Abstract
Streptococcal inhibitor of complement (Sic) is a secreted protein made predominantly by serotype M1 Group A Streptococcus (GAS), which contributes to persistence in the mammalian upper respiratory tract and epidemics of human disease. Unexpectedly, an isogenic sic-negative mutant adhered to human epithelial cells significantly better than the wild-type parental strain. Purified Sic inhibited the adherence of a sic negative serotype M1 mutant and of non-Sic-producing GAS strains to human epithelial cells. Sic was rapidly internalized by human epithelial cells, inducing cell flattening and loss of microvilli. Ezrin and moesin, human proteins that functionally link the cytoskeleton to the plasma membrane, were identified as Sic-binding proteins by affinity chromatography and matrix-assisted laser desorption/ionization time-of-flight mass spectrometry analysis. Sic colocalized with ezrin inside epithelial cells and bound to the F-actin-binding site region located in the carboxyl terminus of ezrin and moesin. Synthetic peptides corresponding to two regions of Sic had GAS adherence-inhibitory activity equivalent to mature Sic and inhibited binding of Sic to ezrin. In addition, the sic mutant was phagocytosed and killed by human polymorphonuclear leukocytes significantly better than the wild-type strain, and Sic colocalized with ezrin in discrete regions of polymorphonuclear leukocytes. The data suggest that binding of Sic to ezrin alters cellular processes critical for efficient GAS contact, internalization, and killing. Sic enhances bacterial survival by enabling the pathogen to avoid the intracellular environment. This process contributes to the abundance of M1 GAS in human infections and their ability to cause epidemics.
Keywords: microbiology‖Serotype M1‖epidemic waves‖ezrin
For most bacterial pathogens, there is relatively little understanding of why certain clonally related isolates cause abundant infections, whereas others do not. Molecular explanation of these phenomena is critical to development of rational methods to limit pathogen emergence, resurgence, and dissemination.
Group A Streptococcus (GAS) is an important human pathogen that causes infections ranging from pharyngitis to necrotizing fasciitis and the postinfectious sequelae acute rheumatic fever and acute glomerulonephritis. GAS are genetically highly heterogeneous. For example, more than 150 distinct M-types have been identified on the basis of antigenic differences in the M protein, an antiphagocytic surface molecule that is an important virulence factor (1). However, GAS expressing relatively few M types cause the majority of human invasive infections. In particular, M1 strains are the most common serotype recovered from invasive disease episodes and also have a propensity to cause epidemics (2).
Streptococcal inhibitor of complement (Sic) is a secreted protein produced by serotype M1 GAS that inhibits the formation of the membrane attack complex (MAC) of complement in vitro by binding to the C5b–C7 complex (3, 4). Sic production is necessary for persistence in the upper respiratory tract of mice (5); however, critical aspects of the contribution of Sic to human–pathogen interactions and M1 epidemics remain unknown. For example, interactions between Sic and host cells have not been analyzed, and the molecular mechanism(s) responsible for the abundance of Sic-expressing M1 strains in human infections is not understood. This study was undertaken to gain insight into these problems. The results provide information about the molecular strategies used by a pathogenic bacterium to cause abundant human infections.
Materials and Methods
Additional methods are published as supporting information on the PNAS web site (www.pnas.org).
Adherence Assays.
To assay GAS adherence to human epithelial cells, 10 μl of purified Sic1.01 (10 μg, 0.32 μM final concentration), purified recombinant Mycobacterium tuberculosis enoyl reductase (10 μg, 0.35 μM final concentration), or PBS was added to wells containing A549 human lung epithelial cells (ATCC CCL-185). After incubation at 37°C for 5 min, GAS (≈3–4 × 108 colony-forming units) were added in 50 μl of Hanks' balanced salt solution and incubation continued for 1 h. The mean number of bacteria per A549 cell was determined by counting the number of individual cell-associated bacteria in 50 randomly chosen A549 cells in four different fields by light microscopy.
Affinity Chromatography and Identification of Sic-Binding Proteins.
A549 cell extracts were applied to a Sepharose 4B-Sic affinity column. Specifically bound proteins were eluted and analyzed by matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) mass spectrometry (6).
Purification of Ezrin and Moesin Fusion Proteins.
The construction of moesin and ezrin proteins fused to glutathione S-transferase (GST) has been described (7, 8).
Peptide Inhibition Assays.
Purified GST-fusion proteins bound to glutathione–agarose were incubated with 50 μl of Tris-buffered saline (TBS) or purified Sic-specific peptides for 15 min with rocking, washed, and resuspended in 0.5 ml of binding buffer. Fifty microliters of TBS or purified Sic1.01 was added (0.32 μM final concentration), and the mixture was incubated for 30 min. Bead-bound proteins were eluted by boiling in sample buffer, blotted to nitrocellulose, and probed with either anti-GST or anti-Sic antibodies. Bands corresponding to Sic or the GST fusion protein were quantified by densitometry.
