6632.1992.tb22788.x. [DOI] [PubMed] [Google Scholar]
- Sternini C., Reeve J. R., Jr, Brecha N. Distribution and characterization of calcitonin gene-related peptide immunoreactivity in the digestive system of normal and capsaicin-treated rats. Gastroenterology. 1987 Oct;93(4):852–862. doi: 10.1016/0016-5085(87)90450-1. [DOI] [PubMed] [Google Scholar]
- Takaki M., Jin J. G., Nakayama S. Possible involvement of calcitonin gene-related peptide (CGRP) in non-cholinergic non-adrenergic relaxation induced by mesenteric nerve stimulation in guinea pig ileum. Brain Res. 1989 Jan 23;478(1):199–203. doi: 10.1016/0006-8993(89)91499-6. [DOI] [PubMed] [Google Scholar]
- Wang X., Jones S. B., Zhou Z., Han C., Fiscus R. R. Calcitonin gene-related peptide (CGRP) and neuropeptide Y (NPY) levels are elevated in plasma and decreased in vena cava during endotoxin shock in the rat. Circ Shock. 1992 Jan;36(1):21–30. [PubMed] [Google Scholar]
- Wong H. C., Lloyd K., Yang H., Sternini C., Walsh J. H. Preparation of a monoclonal antibody to rat alpha-CGRP for in vivo immunoneutralization of peptides. Ann N Y Acad Sci. 1992 Jun 30;657:525–527. doi: 10.1111/j.1749-6632.1992.tb22822.x. [DOI] [PubMed] [Google Scholar]