Polymorphonuclear Leukocyte (PMN) Reactive Oxygen Species (ROS) Production and Phagocytosis.
Human PMNs were isolated as described previously (9). PMN ROS production and phagocytosis were measured with a flow cytometric method described previously (9, 10).
Results
Sic Inhibits Adherence of Serotype M1 and M3 GAS Strains to Human Epithelial Cells.
Lukomski et al. (5) reported that an isogenic mutant M1 strain that does not produce Sic protein was significantly impaired in ability to persist in the mammalian upper respiratory tract. GAS persistence is presumed to involve adherence to host surfaces, a process generally believed to be a crucial step in successful colonization by Gram-positive bacteria. We tested the wild-type strain MGAS5005 (sic1.01) and its isogenic mutant strain MGAS5005sic (5) for their ability to adhere to human A549 epithelial cells. Unexpectedly, significantly more mutant than wild-type bacteria adhered to the host cells (Fig. 1A), indicating that Sic inhibited bacteria–host-cell interactions. Consistent with this finding, addition of purified Sic (10 μg/ml) at the beginning of the incubation period restored to wild-type levels (i.e., decreased) adherence of the mutant bacteria to host cells (Fig. 1A). The Sic-mediated inhibitory effect was dose-dependent (data not shown) and specific for Sic, as enoyl reductase, a control protein with a molecular weight and pI similar to Sic, did not significantly reduce the adherence of the mutant bacteria (Fig. 1A). Analogous results were obtained with Hep-2 human epithelial cells and human primary bronchial/ tracheal epithelial cells (data not shown).
Figure 1.
Sic interacts with human epithelial cells and inhibits adherence of M1 GAS strains. (A) Strain MGAS5005 (black bars) or the isogenic mutant strain MGAS5005sic (white bars) was incubated with A549 cells in the presence of media plus PBS, 10 μg/ml of exogenously added Sic1.01, or enoyl reductase (ER), a M. tuberculosis protein with a molecular weight and pI similar to Sic. Data are presented as percent bacterial adherence relative to MGAS5005sic in the presence of media plus PBS. Analogous results were obtained with Hep-2 human epithelial cells and human primary bronchial/tracheal epithelial cells (data not shown). (B) Antibody to Sic abrogates the inhibitory activity. MGAS5005 (closed circles) or MGAS5005sic (closed squares) were incubated with A549 cells in the presence of the indicated dilution of affinity-purified rabbit anti-Sic antibody. Open circles indicate the adherence of MGAS5005 in the presence of affinity-purified rabbit antibody raised against Spy0843. Data are presented as percent bacterial adherence relative to MGAS5005 in the presence of a 1:200 dilution of anti-Sic antibody. (C) A549 human epithelial cells or the indicated bacterial strains were incubated with 10 μg/ml of purified Sic for 5 min, washed, and then allowed to interact without further addition of Sic.
The Sic gene is present predominantly in serotype M1 GAS strains (3, 11). To determine whether the ability of Sic to inhibit the adherence of GAS was restricted to M1 serotype organisms, we incubated two serotype M3 GAS isolates (these organisms do not have the sic gene) with A549 cells in the presence or absence of purified Sic. Sic significantly inhibited the adherence of the serotype M3 GAS strains to host cells (P = 0.0006 and 0.025, respectively; data not shown), whereas the control protein did not.
Antibody to Sic Abrogates the Inhibition of Adherence of Serotype M1 GAS Strains to Epithelial Cells.
To determine whether antibody to Sic interfered with its inhibitory activity, strain MGAS5005 and MGAS5005sic were incubated with A549 cells in the presence of affinity-purified rabbit anti-Sic1.01 antibody. Addition of increasing amounts of the anti-Sic antibody resulted in increased adherence of the wild-type strain to the A549 cells but did not alter the adherence of MGAS5005sic (Fig. 1B). To determine whether antibody alone promoted adherence of the bacteria to the A549 cells, strain MGAS5005 was incubated with A549 cells in the presence of affinity-purified rabbit antibody raised against Spy0843, a protein present on the cell surface of strain MGAS5005 (12). The antibody to Spy0843 had no effect on adherence of strain MGAS5005.
Sic Interacts with Human Epithelial Cells.
To determine whether Sic affected the host cell or the bacterium, A549 cells or mutant bacteria were preincubated with purified Sic, washed, and then allowed to interact without further addition of Sic. Preincubation of A549 cells (but not GAS) with Sic reduced the adherence of the sic mutant (that is, restored the wild-type phenotype), suggesting that Sic exerts its effect through interaction with the host cell (Fig. 1C).
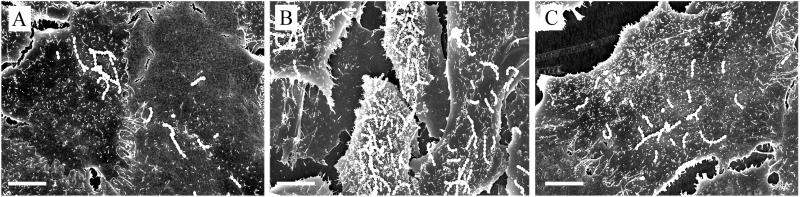
Scanning electron microscopy was used to determine whether infection of host cells with the wild-type strain or the sic mutant produced a phenotypic change. Compared with cells infected with the sic mutant strain, cells infected with wild-type MGAS5005 had fewer adherent bacteria, were flatter, and had fewer and shorter microvilli (Fig. 2). Addition of Sic at the beginning of the incubation period to host cells infected with the sic mutant produced a phenotype resembling that of cells infected with the wild-type bacteria (Fig. 2), demonstrating that Sic was responsible for the cellular changes.
Figure 2.
Phenotype of A549 cells infected with serotype M1 GAS strains. Scanning electron micrographs of A549 cells infected for 1 h with (A) MGAS5005, (B) MGAS5005sic, or (C) MGAS5005sic in the presence of 10 μg/ml of Sic. Note that there are fewer A549 cells visible in A and C because of cell flattening caused by Sic. (Bar = 10 μm.)
Sic Is Very Rapidly Internalized by Host Cells.
Sic may exert its antiadherence effect by blocking a bacterial receptor on the host-cell surface or by entering the cell and interfering with cellular functions. Optical sectioning of A549 cells treated with Sic revealed that Sic was distributed throughout the cell cytoplasm, observable as a punctate staining pattern, as early as 5 min after addition of the protein to the host cells (Fig. 3). Sic treatment resulted in loss of microvilli and increased cell flattening (Fig. 3), as indexed by a significant reduction in the number of host cells present per microscopic field (56.4 ± 3.6 cells per field for Sic treated cells versus 77.0 ± 14.3 cells per field for untreated cells; mean ± SD for five microscopic fields; P = 0.007). This reduction is not due to increased release of A549 cells from the monolayer (data not shown). Hence, Sic is rapidly internalized by host cells, suggesting that it interacts with intracellular proteins to alter cell morphology.
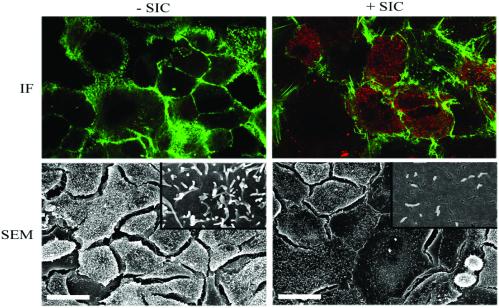
Figure 3.
Sic is rapidly internalized and induces morphological changes in human epithelial cells. (Upper) Immunofluorescence (IF) confocal microscopy of A549 cells treated with or without purified Sic1.01 for 5 min. After Sic treatment, cells were fixed with paraformaldehyde, permeabilized with saponin, and stained for Sic (red) and actin (green). An optical section through a plane near the base of the cell indicates that Sic is present in the cell cytoplasm. No cytoplasmic staining was observed in nonpermeabilized cells (data not shown). (Lower) Scanning electron microscopy (SEM) of A549 cells treated with or without purified Sic1.01 for 5 min. (Bar = 30 μm.) (Insets) Higher magnification of A549 cells demonstrating the loss of cell-surface structures after Sic treatment.
Identification of Ezrin and Moesin as Sic-Binding Proteins.
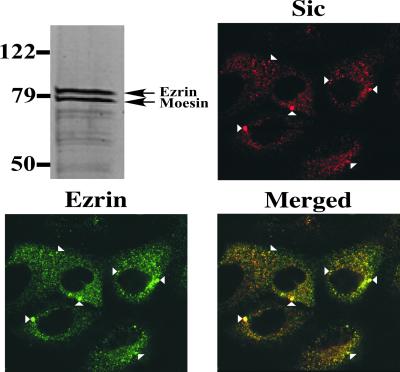
Sic-Sepharose affinity chromatography was used to identify host proteins that interact with Sic. Two proteins with an apparent molecular mass of ≈79 kDa were specifically eluted from the Sic-Sepharose affinity column (Fig. 4) and identified as ezrin [National Center for Biotechnology Information (NCBI) accession no. 4507893] and moesin (NCBI accession no. 4505257) by MALDI-TOF mass spectrometry analysis.
Figure 4.
Sic binds ezrin and moesin. (Top Left) SYPRO ruby red-stained SDS/PAGE analysis of proteins eluted from a Sic-Sepharose affinity column after incubation with an A549 whole cell lysate. Molecular mass markers are shown. Ezrin and moesin were identified by MALDI-TOF mass spectrometry analysis. The other three sections demonstrate the partial colocalization of Sic and ezrin in A549 cells. A549 cells were treated with purified Sic1.01 for 5 min, fixed, and permeabilized. Double fluorescence labeling was conducted with an affinity-purified rabbit polyclonal antibody to Sic (red) and goat anti-ezrin antiserum (green). (Bottom Right) Overlay of the two images, with colocalization visualized as yellow. Specific examples of Sic-ezrin colocalization are indicated by arrowheads.
Ezrin and moesin are members of the ezrin/radixin/moesin (ERM) family of proteins that are present in increased amounts in specialized plasma membrane structures such as filopodia and microvilli (13). Intracellular staining of moesin was poor in permeabilized A549 cells, so the subcellular distribution of Sic and ezrin in Sic-treated cells was examined by confocal immunofluorescence microscopy. Ezrin and Sic colocalized extensively throughout the cell (Fig. 4), suggesting that Sic and ezrin interact intracellularly.
Sic Binds to the Carboxyl-Terminal Regions of Ezrin and Moesin.
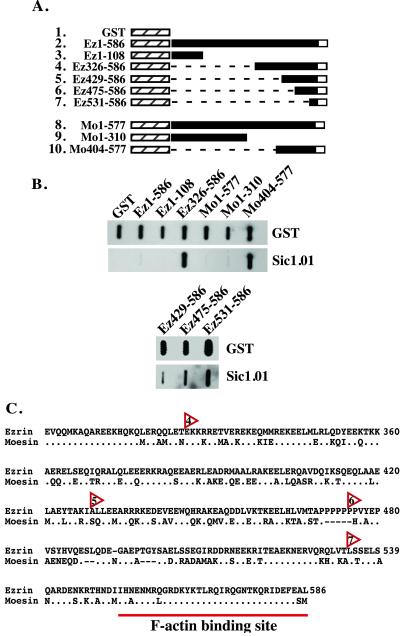
ERM proteins have a highly homologous amino-terminal domain that binds to integral membrane proteins such as CD44 (14), and a very well-conserved F-actin-binding site located at the carboxyl terminus (15). To confirm direct binding of Sic to ezrin or moesin, and to determine whether Sic interacts with the amino- or carboxyl-terminal domains, purified native GST fusion proteins containing full-length or truncated fragments of ezrin and moesin (Fig. 5A) were blotted to nitrocellulose and probed with purified Sic. Sic bound to the carboxyl-terminal domain of ezrin (amino acids 326–586) and moesin (amino acids 404–577) (Fig. 5B) but not to the amino-terminal domain of these proteins or full-length ezrin or moesin. These results are consistent with structural data indicating that the carboxyl-terminal domain is masked by intramolecular interactions in the full-length proteins (7, 16). To map the Sic-binding region present in the carboxyl terminus of ezrin, GST-fusion proteins consisting of progressively smaller fragments of ezrin were tested in the slot blot assay. Sic bound specifically to the carboxyl-terminal 56 amino acids (amino acids 531–586) of ezrin, the region that contains the F-actin-binding site (Fig. 5 B and C) (15).
Figure 5.
Sic binds to the carboxyl terminus of ezrin and moesin. (A) Schematic of ezrin and moesin GST fusion proteins used for immunoblot analysis. Ez1–586, full-length GST-ezrin fusion construct; Mo1–577, full length GST-moesin fusion construct; hatched box, GST; black box, ezrin or moesin; white box, F-actin binding site in the carboxyl terminus of ezrin or moesin; dashed lines, deleted amino acids. (B) GST or the indicated GST fusion proteins were purified in the absence of detergent and 1 μg of each protein vacuum-blotted to nitrocellulose. Blots were incubated with 20 μg of Sic1.01, and bound Sic was detected with specific antibody. Transferred GST fusion proteins were detected on a duplicate blot with anti-GST antiserum. (C) Alignment of the carboxyl termini of ezrin and moesin. The alignment starts at residue 301 of ezrin (National Center for Biotechnology Information accession no. 4507893). Periods indicate identical amino acids, and dashes indicate sequence gaps. Red arrowheads indicate the starting residue of the carboxyl-terminal ezrin GST fusion proteins that bind Sic. Numbers within the arrowheads correspond to row numbers in A.
Adherence-Inhibitory Activity of Sic Synthetic Peptides.
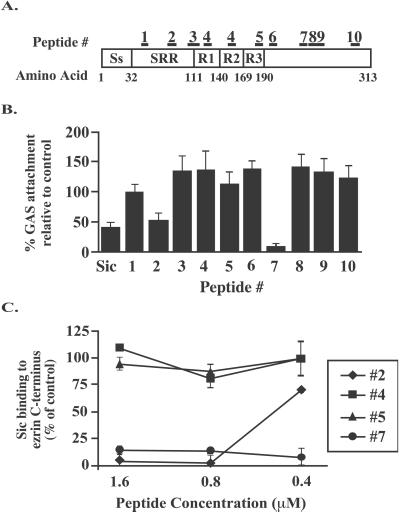
To identify regions of Sic important for the adherence-inhibitory activity, computer modeling was used to identify segments of Sic1.01 that may be surface-exposed. Synthetic peptides corresponding to 10 regions of Sic (Fig. 6A) were studied for ability to restore the antiadherence phenotype of the MGAS5005 Sic-negative isogenic strain. Two of the 10 peptides (peptide 2/YGWSSDKEEWP and peptide 7/SDWGQSEDTPRF) had significant adherence-inhibition activity (P < 0.01 compared with PBS control) (Fig. 6B). This inhibitory activity correlated with the ability of the peptides to inhibit Sic binding to glutathione–agarose beads coated with a GST fusion protein containing amino acids 531–586 of ezrin (Fig. 6C), indicating that these two regions of Sic are important for both activities.
Figure 6.
Sic-specific synthetic peptides inhibit the adherence of M1 GAS to human epithelial cells and Sic binding to ezrin. (A) Schematic of Sic1.01 showing the position of 10 peptides projected to be surface-exposed regions. Ss, signal sequence; SRR, short repeat region; R1, R2, and R2, tandem repeats R1, R2, and R3, respectively. (B) MGAS5005sic was incubated with A549 cells in the presence of Sic1.01 or a 5-fold molar excess (final concentration 1.6 μM) of each of the 10 synthetic peptides. Results are expressed as percent GAS attachment relative to bacterial binding in the absence of peptide. (C) Peptides #2 and #7 inhibit Sic binding to glutathione-agarose beads coated with the GST fusion protein Ez531–586. Results are expressed as percent Sic binding relative to Sic binding in the absence of peptide.
Increased Phagocytosis and Killing of the Sic Mutant Strain by Human PMNs.
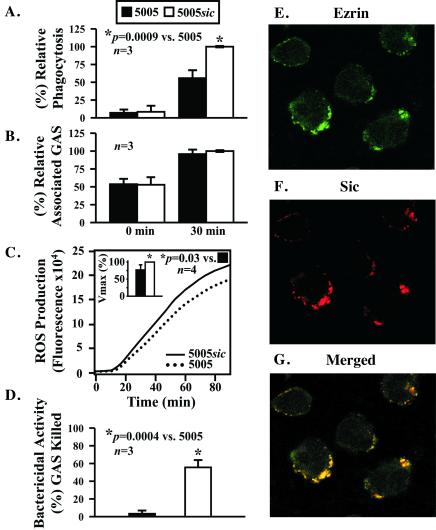
Ezrin and moesin mediate the interaction of the actin-based cytoskeleton with the plasma membrane and thus participate in formation of cellular protrusions and cell motility (13, 17). In PMNs, ezrin and moesin localize to the cytoplasmic surface of the plasma membrane and are present at the posterior pole or uropod of migrating cells (18, 19). Inasmuch as phagocytosis involves the coordination of the cell membrane and the actin cytoskeleton (20), we sought to determine whether expression of Sic detrimentally affected phagocytosis of GAS opsonized with human serum. Phagocytosis of the wild-type strain by human PMNs was significantly lower than the mutant strain (P = 0.0009) (Fig. 7A). The number of adherent bacteria was identical for both strains at 0 and 30 min after initiation of phagocytosis (Fig. 7B); hence, the reduction in phagocytosis was not due to differences in the binding of the wild-type and mutant bacteria to PMNs. Consistent with decreased phagocytosis, the wild-type strain stimulated significantly less production of intracellular reactive oxygen species (22.8 ± 15%, P = 0.03) (Fig. 7C). Moreover, compared with the MGAS5005sic mutant strain, significantly fewer wild-type bacteria were killed by the PMNs (P = 0.0004) (Fig. 7D). Taken together, the results indicate that expression of Sic detrimentally alters the ability of human PMNs to internalize and kill GAS.
Figure 7.
Phagocytosis of serotype M1 GAS and colocalization of Sic with ezrin in human PMNs. Human PMNs were incubated with fluorescently labeled and opsonized MGAS5005 (black bars) or MGAS5005sic (white bars) and the amount of (A) phagocytosis, (B) ingested and bound bacteria, and (C) reactive oxygen species production was determined at the indicated times by flow cytometry. Results are the mean ± SD of three separate experiments. (D) Bactericidal assay. Human PMNs were incubated with MGAS5005 or MGAS5005sic, and bactericidal activity was determined after 2–3 h. Results are the mean ± SD of three separate experiments. *, P = 0.0004 vs. MGAS5005. (E–G) PMNs were treated with purified Sic1.01 for 5 min, fixed, and permeabilized. Double fluorescence labeling was conducted with goat anti-ezrin antiserum (green) and an affinity-purified rabbit polyclonal antibody to Sic (red). G is an overlay of the two images, with colocalization visualized as yellow.
Sic Colocalizes with Ezrin in Human PMNs.
We next examined the subcellular distribution of Sic and ezrin in Sic-treated human PMNs by confocal immunofluorescence microscopy. Ezrin and Sic colocalized predominantly in the periphery of the PMNs (Fig. 7 E–G), strongly suggesting intracellular interaction of these proteins, as observed with epithelial cells.
Discussion
Serotype M1 GAS and Host–Cell Interactions.
Insights into host and pathogen molecular factors contributing to epidemics of human infectious diseases are limited. Our analysis provides information about a GAS protein that participates in epidemics of serotype M1 organisms. The primary discovery is that Sic directly interacts with ERM proteins to reduce the adherence of M1 GAS to human epithelial cells. These results were unexpected given the conventional wisdom in bacterial pathogenesis research that adherence, persistence, and long-term survival are closely intertwined. However, several other observations are consistent with the concept that avoidance of intimate interaction with the host conveys an enhanced survival advantage to GAS strains in the mammalian upper respiratory tract. M1 strains lack the gene (prtF) encoding a major fibronectin-binding protein that promotes adherence to host epithelial cells (21). This gene also was absent in serotype M3 and M18 organisms that commonly cause human infections. In addition, Hagman et al. (22) found that several M1 strains adhered minimally to human laryngeal epithelial cells. Gene expression analysis indicates that sic is maximally expressed in very early log phase (data not shown), suggesting that it acts during initial host–pathogen interactions. Although host-cell adherence undoubtedly contributes to the GAS life cycle, avoidance of excessive host contact early in infection may delay triggering the host inflammatory response, thereby providing a privileged niche for the bacteria to multiply and establish infection.
Several reports have described adherence and internalization of serotype M1 strains by human epithelial cells, in support of the hypothesis that the bacteria adhere to gain access to the intracellular niche (23). This process may enable the bacteria to avoid antibiotic therapy, but this remains speculative. We observed that increased phagocytosis of a sic mutant GAS strain by human PMNs resulted in increased killing of the bacteria. Schrager et al. (24) reported that GAS adherence to human keratinocytes and subsequent internalization also resulted in efficient killing of the pathogen and hypothesized that internalization of GAS is a nonspecific host defense mechanism. Hence, if adherence results in a relatively high frequency of internalization and killing, the antiphagocytosis phenotype mediated by Sic would provide a very effective strategy to enhance long-term survival and dissemination.
Cell Surface Remodeling and Adherence Inhibition.
We discovered that Sic specifically binds ezrin and moesin, two members of the ERM family of proteins that also includes radixin, erythrocyte band 4.1 protein, and merlin/schwannomin (the neurofibromatosis type 2 protein) (13). These proteins are concentrated in specialized membrane structures such as microvilli, filopodia, and lamellipodia, and participate in the formation of these structures through the creation of plasma membrane/actin filament linkages (13, 17). Sic entered A549 cells very rapidly, resulting in striking morphological changes including cell flattening and loss of microvilli. Sic colocalized with ezrin in epithelial cells, and in vitro binding assays determined that Sic interacted with the carboxyl terminus of ezrin and moesin in a region that contains the F-actin-binding site. The suggested association of Sic with full-length ezrin in vivo but not in vitro is most likely a consequence of conformational changes in ezrin that are induced by host-cell signaling events and expose the actin-binding site (13).
Taken together, our results suggest that Sic interferes with the ability of ezrin, and possibly moesin, to bind actin. We hypothesize that binding of Sic to ERM proteins alters ERM-mediated plasma membrane/actin linkages, resulting in the loss of microvilli at the epithelial cell surface. Studies examining the effects of expression of mutant ERM proteins support this hypothesis. For example, overexpression of the amino terminus of ezrin in the polarized epithelial cell line LLC-PK1 prevented formation of microvilli (25), and a similar construct in NIH 3T3 fibroblasts resulted in the formation of fragile filopodia that were defective in protrusion and retraction (26). Remodeling of the host-cell surface in this manner may directly inhibit bacteria–host-cell interactions. GAS are frequently seen in close contact with microvilli (23), an association that may be required for efficient bacterial adherence. Alternatively, adherence may be detrimentally affected indirectly if the loss of membrane projections alters the density or distribution of specific bacterial receptors. Of note, CD44, the hyaluronic acid receptor, is a receptor for the GAS hyaluronic acid capsule on human keratinocytes (27). CD44 also colocalizes with ezrin in microvilli of mouse epithelial cells (28) and cultured fibroblasts (14) because of the interaction of ezrin with the cytoplasmic domain of the receptor (28). If receptor clustering is required for efficient GAS–host-cell contact, the Sic-mediated diffuse arrangement of GAS receptors on the cell surface due to uncoupling of the plasma membrane from the actin cytoskeleton would be expected to decrease tight contact. This interpretation would explain our observation that Sic significantly inhibits, but does not abolish, bacterial adherence.
Sic and Inhibition of Phagocytosis by Human PMNs.
We discovered that in the presence of human serum opsonins, wild-type GAS are more resistant to phagocytosis than a sic mutant strain. Sic also colocalized with ezrin inside human PMNs, strongly suggesting intracellular interaction of the two proteins. Little is known about the role of ERM family members in bacterial internalization by host cells; however, ezrin has been shown to be involved in the uptake of Shigella flexneri by epithelial cells (29). Although the exact molecular mechanism is not yet understood, Sic apparently represents the first GAS antiphagocytic effector molecule to act in the interior of host cells.
Serotype M1 GAS Strains and Pathogen Abundance.
The pathogen and host processes that contribute to epidemics caused by GAS are undoubtedly complex. However, the molecular adaptations that specifically assist M1 strains to survive host defenses and cause widespread human disease are now being revealed. Serotype M1 strains have a naturally occurring single amino acid polymorphism in the extracellular cysteine protease virulence factor that creates a surface-exposed integrin-binding Arg-Gly-Asp (RGD) motif, distinct from the Arg-Ser-Asp sequence found in other GAS organisms (30). Numerically abundant serotype M1 strains contain at least 70 kb of prophage DNA encoding the potent superantigen streptococcal pyrogenic exotoxin A (scarlet fever toxin) (2) and many other molecules. M1 strains express the Sic protein that enhances extracellular life and thereby endows survival advantage on the host mucosa, whereas the sic structural gene is rarely found in other GAS. Thus, we conclude that Sic is an accessory virulence factor that specifically contributes to pathogen persistence and spread by lessening the likelihood that the bacterium will be killed by the host while associated with the mucosa. Together, these diverse adaptations provide several distinct molecular mechanisms to circumvent host defenses, resulting in a facile ability of M1 strains to persist on the host mucosa, cause infections, and spread to new hosts.
Supplementary Material
Acknowledgments
We thank B. Lei for providing purified recombinant M. tuberculosis enoyl reductase, H. Furthmayr (Stanford University) for providing moesin GST fusion proteins, M. Chaussee for conducting MALDI-TOF analysis, A. Mora for helping with figure preparation, T. Hackstadt for critical reading of the manuscript, and S. Lukomski (Baylor College of Medicine) for many helpful discussions.
Abbreviations
- GAS
group A Streptococcus
- Sic
streptococcal inhibitor of complement
- GST
glutathione S-transferase
- PMN
polymorphonuclear leukocyte
- MALDI-TOF
matrix-assisted laser desorption/ionization time-of-flight
- ERM
ezrin/radixin/moesin
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Fischetti V A. In: Gram-Positive Pathogens. Fischetti V A, Novick R P, Ferretti J J, Portnoy D A, Rood J I, editors. Washington, DC: Am. Soc. Microbiol.; 2000. pp. 11–24. [Google Scholar]
- 2.Musser J M, Krause R M. In: Emerging Infections. Krause R M, editor. New York: Academic; 1998. pp. 185–218. [Google Scholar]
- 3.Akesson P, Sjöholm A G, Bjorck L. J Biol Chem. 1996;271:1081–1088. doi: 10.1074/jbc.271.2.1081. [DOI] [PubMed] [Google Scholar]
- 4.Fernie-King B A, Seilly D J, Willers C, Wurzner R, Davies A, Lachmann P J. Immunology. 2001;103:390–398. doi: 10.1046/j.1365-2567.2001.01249.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lukomski S, Hoe N P, Abdi I, Rurangirwa J, Kordari P, Liu M, Dou S J, Adams G G, Musser J M. Infect Immun. 2000;68:535–542. doi: 10.1128/iai.68.2.535-542.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chaussee M S, Watson R O, Smoot J C, Musser J M. Infect Immun. 2001;69:822–831. doi: 10.1128/IAI.69.2.822-831.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gary R, Bretscher A. Mol Biol Cell. 1995;6:1061–1075. doi: 10.1091/mbc.6.8.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang L, Wong T Y, Lin R C, Furthmayr H. J Biol Chem. 1999;274:12803–12810. doi: 10.1074/jbc.274.18.12803. [DOI] [PubMed] [Google Scholar]
- 9.DeLeo F R, Allen L A, Apicella M, Nauseef W M. J Immunol. 1999;163:6732–6740. [PubMed] [Google Scholar]
- 10.van Eeden S F, Klut M E, Walker B A, Hogg J C. J Immunol Methods. 1999;232:23–43. doi: 10.1016/s0022-1759(99)00148-9. [DOI] [PubMed] [Google Scholar]
- 11.Hoe N P, Nakashima K, Lukomski S, Grigsby D, Liu M, Kordari P, Dou S J, Pan X, Vuopio-Varkila J, Salmelinna S, et al. Nat Med. 1999;5:924–929. doi: 10.1038/11369. [DOI] [PubMed] [Google Scholar]
- 12.Reid S D, Green N M, Buss J K, Lei B, Musser J M. Proc Natl Acad Sci USA. 2001;98:7552–7557. doi: 10.1073/pnas.121188598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bretscher A. Curr Opin Cell Biol. 1999;11:109–116. doi: 10.1016/s0955-0674(99)80013-1. [DOI] [PubMed] [Google Scholar]
- 14.Tsukita S, Oishi K, Sato N, Sagara J, Kawai A. J Cell Biol. 1994;126:391–401. doi: 10.1083/jcb.126.2.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Turunen O, Wahlstrom T, Vaheri A. J Cell Biol. 1994;126:1445–1453. doi: 10.1083/jcb.126.6.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pearson M A, Reczek D, Bretscher A, Karplus P A. Cell. 2000;101:259–270. doi: 10.1016/s0092-8674(00)80836-3. [DOI] [PubMed] [Google Scholar]
- 17.Louvet-Vallee S. Biol Cell. 2000;92:305–316. doi: 10.1016/s0248-4900(00)01078-9. [DOI] [PubMed] [Google Scholar]
- 18.Alonso-Lebrero J L, Serrador J M, Dominguez-Jimenez C, Barreiro O, Luque A, del Pozo M A, Snapp K, Kansas G, Schwartz-Albiez R, Furthmayr H, et al. Blood. 2000;95:2413–2419. [PubMed] [Google Scholar]
- 19.Pestonjamasp K, Amieva M R, Strassel C P, Nauseef W M, Furthmayr H, Luna E J. Mol Biol Cell. 1995;6:247–259. doi: 10.1091/mbc.6.3.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.May R C, Machesky L M. J Cell Sci. 2001;114:1061–1077. doi: 10.1242/jcs.114.6.1061. [DOI] [PubMed] [Google Scholar]
- 21.Natanson S, Sela S, Moses A E, Musser J M, Caparon M G, Hanski E. J Infect Dis. 1995;171:871–878. doi: 10.1093/infdis/171.4.871. [DOI] [PubMed] [Google Scholar]
- 22.Hagman M M, Dale J B, Stevens D L. FEMS Immunol Med Microbiol. 1999;23:195–204. doi: 10.1111/j.1574-695X.1999.tb01239.x. [DOI] [PubMed] [Google Scholar]
- 23.Dombek P E, Cue D, Sedgewick J, Lam H, Ruschkowski S, Finlay B B, Cleary P P. Mol Microbiol. 1999;31:859–870. doi: 10.1046/j.1365-2958.1999.01223.x. [DOI] [PubMed] [Google Scholar]
- 24.Schrager H M, Rheinwald J G, Wessels M R. J Clin Invest. 1996;98:1954–1958. doi: 10.1172/JCI118998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Crepaldi T, Gautreau A, Comoglio P M, Louvard D, Arpin M. J Cell Biol. 1997;138:423–434. doi: 10.1083/jcb.138.2.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Amieva M R, Litman P, Huang L, Ichimaru E, Furthmayr H. J Cell Sci. 1999;112:111–125. doi: 10.1242/jcs.112.1.111. [DOI] [PubMed] [Google Scholar]
- 27.Schrager H M, Alberti S, Cywes C, Dougherty G J, Wessels M R. J Clin Invest. 1998;101:1708–1716. doi: 10.1172/JCI2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yonemura S, Tsukita S. J Cell Biol. 1999;145:1497–1509. doi: 10.1083/jcb.145.7.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Skoudy A, Nhieu G T, Mantis N, Arpin M, Mounier J, Gounon P, Sansonetti P. J Cell Sci. 1999;112:2059–2068. doi: 10.1242/jcs.112.13.2059. [DOI] [PubMed] [Google Scholar]
- 30.Kagawa T F, Cooney J C, Baker H M, McSweeney S, Liu M, Gubba S, Musser J M, Baker E N. Proc Natl Acad Sci USA. 2000;97:2235–2240. doi: 10.1073/pnas.040549997. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.